Microfluidic Device for the Separation of Non-Metastatic (MCF-7) and Non-Tumor (MCF-10A) Breast Cancer Cells Using AC Dielectrophoresis
Summary
Breast cancer cells exhibit different dielectric properties compared to non-tumor breast epithelial cells. It has been hypothesized that, based on this difference in dielectric properties, the two populations can be separated for immunotherapy purposes. To support this, we model a microfluidic device to sort MCF-7 and MCF-10A cells.
Abstract
Dielectrophoretic devices are capable of the detection and manipulation of cancer cells in a label-free, cost-effective, robust, and accurate manner using the principle of the polarization of the cancer cells in the sample volume by applying an external electric field. This article demonstrates how a microfluidic platform can be utilized for high-throughput continuous sorting of non-metastatic breast cancer cells (MCF-7) and non-tumor breast epithelial cells (MCF-10A) using hydrodynamic dielectrophoresis (HDEP) from the cell mixture. By generating an electric field between two electrodes placed side-by-side with a micron-sized gap between them in an HDEP microfluidic chip, non-tumor breast epithelial cells (MCF-10A) can be pushed away, exhibiting negative DEP inside the main channel, while the non-metastatic breast cancer cells follow their course unaffected when suspended in cell medium due to having conductivity higher than the membrane conductivity. To demonstrate this concept, simulations were performed for different values of medium conductivity, and the sorting of cells was studied. A parametric study was carried out, and a suitable cell mixture conductivity was found to be 0.4 S/m. By keeping the medium conductivity fixed, an adequate AC frequency of 0.8 MHz was established, giving maximum sorting efficiency, by varying the electric field frequency. Using the demonstrated method, after choosing the appropriate cell mixture suspension medium conductivity and frequency of the applied AC, maximum sorting efficiency can be achieved.
Introduction
A malignant tumor that develops in and around the breast tissue is a frequent cause of breast cancer in women worldwide, causing a critical health problem1. Breast tumors before metastasis can be treated through surgery if detected at an early stage, but if ignored, they can have severe implications on the patient's life by spreading to their lungs, brain, and bones. The treatments offered at later stages, such as radiation and chemical-based therapies, have severe side effects2. Recent studies have reported that an early diagnosis of breast cancer reduces the mortality rate by 60%3. Hence, it is imperative to work toward personalized early detection methods. To this end, researchers working in different fields of science and technology have used microfluidics to develop devices for the early diagnosis of breast cancer4. These methods include cell affinity micro-chromatography, magnetic-activated micro-cell sorters, size-based cancer cell capture and separation, and on-chip dielectrophoresis (DEP)5,6. These microfluidic techniques reported in the literature enable precise cell manipulation, real-time monitoring, and sorting of well-defined samples, which serve as an intermediate step in many diagnostic and therapeutic applications5. The integration of these sorting mechanisms with microfluidics offers flexible and reliable manipulation of the target cells7,8,9,10. One of the main advantages of such an integration is the ability to work with fluid samples in nano to microliter volumes and also being able to manipulate the electrical properties of the sample fluid. By adjusting the conductivity of the suspending fluid inside microfluidic devices, the biological cells can be sorted based on their sizes and differences in their dielectric properties11,12.
Among these techniques, on-chip DEP is often preferred as it is a label-free cell sorting technique that exploits the electric properties of the biological samples. DEP has been reported to manipulate bio-samples such as DNA13, RNA14, proteins15, bacteria16, blood cells17, circulating tumor cells (CTCs)18, and stem cells19. Microfluidic devices that employ DEP for sorting biological samples have been reported extensively in literature20. Reservoir-based DEP microfluidic (rDEP) devices for sorting viable and non-viable yeast cells have been reported that protect the cells from the adverse effects of electrochemical reactions21,22. Piacentini et al. reported a castellated microfluidic cell sorter that separated red blood cells from platelets with an efficiency of 97%23. On-chip DEP devices with asymmetric orifices and embedded electrodes have also been reported to sort viable and non-viable cells24. Valero and Demierre et al. modified the castellated microfluidic cell sorter by introducing two arrays of microelectrodes on both sides of the channel25,26. This helped in focusing the cells in the center of the channel. Zeynep et al. presented a DEP-based microfluidic device to separate and concentrate MCF7 breast cancer cells from leukocytes27. They reported an efficiency of extracting MCF7 cells from leukocytes between 74%-98% with a frequency of 1 MHz and an applied voltage ranging from 10-12 Vpp. Supplementary Table 1 represents a qualitative and quantitative comparison between the DEP-based microfluidic sorting devices based on their design, electrode configuration, and operating parameters (applied frequency and voltage).
More recently, researchers have tried to measure the differences in the dielectric behavior of breast epithelial cells (MCF-10A) and non-metastatic breast cancer cells (MCF-7) inside a microfluidic chip28,29. Jithin et al. also characterized the dielectric responses of different cancer cell lines using an open-ended coaxial probe technique with frequencies between 200 MHz and 13.6 GHz30. These differences in the dielectric responses of MCF-7 and MCF-10A cell lines can be exploited to separate them in runtime and can lead to the development of personalized early-stage diagnosis devices.
In this article, we simulate the controlled sorting of non-metastatic breast cancer cells (MCF-7) and non-tumor breast epithelial cells (MCF-10A) using AC dielectrophoresis. The region of change in the electric field influences the sorting inside the microfluidic chip. The proposed technique is easy to implement and allows for the integration of the sorting technique into various microfluidic chip layouts. Computational fluid dynamics (CFD) simulations were carried out to study the separation of non-metastatic breast cancer cells and non-tumor breast epithelial cells by varying the conductivity of the fluid medium in which cells were suspended. In these simulations, it is shown that, by keeping the conductivity constant and by changing the applied frequency, the separation of cancer cells and healthy cells can be controlled.
