Profiling the Bacterial Community of Fermenting Traminette Grapes during Wine Production using Metagenomic Amplicon Sequencing
Summary
Here, we present a protocol to describe amplicon metagenomic for determining the bacterial community of Traminette grapes, fermenting grapes, and final wine.
Abstract
Advances in sequencing technology and the relatively easy access to the use of bioinformatics tools to profile microbial community structures have facilitated a better understanding of both culturable and non-culturable microbes in grapes and wine. During industrial fermentation, microbes, known and unknown, are often responsible for product development and off-flavor. Therefore, profiling the bacteria from grape to wine can enable an easy understanding of in situ microbial dynamics. In this study, the bacteria of Traminette grapes must undergoing fermentation, and the final wine were subjected to DNA extraction that yielded 15 ng/µL to 87 ng/µL. The 16S amplicon of the hypervariable region of the V4 region was sequenced, relatively abundant bacteria consisting of phyla Proteobacteria, Actinobacteriota, Firmicutes, Bacteroidota, Fusobacteriota and followed by the Verrucomicrobiota, Halobacterota, Desulfobacterota, Myxococcota, and Acidobacteriota. A Venn diagram analysis of the shared unique operational taxonomic units (OTU) revealed that 15 bacteria phyla were common to both grape must, fermenting stage, and final wine. Phyla that were not previously reported were detected using the 16S amplicon sequencing, as well as genera such as Enterobacteriaceae and Lactobacillaceae. Variation in the organic nutrient use in wine and its impact on bacteria was tested; Traminette R tank containing Fermaid O and Traminette L stimulated with Stimula Sauvignon blanc + Fermaid O. Alpha diversity using the Kruskal-Wallis test determined the degree of evenness. The beta diversity indicated a shift in the bacteria at the fermentation stage for the two treatments, and the final wine bacteria looked similar. The study confirmed that 16S amplicon sequencing can be used to monitor bacteria changes during wine production to support quality and better utilization of grape bacteria during wine production.
Introduction
Traminette grape is characterized by production of superior wine quality, in addition to appreciable yield and partial resistance to several fungal infections1,2,3. The natural fermentation of grapes relies on associated microorganisms, wine production environment, and fermentation vessels4,5. Oftentimes, many wineries rely on wild yeast and bacteria for fermentation, production of alcohol, esters, aroma, and flavor development6.
The goal of this study is to examine the bacterial composition of grapes and monitor their dynamics during fermentation. Although, the modern use of starter cultures such as Saccharomyces cerevisiae for primary fermentation, where alcohol is produced, is common to different wine styles7. In addition, secondary fermentation, where malic acid is decarboxylated by Oenococcus oeni to lactic acid, improves the organoleptic and taste profile of the wine and reduces the acidity of wine8,9. With the recent advances in the use of culture-independent methods, it is now possible to determine different microbes associated with the wine grape and the species that are transferred to must and participate in the fermentation at different times up to the final product10.
The roles and dynamics of wild bacteria from different grapes transferred to the must during wine fermentation are poorly understood. The taxonomy of many of these bacteria is not even known, or their phenotypic properties are uncharacterized. This makes their application in coculture fermentation still poorly underutilized. However, microbiological culture-based analysis has been used to determine the bacterial population associated with grapes and wine10. It is widely known that selective culture plating is tedious, prone to contamination, has a low reproducibility, and output can be doubtful; it also misses bacterial species whose growth requirements are unknown. Previous studies indicate that culture-independent, 16S rRNA gene-based methodologies offer a more dependable and cost-effective approach to characterizing complex microbial communities11. For example, sequencing the hypervariable regions of the 16S rRNA gene has been successfully employed to study bacteria in grapes leaves, berries, and wine12,13,14. Studies have shown that the use of either 16S rRNA metabarcoding or whole metagenomic sequencing is suitable for microbiome studies15. There is emerging information about the possible linkage of bacterial diversity to their metabolic attributes during wine production, which could help in the determination of oenological properties and terroir16.
The need to maximize the advantages of the metagenomic tools using next-generation sequencing (NGS) to study the grape and wine microbial ecology has been emphasized16,17. Also, the use of culture-independent methods based on high throughput sequencing to profile microbial diversity of the food and fermentation ecosystem has become very relevant and valuable to many laboratories and is recommended for industrial use18,19. It provides an advantage of detection and taxonomic profiling of the present microbial populations and the contribution of environmental microbes, their relative abundance, and alpha and beta diversity20. The sequencing of the variable region of the 16S region has become an important gene of choice and has been used during different microbial ecological studies.