Protocol
NOTE: The protocol here uses COMSOL, a multiphysics simulation software, to simulate the controlled sorting of non-metastatic breast cancer cells (MCF-7) and non-tumor breast epithelial cells (MCF-10A) using AC dielectrophoresis.
1. Chip design and parameter selection
- Open multiphysics software and select Blank Model. Right-click on the Global Definitions and select Parameters. Import the parameters given in Table 1 into global definitions as a text file or enter the values individually.
- Select Add Component from the home tab and add a 2D Component. Right-click on geometry and import the model file by double-clicking on the file.
- Choose a blank material and use the material properties from Table 1.
- Select Add Physics from the home tab, and type AC/DC. Under the AC/DC node, choose electric currents as Physik under the sub-node of electric fields and currents.
- Right-click on the Electric Current and choose the Current Conservation, Insulate, and Electric Potential sub-nodes to insulate the channel walls to assign potential to the electrodes.
- Select Add Physics from the home tab, and under the Fluid Flow node, choose Creeping Flow Physics under the sub-node of Single-Phase Flow. Right-click on Single-Phase Flow and render the chip boundaries as walls by using the Wall sub-node.
- Right click on Single-Phase Flow and add two inlet sub-nodes and one outlet sub-node.
- Assign the inlets using the inlet sub-node and use normal in Flow Velocity as the Boundary Condition. Assign the outlet using the outlet sub-node.
- Select Add Physics from the home tab, and under the Fluid Flow node, choose Particle Tracing Flow Physics under the sub node of Particle Tracing.
- Right-click on the Particle Tracing node and add the sub-nodes wall, two-particle property sub-nodes, two inlet sub-nodes, one outlet sub-node, two dielectrophoresis force sub-nodes, and one drag force sub-node.
- Set Particle Properties for both MCF-7 and MCF-10A cells using the Particle Properties sub-node. Choose the particle properties from parameters under the Global Definition section.
- Add the Drag Force sub-node to assign the dielectrophoretic force to both types of cells.
- Add Particle Properties in this case from the parameter section. Add the Shell sub-node to model mammalian cells.
- From the home tab, choose Add Mesh and select Fine Mesh. Choose Build Mesh from the home tab to build a mesh.
- From the home tab, click on Add Study to add three study steps. Study Step 1 is for simulating a frequency response; use a Frequency Domain sub-node.
- To simulate creeping flow, choose a Stationary Study node. Add two time-dependent steps to simulate conditions with dielectrophoretic force and without dielectrophoretic force.
- For the no dielectrophoretic condition, choose Physics and Variables Selection, check the box titled Modify Model Configuration for the study step, and disable the Dielectrophoretic Step. For dielectrophoretic conditions, do not disable. Save the file and press Compute for the simulation to run.
NOTE: The microfluidic chip designed for the sorting of non-metastatic breast cancer cells (MCF-7) and non-tumor breast epithelial cells (MCF-10A) has two separate inlets for cell mixture flow and for hydrodynamic flow focusing, respectively, with widths of 20 µm and 40 µm, respectively, as shown in Supplementary Figure 1 and Supplementary Figure 2. - Assign frequency (f0) under the Frequency Domain sub-node and voltage using the Electric Potential sub-node to the planer electrodes (295 µm in width) placed along the top side wall of the sorting chamber. At the outlet, use the "freeze" wall condition to visualize the sorted particles.
2. Mathematical model and computational analysis
- Verify the operating parameters for separating non-metastatic breast cancer cells and non-tumor breast epithelial cells inside the microfluidic device by setting up a computational fluid dynamic (CFD) study.
NOTE: Multiphysics software (AC/DC, Microfluidics, and Particle Tracking modules) was used for this purpose. The governing equations and theoretical background are given in detail in Supplementary File 1. The model was tested by using the dielectric properties of non-metastatic breast cancer cells (MCF7) and non-tumor breast epithelial cells (MCF-10A) reported in literature31,32, which are summarized in Table 1. - Perform the CFD simulations by introducing non-metastatic breast cancer (MCF7) and non-tumor breast epithelial (MCF-10A) cell lines with a ratio of 1:1 at the cell mixture inlet.
- Initially, perform a mesh independence study to optimize the mesh size for the simulations33.
NOTE: A mesh independence study was performed to find the best solution for the operating parameters. A set of five different mesh sizes was chosen to quantify the best possible element size for the convergence of the solution. It was observed that, when the total number of elements defining a mesh was 635 (coarser mesh), as shown in Supplementary Figure 3A, the sorting efficiency was at its lowest, with some of the MCF7 cells moving to the bottom outlet, as depicted in Supplementary Figure 3B. When the mesh size was increased to fine, the number of elements defining the mesh also increased to 2,288. The sorting efficiency was at its maximum in this case, with both MCF7 and MCF-10A cells moving toward their respective outlets. The finer mesh was also simulated, with the number of elements defining the mesh increasing to 3,188. The sorting efficiency remained unaffected beyond this point. Hence, we can safely say that fine mesh size works the best in our case. - Solve two sets of CFD studies.
- For the first set, right -click on Study 1 and add the Parametric Sweep sub-node. Press the + sign to add fluid medium conductivity "σm" as the sweep variable. Perform a parametric sweep study for the fluid medium conductivity σm ranging from 0.01 S/m to 2.5 S/m, keeping the applied frequency, f (Hz), constant at a value of 800 kHz.
- For the second set, conduct a Parametric Sweep study by varying the applied AC frequency from 100 kHz to 100 MHz while keeping the conductivity of the fluid medium, σm, fixed at 0.4 S/m for each case. This σm value was chosen based on the results of the first CFD study as a maximum separation between MCF-7 and MCF-10A was observed at this value.