While many studies focus on fungi, especially yeast, during wine fermentation21, this study reported the 16S amplicon sequencing and bioinformatic tools used to study the bacteria during Traminette grapes fermentation for wine production.
Protocol
1. Experimental wine production
- Obtain Traminnette grapes from Dynamis Estate wine in Jonesville, North Carolina vineyard, destem, and crush to release must into two separate 600 L open-top fermenters and leave on skins for about 4 days with the lids on.
- Punch the caps down once a day to keep skins wet and limit volatile acid (VA) production.
NOTE: The idea is that a lot of the aromatic precursors (monoterpenes) are situated in the skins and leave the juice in contact with the skin to increase the aromatic potential of the wine. - After 4 days, pitch Saccharomyces cerevisiae var cerevisiae QA23 (Lallemand). This yeast was selected as it is a strong fermenter and is associated with enhancing the varietal character in the wine from the grapes.
- In one tank, Traminette R, add 20 g/hL Fermaid O when the brix is at 17.7 and 20 g/hL Fermaid O and 12.5 g/hL Fermaid K at 13.1 g/hL to achieve fermentation security, while the other tank Traminette L, add 40 g/hL Stimula Sauvignon blanc when the brix is at 17.1. Also add 20 g/hL Fermaid O when the brix is at 13.4 to enhance the varietal character of the wine.
- Take duplicate samples of must (day 1), yeast added (day 4), fermented must (day 15), and finished wine samples (day 30) and keep at -20 °C before DNA extraction and metagenomic analysis.
2. DNA extraction for metagenomics
- Keep all duplicate samples obtained above on ice until the first centrifugation.
- Transfer 20 mL of the fermented sample into a sterile 50 mL screw cap tube.
- Add 10 mL of cold water and homogenize (vortex). Centrifuge for 1 min at 800 x g at 4 °C and transfer the supernatant to a new 50 mL tube.
NOTE: This step will remove the solids of the sample. - Repeat step 2.3 two times and pool the supernatants in the same tube.
- Centrifuge supernatant at 3,000 x g at 4 °C for 20 min to pellet the cells. Discard the supernatant.
- Resuspend the pellet in 1 mL of PBS, transfer to a 2 mL screw-cap tube, and centrifuge at 14,000 x g for 2 min. Perform two more washes using PBS.
- At this step, freeze the pellets at -20°C or follow step 2.8. It is important that all the samples are treated in the same way.
- Add 978 µL of sodium phosphate buffer. Prepare sodium phosphate buffer, add 3.1 g of NaH2PO4·H2O and 10.9 g of Na2HPO4 (anhydrous) to distilled H2O to make a volume of 1 L, and adjust pH to 7.4.
- Add 122 µL of DNA buffer (DNA spin kit) and vortex.
- Let it stand in the refrigerator (4 °C) for 45 min-1 h. Vortex the samples every 15 min. This step can be left overnight (o/n) or until ready to continue using the DNA spin kit.
- Transfer 1 mL of sample into a Lysis solution 1 tube (DNA spin kit) and tighten the cap (write the sample number on the side of the tube and not on the cap; the kit reagents will remove anything written on the cap).
- Homogenize the sample in a bead-beating grinder (6.5 m/s, CY: 24 x 2) for 60 s three times. If the bead-beating grinder has the device to put ice, put 50 g of dry ice under the tubes. If not, keep the samples on wet ice for 5 min between each homogenization step.
- Centrifuge Lysing Matrix E tubes (DNA spin kit) for 1 min at 16800 x g(or max speed).
- Transfer the supernatant into a clean microtube. Thereafter, add 250 µL of protein precipitation solution (PPS) reagent (DNA spin kit) and mix by shaking the tube by hand 10 times.
- Centrifuge for 5 min at 16800 x g to pellet the precipitate.
- Transfer the supernatant into a sterile 15 mL tube. Add 1 mL of binding matrix suspension (DNA spin kit) to the supernatant.
NOTE: Resuspend the binding matrix suspension before use until it is homogeneous. - Invert the tubes by hand for 2 min, then let the tubes stand in a rack for 3 min (to allow settling of silica matrix).
- Carefully remove 1 mL of the supernatant. Resuspend the matrix in the remaining supernatant.
- Transfer 600 µL of the mixture into a spin filter tube (DNA spin kit) and centrifuge for 1 min at 14500 x g.
- Add the remaining mixture and centrifuge.
- Decant flow-through and add 500 µL of wash solution (DNA spin kit) into the spin filter tube and centrifuge for 1 min at 14500 x g(ensure to add EtOH to wash solution; see the product handbook). Decant the flow-through and repeat the wash two more times (3 washes in total).
- Decant the flow-through and centrifuge for an additional 2 min at 14,500 x g to dry the matrix of residual wash solution.
- Remove the spin filter and place it in a fresh catch tube (DNA spin kit). Air dry the spin filter for 5 min at room temperature (RT).
- Warm the DNAse-free water (DNA spin kit) at 55 °C for 5 min. Add 50 µL of DNAse-free water and gently stir the matrix on the filter membrane with a pipette tip for efficient elution of the DNA. Let samples stand at RT for 1 min, then centrifuge for 1 min at 14500 x g to elute DNA.
- Place the samples on ice. Load the sample mixture (2 µL of sample + 2 µL of water + 1 µL of loading buffer) into a gel. Measure the DNA concentration.
- Store the samples at -20 °C.
- Use a spectrophotometer to quantify the DNA extracted.
NOTE: A 1 µL volume of DNA was dropped and quantified, and the quality of DNA was noted at an A260/A280 ratio.
3. DNA electrophoresis
- Prepare 1% agarose gel in 0.5 Tris/Borate/EDTA (TBE) buffer (20 mL for a mini gel, 70-100 mL for a medium gel). Weigh agarose+buffer, boil in microwave to melt agarose, reweigh, and add dH2O to the original weight. Cool the agarose solution to 55-60 °C and pour into the gel former with comb assembly. Leave the gel to solidify.
- Add 1 µL of 10x gel loading buffer to the sample (10 µL) and load onto the gel next to a molecular weight marker. Run from negative (black) to positive (red) at 100-115 V (large gel) or 90 V (mini gel) until the blue dye is near the bottom.
- Stain gel for 30 min in 1 µL/mL ethidium bromide or SYBR green (nitrile gloves and goggles needed), rinse in water, and view under an ultraviolet (UV) light source. From the duplicate samples, select the highest yield for further processing.
4. High throughput sequencing
- Amplify the V4 hypervariable region of the 16S rRNA gene using specific primers 515F (5' -GTGYCAGCMGCCGCGGTAA-3') and 806R (5'-GGACTACNVGGGTWTCTAAT-3')22. Set the polymerase chain reaction (PCR) conditions at 94 °C for 2 min, 30 cycles of 94 °C for 20 s, 58 °C for 20 s, 65 °C for 1 min, and 65 °C for 10 min (final extension).
- Carry out PCR reactions with a high-fidelity PCR master mix (Table of Materials).
- Generate libraries with DNA library preparation kit and then sequence using paired-end sequencing on a sequencing platform.
NOTE: Here, the libraries generated with NEBNext Ultra II DNA Library Prep Kit were sequenced using paired-end Illumina sequencing (2 × 250 bp) on the novaseq6000 platform. - Follow the sequencing steps:
- Step 1: Shear the DNA with an enzyme, usually by a method that selects for a general size.
- Add 3.5 µL of sequencing buffer and 1.5 µL of enzyme mix to 25 µL of DNA (ca. 1 µg,) mix 10 times with a pipette and spin quickly.
- Place in a thermocycler with the following conditions: preheat lid at 75°C, (1) 30 min for 20 °C (2) 30 min for 65 °C (3) hold at 4°C.
- Step 2: Add adaptors via ligation
- Add 15 µL of ligation master mix, 1.25 µL of adapter, 0.5 µL of ligation enhancer, and 30 µL of end prep DNA mix with a pipette.
- Incubate at 20 °C for 15 min in a thermocycler and then add 1.5 µL uracil-specific excision reagent (USER) enzyme to the ligation mix to obtain a total of 48.26 µL. Incubate 37 °C for 15 min.
- Add 43.5 µL of magnetic beads to the adapter ligation DNA, mix 20 times with a pipette, and incubate for 5 min at RT. Separate the beads with a magnetic rack, then incubate for 5-10 min, and discard the supernatant.
- Wash the pellet with 100 µL of 80% ethanol, dry for 30 s, and wash again. Elute beads and pellet in 8.5 µL of 10 mM Tris HCl/0.1x Tris-EDTA(TE)/HPLC H20, mix with a pipette and incubate at RT for 2 min.
- Incubate in the magnetic rack and remove 7.5 µL of eluted DNA as supernatant. Place the elute in a new tube and conduct a PCR step.