- The strength of the dielectrophoresis (DEP) force, FDEP (-), exerted on a dielectric spherical particle in a conductive medium is given by Equation 1T34:
FDEP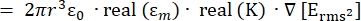 [1]
[1]
Use Equation 1 under the dielectrophoretic force sub-node. In equation 1, r shows the radius of the particle on which FDEP is applied; K (-) is known as the Clausius-Mossotti factor; εm (-) shows the dielectric permittivity of the medium; and E(V/m) is the root mean square value of the electric field. - Use Equation 2 for a spherical particle under the dielectrophoretic force sub-node.
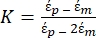 [2]
[2]
In Equation 2,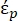 (-) shows the complex permittivity of the particle on which the DEP force is applied;
(-) shows the complex permittivity of the particle on which the DEP force is applied; 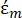 (-) shows the complex permittivity of the fluid surrounding the particle. The complex permittivity
(-) shows the complex permittivity of the fluid surrounding the particle. The complex permittivity 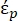 and
and 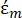 are defined as follows35:
are defined as follows35: - Use Equation 3 for a spherical particle under the dielectrophoretic force sub-node:
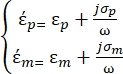 [3]
[3]
In Equation 3, εp (-) shows the real part of the complex permittivity of the particle; εm (-) shows the real part of the complex permittivity of the fluid surrounding the particle; σp (S/m) shows the particle conductivity; σm (S/m) shows the conductivity of the medium surrounding the particle; and ω (Hz) is the frequency of the applied electric field.
NOTE: The sign of Re(K) determines the polarity of the FDEP. If the sign of Re(K) is negative, then the particle experiences a negative dielectrophoretic force (nDEP); contrary to this, if the sign of Re(K) is positive, it implies a positive dielectrophoretic force (pDEP). For the Clausius-Mossotti factor (K), the variation lies within the range of -1 to 1.
- Initially, perform a mesh independence study to optimize the mesh size for the simulations33.
- Use a modified form of Equation 3 to model biological cells such as mammalian cells, which are more complex and have a multilayered structure.
K (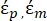 ) =
) = 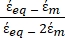 [4]
[4]
In Equation 4,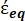 (-) incorporates both the complex permittivity of the cytoplasm,
(-) incorporates both the complex permittivity of the cytoplasm, 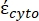 (-), and the complex permittivity of the cell membrane,
(-), and the complex permittivity of the cell membrane, 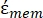 (-), and is given as follows:36
(-), and is given as follows:36 - Use Equation 5 to solve "
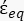 ":
":
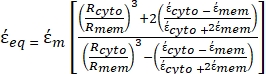 [5]
[5]
In Equation 5, Rcyto (m) and Rmem (m) show the radius of the cell cytoplasm and cell membrane, respectively. - Then, use Equation 4 to plot Re(K) as a function of the applied electric field for both cancer and healthy cells. Calculate the real part of the Clausius-Mossotti (CM) factor, Re(K), to quantify the dielectrophoretic force (DEP) that the particle experiences.
- Right-click on the Ergebnisse node, add the Particle Evaluation sub-node, and in the expression section, type fpt.deff1.K to plot the CM factor for particle 1 and fpt.deff2.K for particle 2.
NOTE: All the protocol steps listed in the main text can be viewed in the protocol video (Video 1).
Representative Results
Investigating the optimal operational parameters for effective DEP-based sorting of non-metastatic breast cancer (MCF-7) and non-tumor breast epithelial (MCF-10A) cells
To achieve a successful separation of non-metastatic breast cancer (MCF-7) and non-tumor breast epithelial (MCF-10A) cells with divergent dielectric properties when undergoing dielectrophoresis, their K factors should be distinct by keeping the applied frequency fixed37,38. The quantification of the dielectric responses of non-metastatic breast cancer cells and non-tumor breast epithelial cells under an applied electric field and the calculation of the "K" factor as a function of the applied frequency for both cell lines were achieved by using Equation 4. The results shown in Figure 1 were generated by keeping all the dielectric parameters of the non-metastatic breast cancer cells and non-tumor breast epithelial cells fixed while the applied frequency of the electric field was varied for three different values of conductivity of the cell suspension media, σm.
As shown in Figure 1, in each case, the value of K is within the range of -1 to 1, in line with previous studies39,40. Nonetheless, the plot of real(K) versus the frequency changes for both non-metastatic breast cancer cells (MCF-7) and non-tumor breast epithelial cells (MCF-10A) according to the value of the medium conductivity (σm). The results shown in Figure 1 are in agreement with a recent study in which the effect of σm on Re(K) for MCF-7 cells was quantified41.
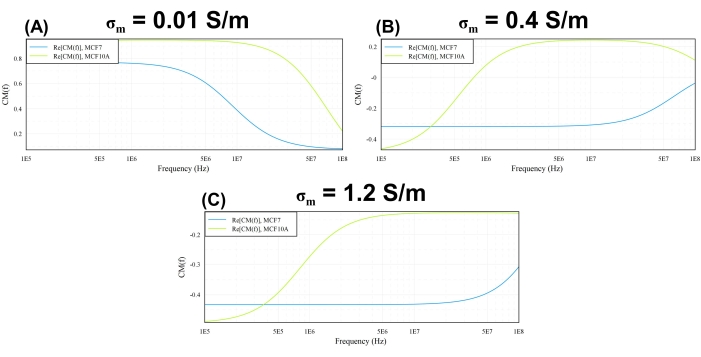
Figure 1: Clausius-Mossotti factor. The real part of the Clausius-Mossotti factor, K, plotted as a function of frequency, for MCF-7 and MCF-10A cells suspended in a medium characterized by a conductivity of (A) σm = 0.01 S/m; (B) σm = 0.4 S/m; (C) σm = 1.2 S/m. Please click here to view a larger version of this figure.