- First, add 12.5 µL of master mix, 2.5 µL of index primer i7 primer, and 2.5 µL of universal PCR primer/i5 primer to 7.5 µL adapter-ligated DNA to obtain 25 µL of PCR reaction mix. Use the following PCR condition: preheat the lid to 103 °C. 98 °C for 30 s, 98 °C for 10 s, 65 °C for 75 s, 65 °C for 5 min and hold at 4 °C after 10 cycles.
- Clean the 25 µL amplified DNA. Add 22.5 µL of magnetic beads and mix 20 times. Incubate at RT for 5 min and remove 50 µL of the supernatant. Wash the beads with 100 µL of 80% ethanol, and remove all ethanol by drying the beads gently for 30 s.
- Resuspend the dark brown beads in 16 µL of water, mix 20 times with a pipette, and place in the magnetic rack for 30-60 s. Transfer 15 µL to a new tube.
- Determine the DNA concentration using a fluorometer. Mix 90 µL of buffer + 1 µL dye + 2 µL of DNA library sample, and read the concentration. Dilute the DNA to 2 nM, load 27-30 µL to the flow cell, and place it in the sequencer.
- Step 3: Sequence the adaptors, hybridize the libraries generated above to the matrix of the patterned flow cell, and capture DNA per the manufacturer's instructions. Use it as the template for the second synthesis.
- Then, amplify the second strand into a clonal cluster. Linearize the cluster and block the active sites. Add sequencing primer to provide a site for sequencing by synthesis in the sequence per the manuacturer's protocol.
NOTE: Chemically, the modified nucleotides bind to the DNA template strand using natural complementarity. Each nucleotide has a fluorescent tag and a reversible terminator that blocks the inclusion of the next base. Therefore, the presence of a fluorescent signal indicates which nucleotide is inserted, and the terminator is cleaved for the next base to bind.
- Then, amplify the second strand into a clonal cluster. Linearize the cluster and block the active sites. Add sequencing primer to provide a site for sequencing by synthesis in the sequence per the manuacturer's protocol.
- Step 4: Amplify the single-bound strand to give clusters of sequences that are sequenced in tandem by synthesis.
NOTE: The clusters give a brighter signal than a single strand through bidirectional scanning and two-channel sequencing chemistry. The sequencing software automatically transfers base call (*.cbcl) files to the specified output folder location for data analysis.
- Step 1: Shear the DNA with an enzyme, usually by a method that selects for a general size.
5. Bioinformatics
- Generate the sequence data as FASTQ files. Analyze to include the following parts.
- Part I:
- Save the FASTQ files obtained from the sequencer, click to open a spreadsheet file, and create mapping/metadata files.
- Open the Nephele website (https://nephele.niaid.nih.gov/), upload the FASTQ files, read QC, filtering, and trimming in QIIME222. Deposit the raw sequences as sequence read archive (SRA) and GenBank in NCBI BioProject (https://submit.ncbi.nlm.nih.gov/subs/bioproject/)
- Part II: Go to the Nephele website (https://nephele.niaid.nih.gov/), do the demultiplexed paired-end reads, filter substitution, and chimera errors, and merge using DADA223. Use the Naive Bayes classifier trained on the Silva version 132 99% OTU database to perform bacterial taxonomic assignment at 97% similarity24.
- Part III: Open the MicrobiomeDB website, click to upload files, and generate OTU alpha-diversity, beta-diversity, relative abundance, and rarefaction curves (https://microbiomedb.org/mbio/app).
- Part IV: Open a spreadsheet and transfer data to make heatmaps, Venn diagrams, and linear discriminant analysis effect size (https://microbiomedb.org/mbio/app).
- Part I:
6. Statistical analysis
- Open QIIME222, and determine the significant differences in alpha diversity between groups using the alpha-group-significance script. This will also perform the Kruskal-Wallis test.
- Determine the differences in beta diversity between groups by using PERMANOVA, including a pairwise test25.
- Obtain the significant differences in the bacterial community structure amongst the groups using MicrobiomeDB. A p-value ≤0.05 was considered statistically significant.
Representative Results
The quantity and quality of DNA extracted from grapes must, fermenting wine, and final wine were first determined; the quantity value ranges from 15-87 ng/µL (Table 1).