Figure 1 was plotted using the MyDEP tool for three different σm values, keeping and varying the applied AC frequency from 100 kHz to 100MHz. Initially, the σm was chosen to be 0.01 S/m, and the applied AC frequency was varied between 100 kHz and 100 MHz, as shown in Figure 1A. At an applied AC frequency of 100 kHz, the value of Re(K) for MCF-10A cells turned out to be 0.82, which means they experience positive dielectrophoresis (pDEP) and should move toward an area of high electric field strength. Similarly, the MCF-7 cells at 100 kHz also experience pDEP with a Re(K) value of 0.76. The frequency was increased in steps of 100 kHz, and the value of the CM factor for both cell types remained on the positive side throughout the applied frequency spectrum. By keeping all the other operating parameters constant, the medium conductivity was increased to 0.4 S/m to plot the Re(K), as shown in Figure 1B. MCF-10A and MCF-7 demonstrated negative dielectrophoresis (nDEP) behavior with Re(k) values of -0.46 and -0.31, respectively, at 100 kHz. As the frequency was increased to 0.8 MHz, the DEP response of MCF-10 cells changed, and they experienced pDEP with an Re(K) value of 0.014. This behavior of MCF-7 cells is caused by the Maxwell-Wagner polarization at the interface between the cell membrane and the surrounding cell suspension medium39,41. The frequency where this change in DEP response is observed is known as the cross-over frequency, as shown in Figure 1A42,22. The MCF-7 cells, in this case, experienced nDEP. The frequency was further increased up to 100 MHz, but both cell types did not change their DEP behavior and, thus, remained unaffected by the variations in the applied electric field frequency. When the conductivity was increased to 1.2 S/m, the MCF-10A and MCF-7 cells experienced nDEP at 100 kHz. The Re(k) values for MCF-10A and MCF-7 cells, in this case, were -0.49 and -0.43, respectively, as shown in Figure 1C. As the frequency was increased to 0.8 MHz, the DEP response of the cells did not change, as they kept experiencing nDEP. The negative DEP behavior of both MCF-7 and MCF-10A cell lines at high values of cells suspension medium conductivity is in agreement with previously reported studies39,43,44. The DEP behavior of the cells at frequencies higher than the first cross-over frequency is governed by the interaction between cytoplasmic conductivity and the suspending solution45,46. On the other hand, at frequencies lower than the first cross-over frequency, the dielectric response of the cells is determined by the interaction between the cell membrane conductivity and the cell suspension medium.
Based on the results shown in Figure 1, COMSOL simulations were set up. Initially, the electric field strength was quantified using this simulation software, as shown in Figure 2. It can be seen that the maxima of the magnitude of the total electric field generated by two electrodes placed side-by-side on the top wall of the sorting channel are located near the electrode edges. The arrows show the direction of the electric field.
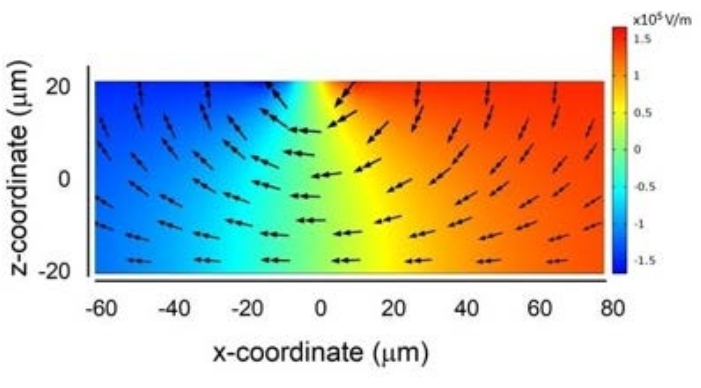
Figure 2: Electric field strength. The electric field generated by two electrodes placed side-by-side. Please click here to view a larger version of this figure.
The simulations to sort MCF-7 and MCF-10A cells were set up by keeping the applied AC frequency fixed at 0.8 MHz (cross-over frequency) and changing the value of σm. Three values for σm were chosen in accordance with the Re(K) plots shown in Figure 1. Initially, when σm was 0.01 S/m, both cell types experienced positive DEP, moved toward the region of high electric field strength, and moved out from the top outlet, as shown in Figure 3A. The cytoplasmic conductivities σcytoplasm for both cell lines were higher than the medium conductivity σm in this particular case, thus forcing both cell lines to move closer to the electrodes at the top of the microfluidic channel47. The DEP response of the MCF-10A cells changed, and they experienced negative DEP, when the σm was increased to 0.4 S/m with the applied frequency fixed at 0.8 MHz. Figure 3B shows that MCF-10A cells move to the top outlet, while MCF-7 cells move to the bottom outlet. The reason for this separation is that MCF-7 cells are more polarized compared to MCF-10A as their cytoplasmic conductivity σcytoplasm is greater than the medium conductivity σm, as shown in the cell sorting video (Video 2).