Sequencing and bioinformatics
The Illumina high throughput sequencer generated a FASTQ file that was imported to the Nephele and viewed on QIIME 2 platform26. Firstly, FastQC software was used to check for the sequence quality. Then, it was trimmed at both 5'- and 3'- ends to eliminate poor quality nucleotides, denoised, merged, and depleted of chimeric sequences before clustering into OTUs (operational definition for a species) using the DADA2 denoiser23. They were mapped to bacterial taxa using the SILVA v138.1 database. The sequences were clustered into OTU based on 99% nucleotide sequence similarity. Figure 1 shows the DADA2 rarefaction curves for the OTUs; it reached a plateau. This indicated that enough sequences were covered to indicate the majority of the microbial diversity. Replicate analysis of the sequences did not generate any significant difference.
The heatmap based on the relative abundance of the bacteria at different regimes from grape must to wine is shown in Figure 2. The most dominant phyla indicated in red consist of Proteobacteria, Actinobacteriota, Firmicutes, Bacteroidota, Fusobacteriota, and, followed by the Verrucomicrobiota, Halobacterota, Desulfobacterota, Myxococcota, and Acidobacteriota. The least abundant bacteria were indicated in blue. Many of these groups were present in must, when yeast was added but absent during fermentation, especially Nitrospirota, Bdellovibrionota, Nitrospinota, Armatimonadota, Gemmatimonadota, Chloroflexi, Campilobacterota, Deinococcota, Patescibacteria, Planctomycetota, Abditibacteriota, Crenarchaeota, Spirochaetota, Dependentiae, Thermotogota, Methylomirabilota and Elusimicrobiota. The relative abundance was also determined at the genus level, and the results indicated important genera such as Enterobacteriaceae and Lactobacillaceae, as shown in Figure 3. A Venn diagram analysis of the shared unique OTU revealed 15 bacteria were present from the wine grape must to the final wine (Figure 4). The results of the amplicon sequencing based on the V4 variable region also indicated the alpha diversity in the two Traminette R and L. Figure 4 shows the species evenness as a mark of distribution, which is different between the two nutritional treatments. The data showed that the diversity shift in the Stimula Sauvignon blanc + Fermaid O was lower than 1 at 13.4 brix, and evenness approaches zero as relative abundances vary. The data was also analyzed for beta diversity; Figure 5 shows the difference between the two fermentation Traminette R and L; the bacteria were similar at the must and yeast-added stages. There was a shift in the bacteria at the fermentation stage for the two treatments, and it looks similar in the final wine (Figure 6).
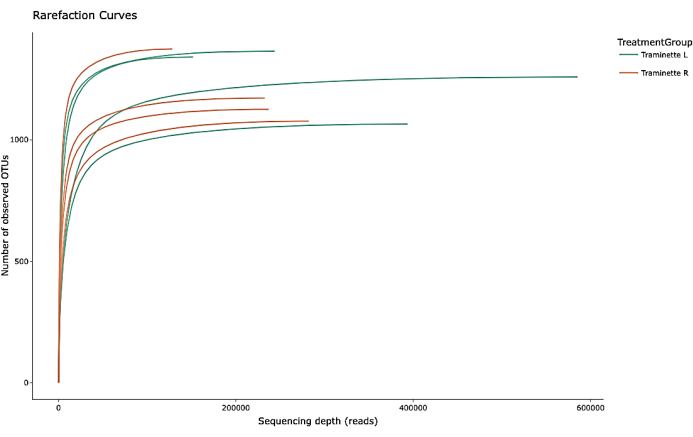
Figure 1: Rarefaction plots of observed OTUs. Please click here to view a larger version of this figure.
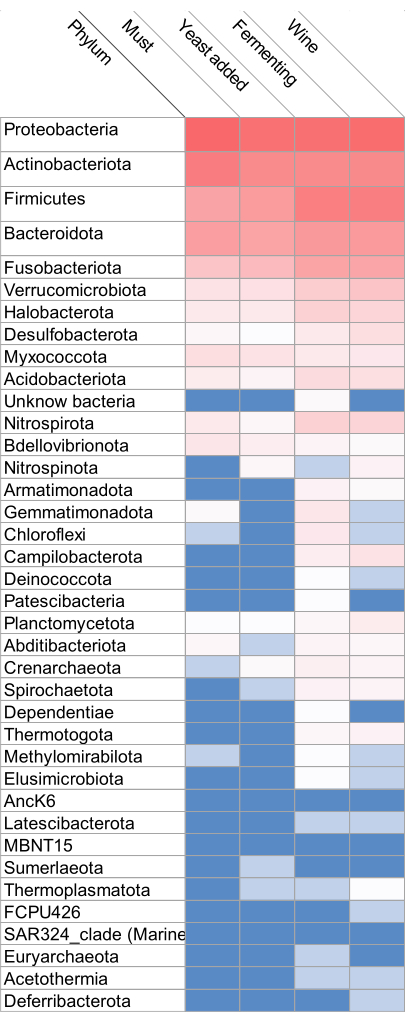
Figure 2: Relative abundance heatmap. The heatmap of the relative abundance at phylum levels R and L were not significantly different (p ≤0.05). The legend bar indicates the key score. Please click here to view a larger version of this figure.