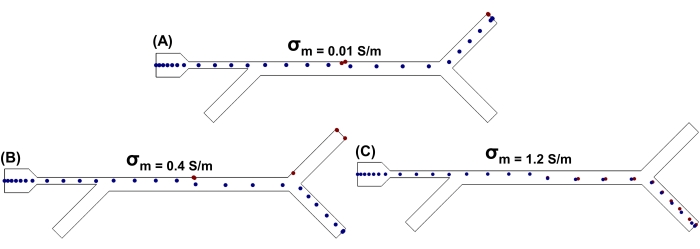
Figure 3: Cell sorting fixed frequency. Simulation of MCF-7 and healthy cell separation over time by DEP in the microfluidic device designed. MCF-7 and healthy cell separation at three different values of conductivity of the suspended medium: (A) 0.01 S/m; (B) 0.4 S/m; (C) 1.2 S/m. In each case, the applied frequency was 0.8 MHz, the applied voltage Vpp was 1.5 V, and the flow velocity at the injection inlet was 184 µm/s and 853 µm/s at the flow focusing inlet. In the simulation, MCF-7 cells and MCF-10A cells are represented with blue and red circles, respectively. Please click here to view a larger version of this figure.
As the medium conductivity was further increased to 1.2 S/m, both MCF-7 and MCF-10A cells both became less polarizable than the medium surrounding them due to lower cytoplasmic conductivity σcytoplasm values. Consequently, they experienced nDEP and moved away from regions of high electric field, as shown in Figure 3C.
These results demonstrate that the conductivity of the medium plays an important role in separating the MCF-7 non-metastatic breast cancer cells from MCF-10A non-tumor breast epithelial cells based on DEP. Moreover, as shown in Figure 3B, to achieve an effective separation, the medium conductivity needs to be adjusted in a way that the cells experience either pDEP or nDEP, based on their respective dielectric properties.
Finally, the effect of the applied dielectrophoretic force, FDEP, on the sorting behavior of both the cell lines was investigated by keeping the medium conductivity constant at 0.4 S/m. FDEP is a function of the frequency of the applied electric field48,49, and as the frequency of the applied electric field is changed, the cells change their DEP behavior. The simulations were started by setting the frequency at 100 kHz, and it was observed that both MCF-10A and MCF-7 cell lines experienced nDEP and moved away from the region of high electric field toward the bottom outlet, as shown in Figure 4A. As the frequency was increased, the DEP behavior of both cell lines remained unchanged until 0.8 MHz, when MCF-10A changed their DEP behavior and crossed-over to the pDEP region. This is the point with the maximum separation between the DEP response cells under investigation and maximum sorting efficiency, as shown in Figure 4B. When the frequency was increased to 100 MHz, it was observed that both cell lines experienced pDEP and moved toward the top outlet, as shown in Figure 4C. At higher frequencies above 0.8 MHz, the cells started to immobilize at the channel walls. The immobilization of cells at the channel walls can lead to cell loss during the sorting process, which, in turn, has an effect on the overall efficiency of the device. The effect of these forces can also cause serious loss in cell viability if exposed for a longer period of time. Yang et al. quantified the effect of DEP forces on a Listeria monocytogenes cell line by exposing them to an AC electric field of 5 MHz and a peak to peak voltage of 20 VPP50. Their results indicated a viable cell loss of 56%-89% when kept under the effect of DEP force for 4 h. Similarly, DEP forces have also been reported to have an effect on the movement of cells when suspended in a polarizable medium and have been used to immobilize cells. Ettehad et al. reported a microfluidic device with interdigitated electrodes (IDEs) that used an AC frequency of 1 MHz and 20 VPP to immobilize yeast cells51. They showed that immobilization of their yeast cells was dependent on the aspect ratio of the spacing between their IDEs and applied voltage. The increase in the aspect ratio of IDE spacing resulted in a sharp decrease in cell immobilization, and in order to immobilize cells in devices with greater spacing between IDEs, higher VPP was required. Cell immobilization is a desired application when cells are required to be trapped for analysis or growth. The previous results clearly showed that applied AC frequency and voltage have an effect on cell immobilization. In applications where high-throughput sorting or screening is the desired outcome, cell immobilization results in cell loss and reduces the output efficiency of the device.
In order to quantify the effect of applied frequency and voltage on cell immobilization, a set of simulations were run from the kilohertz to megahertz frequency range at a fixed applied voltage of 1.5 VPP. The results are shown in Supplementary Figure S4. The results revealed that, at frequencies in the kHz range, the immobilization of the cells at the channel walls was far less compared to frequencies in the MHz range. As DEP force is directly proportional to the applied AC frequency, we can deduce that, at high DEP force, the immobilization of cells is more pronounced. For this microfluidic device, there will be a cell loss during the sorting of MCF7 and MCF-10A cells as it is required to operate at frequencies greater than 0.8 MHz. The effect of the random distribution of cells at the inlet was further investigated by choosing a random distribution boundary condition. More cell-channel wall interactions were observed in this case, as shown in Supplementary Figure 5.
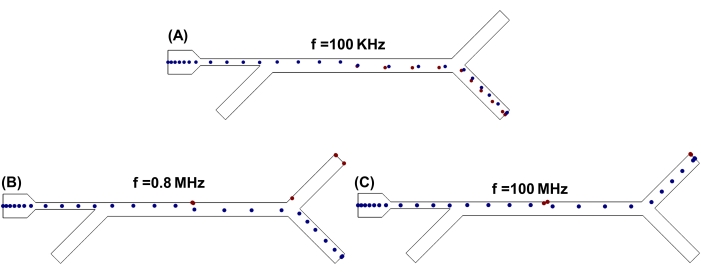
Figure 4: Cell sorting with fixed medium conductivity. Effect of the frequency of the applied AC field on the separation of non-metastatic breast cancer cells (MCF-7) and non-tumor breast epithelial cells (MCF-10A) in the microfluidic device simulated. (A) f= 100 KHz; (B) f= 0.8 MHz; (C) f= 100 MHz. The fluid medium conductivity was fixed at σm = 0.4 S/m. Please click here to view a larger version of this figure.