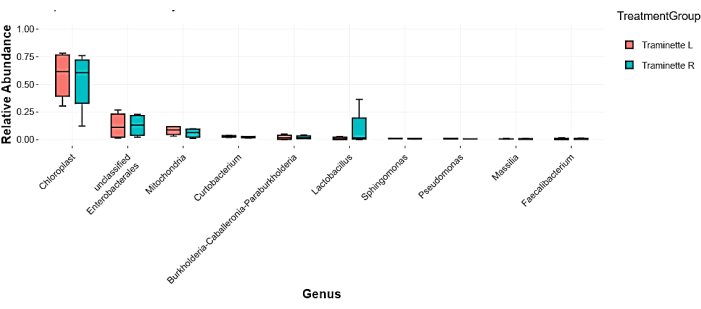
Figure 3: Relative abundance of the top ten taxa. The relative abundance at genus level of top ten taxa ranked by media with Wilcoxon rank sum test (p ≤0.05). Please click here to view a larger version of this figure.
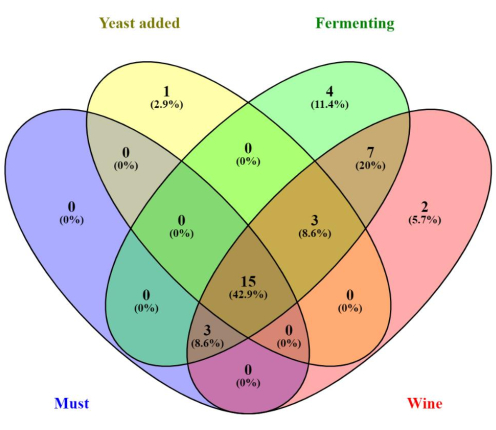
Figure 4: Venn diagram. The unique and shared bacteria among the must, yeast added, fermentation, and wine. Please click here to view a larger version of this figure.
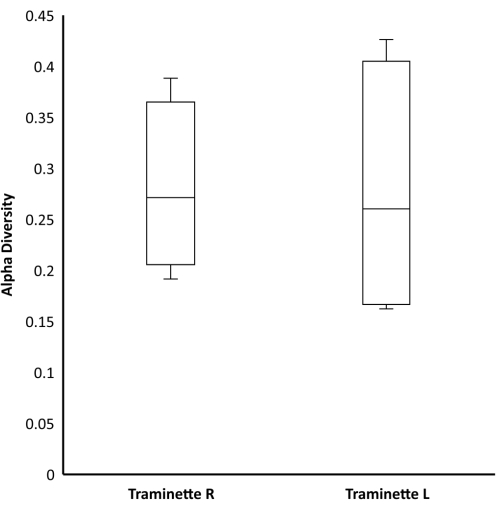
Figure 5: Alpha diversity of Traminette R and Traminette L. Alpha diversity of Shannon's H index of the two nutritional Fermaid O and Stimula Sauvignon blanc and Fermaid O (Dunn > 0.05) based on the analysis of Kruskal-Wallis test. Please click here to view a larger version of this figure.
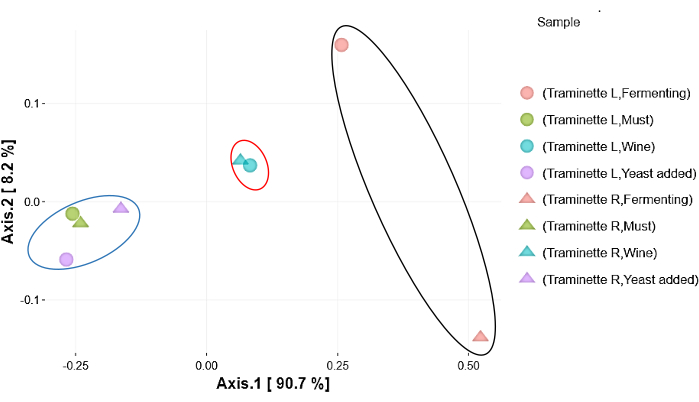
Figure 6: Principal component analysis. Principal component analysis of 16S rRNA data of beta diversity based on Bray-Curtis of the two nutritional Fermaid O and Stimula Sauvignon Blanc and Fermaid O at different stages of wine production. The blue circle represents a cluster of must and yeast addition stages. The red circle indicates the final wine and the black circle is the fermentation stage in the two treatments. Axis 1 and 2 are variations. Please click here to view a larger version of this figure.