| Dielectric properties for simulation | εcytoplasm | σcytoplasm (S/m) |
εmembrane | σmembrane (S/m) |
| MCF-7 | 50 | 0.8 | 11 | 6× 10-6 |
| MCF-10A | 100 | 0.1 | 11 | 6 |
| f0 | 800 [kHz] | 1.2 x 103 Hz | Frequency of the electric field |
| sigma_f | 0.4 [S/m] | 0.8 S/m | Fluid medium conductivity |
| epsilon_f | 80 | 80 | Fluid relative permittivity |
| rho_f | 1000 [kg/m3] | 1000 kg/m³ | Fluid density |
| mu_f | 1 x 10-3 [Pa·s] | 0.001 Pa·s | Fluid dynamic viscosity |
| rho_p | 1050 [kg/m3] | 1050 kg/m³ | Particle density |
| dp1 | 17 [µm] | 1.7 x 10-5 m | Particle diameter |
| dp2 | 16 [um] | 1.6 x 10-5 m | Particle diameter |
| sigma_p1 | 0.8 [S/m] | 0.6 S/m | Particle conductivity |
| sigma_p2 | 0.1 [S/m] | 1.1 S/m | Particle conductivity |
| epsilon_p1 | 50 | 55 | Healthy relative permittivity |
| epsilon_p2 | 100 | 65 | Cancer relative permittivity |
| sigma_s1 | 6 x 10-6 [S/m] | 6 x 10-6 S/m | Shell electrical conductivity |
| sigma_s2 | 6 [S/m] | 6 S/m | Shell electrical conductivity |
| epsilon_s1 | 11 | 11 | Shell relative permittivity |
| epsilon_s2 | 11 | 11 | Shell relative permittivity |
| th_s1 | 7 [nm] | 7 x 10-9 m | Shell thickness |
| th_s2 | 7 [nm] | 7 x 10-9 m | Shell thickness |
Table 1: Operating parameters. Dielectric properties of MCF-7 and MCF-10A
Video 1: A video showing the protocol steps. Please click here to download this Video.
Video 2: Cell sorting video. Please click here to download this Video.
Supplementary File 1: The governing equations and theoretical background. Please click here to download this File.
Supplementary Figure 1: Device design and parameters. Microfluidic device design highlighting different parts of the device. Please click here to download this File.
Supplementary Figure 2: Gap between electrodes. The gap between two patch electrodes. Please click here to download this File.
Supplementary Figure 3: Mesh independence study. A mesh independence study depicting the effect of different mesh sizes on the sorting of MCF-7 and MCF-10A cells. (A) The different mesh sizes for the microfluidic device, depicting the number of elements for each mesh. The number of elements that constitutes the mesh increase from coarser to finer mesh. (B) The sorting of MCF7 and MCF-10A cells on different mesh sizes by keeping all the other operating parameters constant. The fine and finer mesh sizes produce the best results for sorting. Please click here to download this File.
Supplementary Figure 4: Cell immobilization and random distribution test. Simulations performed for frequencies between 10 KHz and 6 MHz to validate the effect of DEP force on cell immobilization. (A) At f = 10 kHz, no sorting and cell immobilization are observed. (B) At f = 200 kHz, no sorting and cell immobilization are observed. (C) At f = 0.8 MHz, sorting and cell immobilization at the outlet walls are observed. Please click here to download this File.
Supplementary Figure 5: Random distribution. Randomly distributed particles at the inlet of the chip. Please click here to download this File.
Supplementary Table 1: Comparison of different DEP-based microfluidic sorting devices. Please click here to download this File.
Discussion
Microfluidic devices have been reported previously for cell culture, trapping, and sorting47,52,53. The fabrication of these devices in the cleanroom is an expensive process, and it is imperative to quantify the output and efficiency of a proposed microfluidic device through CFD simulations. This study presents the design and simulations of an AC-dielectrophoretic microfluidic device for the continuous separation of non-metastatic breast cancer cells (MCF-7) and non-tumor breast epithelial cells (MCF-10A) based on their dielectric properties23.
The device is operated by applying an AC electric field via a set of two microelectrodes embedded into a single microfluidic sorting channel to separate MCF-7 and MCF-10A cells based on their dielectric properties. The separation efficacy of the device was computationally simulated for different values of medium conductivity and for a range of applied AC frequencies. The optimal applied AC frequency and media conductivity values were found to be 0.8 MHz and 0.4 S/m, respectively. A low voltage of 1.5 Vp-p was used throughout the simulations. The applied AC frequency range and the applied voltage are comparable to previously reported literature23,47. At frequencies above 1 MHz, the cell immobilization effect was observed, which should be taken into consideration for future device designs and fabrication. We list this cell immobilization as a limitation of our method in the context of cell sorting applications. We believe cell immobilization at higher frequencies can be used for cell differentiation as previously reported in literature54, giving this proposed design a new direction. This application would be of great interest to researchers in synthetic biology.
The critical steps for the correct implementation of this protocol include the choice of suitable physics nodes and sub-nodes (steps 1.5-1.9). These steps form the basis of the entire simulation protocol and help to choose the parameter values for each cell type, applied force, and applied voltage. Another critical step is to choose the correct fluid medium conductivity and applied frequency. This can be achieved by running a troubleshooting step of the parametric sweep. The parametric sweep of these two parameters can assist in deciding the optimal values for any future simulations. Lastly, a mesh independence study is also critical in the context of choosing the right mesh size for any future simulations. It is highly recommended that a mesh independence study is performed as a troubleshooting step before finalizing any future simulations.
This study provides the first simulation-based example of inline separation of non-metastatic breast cancer cells (MCF-7) and non-tumor breast epithelial cells (MCF-10A) based on their dielectric properties. We believe this design can be further implemented for viable and non-viable cell sorting.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This study was supported by the Higher Education Commission of Pakistan.