| Sampling date | Sample | A260/A280 | Concentration ng/µL Traminette R | A260/A280 | Concentration ng/µL Traminette L |
| 8/30/21 | Grape | 1.762 | 37 | 1.75 | 28 |
| 8/31/31 | Must | 1.769 | 23 | 1.818 | 20 |
| 09-02-2021 | Rest | 1.667 | 15 | -1 | 1 |
| 09-04-2021 | Yeast added | 2.185 | 59 | 1.968 | 61 |
| 09-06-2021 | Fermenting | 2.023 | 87 | 2.048 | 43 |
| 09-09-2021 | Fermenting | 3.4 | 17 | 2.048 | 43 |
| 9/14/21 | Final wine | 2.143 | 30 | 2.042 | 49 |
Table 1: The quantity and quality of DNA extracted from grapes must, fermenting wine, and final wine.
Discussion
The protocol of metagenomics starts from the sampling of the grape must, and when yeast was added to the must, the fermenting wine and final wine samples. This was followed by duplicate DNA extraction that was successfully extracted from these samples. The quantities obtained varied in concentration from 15 ng/ µL to 87 ng/ µL. This shows that the DNA extraction protocol is effective for metagenomic studies of wine. Although the quality of the DNA at A260/A280 varies, this may be attributed to different parameters that may affect extraction, such as alcohol concentration and other fermentation metabolites that developed during and post-fermentation, as well as other organic plant materials that may inhibit genome extraction. Previous studies have reported a similar observation on the metagenomic plant material27. However, the DNA extracted in the protocol described achieved a quantity that was enough for the next step of the analysis. The PCR barcoding needed for metagenomic studies, especially using the Illumina sequencing platform, requires that the minimum DNA quantity be set at 1 ng/L. The V4 region was sequenced, and the determination of the bacterial diversity requires the use of 16S rRNA gene sequencing; this gene has different variable regions from V1-V923. A total of 6,393,443 paired-end sequence reads with an average of 399590.1875 ± 138442.6148 from the DNA extracted from the different samples. V4 has been commonly used, and it is referred to as the hypervariable region of the gene that is good for bacterial taxonomic and diversity analysis. During sequencing, paired Illumina sequencing ca. 200-300 bp sequences were generated. There are different platforms used for sequencing DNA extracted from food and fermented beverages28. The reasons for using the Illumina platform are the following: (1) Illumina is a 2nd second-generation sequencing platform with excellent output, and its chemistry gives a low error rate and profile. It is also affordable compared to available commercial kits for library prep, and its relatively short reads are ideal for differential expression. The final step of the protocol was bioinformatics, an important component of the use of amplicon 16 sequencing for the determination of microbial diversity. The quality check of the sequences FASTQ file obtained was very good, and the OTUs obtained from QIIME resulted in a taxonomic analysis shown in the results of relative abundance, alpha, and beta diversity.
Next-generation sequencing (NGS) has become an important tool for profiling the bacteria of different fermentation microbial communities. In this study, the 16S amplicon classification was first done at the phylum level, and a more diverse group of bacteria was noticed than previously reported12 during grape wine fermentation; 15 phyla were also shared, as indicated from the analysis of the Venn result. Also, analysis at the genus level showed the presence of important genera, such as Enterobacteriaceae and Lactobacillaceae that were more abundant in the Traminette R tank. We limited our analysis to genus-level taxonomy; there are studies where the method has been used for identification at species-level taxonomy through high-resolution sample inference23. One of the limitations of amplicon sequencing is that the sequence data cannot determine strain-level taxonomy because of the short length of the 16S rRNA sequence. Another limitation is the detection of chloroplast and mitochondria. It will be desirable if the bioinformatic procedure can be used to eliminate these sequences since they are contaminants of grape plants. However, amplification protocol utilizing PNA-DNA clamps to maximize chloroplast and mitochondria contamination29. The high abundance of Firmicute in grapes must, during fermentation and final wine, agree with the previous studies on wine grape30. The results of the alpha diversity analysis indicated the bacteria evenness of each fermentation nutritional treatment; it appears the Stimula Sauvignon blanc + Fermaid O nutrient generated a more diverse bacterium. In addition, the beta diversity data showed the shift in the bacteria at the fermentation stage; dynamic changes indicate that the fermentation stage is a very important period where the nutritional changes affect bacterial selection. The technique can be adapted and used by industries to monitor fermentation and improve quality and consistency28. However, further studies will be needed to better understand the impact of organic nutrients on microbial diversity during wine fermentation.