Referenzen
- Liang, L., et al. Microfluidic-based cancer cell separation using active and passive mechanisms. Microfluidics and Nanofluidics. 24 (4), 26 (2020).
- Damiati, S., Kompella, U. B., Damiati, S. A., Kodzius, R. Microfluidic devices for drug delivery systems and drug screening. Genes. 9 (2), 103 (2018).
- Pashayan, N., et al. Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nature Reviews Clinical Oncology. 17 (11), 687-705 (2020).
- Panesar, S., Neethirajan, S. Microfluidics: Rapid diagnosis for breast cancer. Nano-micro Letters. 8 (3), 204-220 (2016).
- Chen, J., Li, J., Sun, Y. Microfluidic approaches for cancer cell detection, characterization and separation. Lab on a Chip. 12 (10), 1753-1767 (2012).
- Beech, J. P., Holm, S. H., Adolfsson, K., Tegenfeldt, J. O. Sorting cells by size, shape and deformability. Lab on a Chip. 12 (6), 1048-1051 (2012).
- Kang, Y., Li, D. Electrokinetic motion of particles and cells in microchannels. Microfluidics and Nanofluidics. 6 (4), 431-460 (2009).
- Schmid, L., Weitz, D. A., Franke, T. Acoustic microfluidic fluorescence-activated cell sorter. Lab on a Chip. 14 (19), 3710-3718 (2014).
- Yu, B. Y., Elbuken, C., Shen, C., Huissoon, J. P., Ren, C. L. An integrated microfluidic device for the sorting of yeast cells using image processing. Scientific Reports. 8, 3550 (2014).
- Asiaei, S., Darvishi, V., Davari, M. H., Zohrevandi, D., Moghadasi, H. Thermophoretic isolation of circulating tumor cells, numerical simulation and design of a microfluidic chip. Journal of Thermal Analysis and Calorimetry. 137 (3), 831-839 (2019).
- Song, Y., Li, M., Pan, X., Wang, Q., Li, D. Size-based cell sorting with a resistive pulse sensor and an electromagnetic pump in a microfluidic chip. Electrophoresis. 36 (3), 398-404 (2014).
- Giraud, G., et al. Dielectrophoretic manipulation of ribosomal RNA. Biomicrofluidics. 5 (2), 024116 (2011).
- Valero, A., Braschler, T., Demierre, N., Renaud, P. A miniaturized continuous dielectrophoretic cell sorter and its applications. Biomicrofluidics. 4 (2), 022807 (2010).
- Allahrabbi, N., Chia, Y. S. M., Saifullah, M. S. M., Lim, K. M., Lanry Yung, L. Y. A hybrid dielectrophoretic system for trapping of microorganisms from water. Biomicrofluidics. 9 (3), 034110 (2015).
- Vykoukal, D. M., Gascoyne, P. R. C., Vykoukal, J. Dielectric characterization of complete mononuclear and polymorphonuclear blood cell subpopulations for label-free discrimination. Integrative Biology: Quantitative Biosciences from Nano to Macro. 1 (7), 477-484 (2009).
- Shim, S., et al. Antibody-independent isolation of circulating tumor cells by continuous-flow dielectrophoresis. Biomicrofluidics. 7 (1), 11807 (2013).
- Jeon, H. J., Lee, H., Yoon, D. S., Kim, B. M. Dielectrophoretic force measurement of red blood cells exposed to oxidative stress using optical tweezers and a microfluidic chip. Biomedical Engineering Letters. 7 (4), 317-323 (2017).
- Song, H., et al. Continuous-flow sorting of stem cells and differentiation products based on dielectrophoresis. Lab on a Chip. 15 (5), 1320-1328 (2015).
- Tsai, S. L., Chiang, Y., Wang, M. H., Chen, M. K., Jang, L. S. Battery-powered portable instrument system for single-cell trapping, impedance measurements, and modeling analyses. Electrophoresis. 35 (16), 2392-2400 (2014).
- Chan, J. Y., et al. Dielectrophoresis-based microfluidic platforms for cancer diagnostics. Biomicrofluidics. 12 (1), 011503 (2018).
- Patel, S., et al. Microfluidic separation of live and dead yeast cells using reservoir-based dielectrophoresis. Biomicrofluidics. 6 (3), 34102 (2012).
- Yildizhan, Y., Erdem, N., Islam, M., Martinez-Duarte, R., Elitas, M. Dielectrophoretic separation of live and dead monocytes using 3D carbon-electrodes. Sensors. 17 (11), 2691-2704 (2017).
- Piacentini, N., Mernier, G., Tornay, R., Renaud, P. Separation of platelets from other blood cells in continuous-flow by dielectrophoresis field-flow-fractionation. Biomicrofluidics. 5 (3), 34122 (2011).
- Zhao, K., Duncker, B. P., Li, D. Continuous cell characterization and separation by microfluidic alternating current dielectrophoresis. Analytical Chemistry. 91 (9), 6304-6314 (2019).
- Valero, A., et al. Tracking and synchronization of the yeast cell cycle using dielectrophoretic opacity. Lab on a Chip. 11 (10), 1754-1760 (2011).
- Demierre, N., Braschler, T., Muller, R., Renaud, P. Focusing and continuous separation Of cells in a microfluidic device using lateral dielectrophoresis. International Solid-State Sensors, Actuators and Microsystems Conference. 430 (98), 1777-1780 (2007).
- Arslan, Z. C., Yalçın, Y. D., Külah, H. Label-free enrichment of MCF7 breast cancer cells from leukocytes using continuous flow dielectrophoresis. Electrophoresis. 43 (13-14), 1531-1544 (2022).
- Turcan, I., Olariu, M. A. Dielectrophoretic manipulation of cancer cells and their electrical characterization. ACS Combinatorial Science. 22 (11), 554-578 (2020).
- Park, J., et al. Sequential cell-processing system by integrating hydrodynamic purification and dielectrophoretic trapping for analyses of suspended cancer cells. Micromachines. 11 (1), 47 (2020).
- Hussein, M., et al. Breast cancer cells exhibits specific dielectric signature in vitro using the open-ended coaxial probe technique from 200 MHz to 13.6 GHz. Scientific Reports. 9, 4681 (2019).
- Fornes-Leal, A., Garcia-Pardo, C., Frasson, M., Pons Beltrán, V., Cardona, N. Dielectric characterization of healthy and malignant colon tissues in the 0.5-18 GHz frequency band. Physics in Medicine and Biology. 61 (20), 7334-7346 (2016).
- Çetin, B., Li, D. Dielectrophoresis in microfluidics technology. Electrophoresis. 32 (18), 2410-2427 (2011).
- Khan, S., Khulief, Y. A., Al-Shuhail, A. A. Effects of reservoir size and boundary conditions on pore-pressure buildup and fault reactivation during CO2 injection in deep geological reservoirs. Environmental Earth Sciences. 79, 294 (2020).
- Adams, T. N. G., Turner, P. A., Janorkar, A. V., Zhao, F., Minerick, A. R. Characterizing the dielectric properties of human mesenchymal stem cellsand the effects of charged elastin-like polypeptide copolymer treatment. Biomicrofluidics. 8 (5), 054109 (2014).
- Lo, Y. J., et al. Measurement of the Clausius-Mossotti factor of generalized dielectrophoresis. Applied Physics Letters. 104, 083701 (2014).
- Lo, Y. J., Lei, U. Measurement of the real part of the Clausius-Mossotti factor of dielectrophoresis for Brownian particles. Electrophoresis. 41 (1), 137-147 (2020).
- Ohta, A. T., et al. Optically controlled cell discrimination and trapping using optoelectronic Tweezers. IEEE Journal of Selected Topics in Quantum Electronics. 13 (2), 235-242 (2007).
- Sun, T., Morgan, H. Single-cell microfluidic Impedance cytometry. Microfluidics and Nanofluidics. 8 (4), 423-443 (2010).
- Weng, P. Y., et al. Size-dependent dielectrophoretic cross-over frequency of spherical particles. Biomicrofluidics. 10 (1), 1909-1921 (2016).
- Lu, Y. W., Sun, C., Kao, Y. C., Hung, C. L., Juang, J. Y. Dielectrophoretic cross-over frequency of single particles: Quantifying the effect of surface functional groups and electrohydrodynamic flow drag force. Nanomaterials. 10 (7), 1364 (2020).
- Henslee, E. A., Sano, M. B., Rojas, A. D., Schmelz, E. M., Davalos, R. V. Selective concentration of human cancer cells using contactless dielectrophoresis. Electrophoresis. 32 (18), 2523-2529 (2011).
- Chan, J. Y., et al. Dielectrophoresis-based microfluidic platforms for cancer diagnostics. Biomicrofluidics. 12 (1), 11503-11525 (2018).
- Gascoyne, P. R. C., Shim, S. Isolation of circulating tumor cells by dielectrophoresis. Cancers. 6 (1), 545-579 (2014).
- Liang, W., et al. Determination of dielectric properties of cells using ac electrokinetic-based microfluidic platform. Micromachines. 11 (5), 513-537 (2020).
- Frusawa, H., et al. Frequency-modulated wave dielectrophoresis of vesicles and cells periodic U-turns at the crossover frequency. Nanoscale Research Letters. 13 (169), 2583-2589 (2018).
- Wei, M. T., Junio, J., Ou-Yang, D. H. Direct measurements of the frequency-dependent dielectrophoresis force. Biomicrofluidics. 3 (1), 12003 (2009).
- Mustafa, A., Pedone, E., Marucci, L., Moschou, D., Lorenzo, M. D. A flow-through microfluidic chip for continuous dielectrophoretic separation of viable and non-viable human T-cells. Electrophoresis. 43 (3), 501-508 (2021).
- Wang, L., et al. Dual frequency dielectrophoresis with interdigitated sidewall electrodes for microfluidic flow-through separation of beads and cells. Electrophoresis. 30 (5), 782-791 (2021).
- Alazzam, A., Mathew, B., Alhammadi, F. Novel microfluidic device for the continuous separation of cancer cells using dielectrophoresis. Journal of Separation Science. 40 (5), 1193-1200 (2017).
- Yang, L., Banada, P. P., Bhunia, A. K., Bashir, R. Effects of dielectrophoresis on growth viability and immuno-reactivity of listeria monocytogenes. Journal of Biological Engineering. 2, 6 (2008).
- Matbaechi, H., Soltani, P., Hölzel, R., Wenger, C. Dielectrophoretic immobilization of yeast cells using CMOS integrated microfluidics. Micromachines. 11 (5), 501-518 (2020).
- Mustafa, A., Pedone, E., La Regina, A., Erten, A. A., Marucci, L. Development of a single layer microfluidic device for dynamic stimulation, culture and imaging of mammalian cells. bioRxiv. , (2022).
- Mustafa, A., et al. Enhanced dissolution of liquid microdroplets in the extensional creeping flow of a hydrodynamic trap. Langmuir. 32 (37), 9460-9467 (2016).
- Chang, H. F., Chou, S. E., Cheng, J. Y. Electric-field-induced neural precursor cell differentiation in microfluidic devices. Journal of Visualized Experiments. (170), e61917 (2021).