This study is the first to examine the bacteria diversity during the fermentation of Traminette grapes and wine using the 16S amplicon barcode sequencing to determine the bacteria changes. This confirmed the diversity of bacteria at the phylum and genus levels. Proteobacteria, followed by Firmicutes, were the most abundant as the fermentation progressed to the final wine. Many phyla that were not previously reported were detected using the 16S amplicon sequencing, and this confirmed that the method can be used to monitor wine production. Alpha diversity showed the role of nutritional change; it impacted the fermentation bacteria, while beta diversity indicates the changes during the fermentation stage. Further studies are needed to investigate the functional roles that many of the described phyla and genera play in wine fermentation.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
Funding from the Appalachian State University Research Council (URC) grant and CAPES Print Travel fellowship that supported the visit of FAO to Universidade de São Paulo, Ribeirão Preto – São Paulo, Brazil, are gratefully acknowledged. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. ECPDM is grateful for the CAPES Print Travel grant that supported her visit to Appalachian State University. ECPDM is a research fellow 2 from the Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brasil (CNPq).
Materials
| Agarose gel | Promega, Madison, WI USA | V3121 | Electrophoresis |
| FastPrep DNA spinKit for soil | MP Biomedicals, Solon, OH USA | 116560-200 | DNA extraction |
| FastQC software | Babraham Institute, United Kingdom | Bioinformatics | |
| Fermaid O | Scott Laboratory, Petaluma, CA USA | Fermentation | |
| High-Fidelity PCR Master Mix | New England Biolabs, USA | F630S | Polymerase chain reaction for sequencing |
| NEBNext Ultra | New England Biolabs, USA | NEB #E7103 | DNA Library Prep |
| NEBNext Ultra II DNA Library Prep Kit | Illumina, San Diego, CA USA | DNA sequencing | |
| NovaSeq Control Software (NVCS) | Illumina, San Diego, CA USA | DNA sequencing | |
| Novaseq6000 platform | Illumina, San Diego, CA USA | DNA sequencing | |
| QuiBit | Thermoscientific, Waltham, MA, USA | DNA quantification | |
| Quickdrop spectrophotometer | Molecular device, San Jose, CA, USA | DNA quantification | |
| Sodium Phosphate | Sigma Aldrich | 342483 | DNA extraction buffer |
| Stimula Sauvignon Blanc | Scott Laboratory, Petaluma, CA USA | Fermentation |
Referenzen
- Skinkis, P. A., Bordelon, B. P., Wood, K. V. Comparison of monoterpene constituents in traminette, gewürztraminer, and riesling winegrapes. American Journal of Enology and Viticulture. 59, 440-445 (2008).
- Bordelon, B. P., Skinkis, P. A., Howard, P. H. Impact of training system on vine performance and fruit composition of Traminette. American Journal of Enology and Viticulture. 59, 39-46 (2008).
- Reisch, B. I., et al. . Traminette’ Grape. New York Food and Life Science Bulletin. , (1996).
- Clemente-Jimenez, J. M., Mingorance-Cazorla, L., Martı́nez-Rodrı́guez, S., Las Heras-Vázquez, F. J., Rodrı́guez-Vico, F. Molecular characterization and oenological properties of wine yeasts isolated during spontaneous fermentation of six varieties of grape must. Food Microbiology. 21, 149-155 (2004).
- Attila, K. &. #. 1. 9. 5. ;., Kluz, M. Natural microflora of wine grape berries. Journal of Microbiology, Biotechnology and Food Sciences. 4 (special issue 1), 32-36 (2015).
- Swiegers, J. H., Bartowsky, E. J., Henschke, P. A., Pretorius, I. S. Yeast and bacterial modulation of wine aroma and flavor in Microbial modulation of wine aroma and flavour. Australian Journal of Grape and Wine Research. 11 (2), 139-173 (2008).
- Alonso-del-Real, J., Pérez-Torrado, R., Querol, A., Barrio, E. Dominance of wine Saccharomyces cerevisiae strains over S. kudriavzevii in industrial fermentation competitions is related to an acceleration of nutrient uptake and utilization. Environmental Microbiology. 21 (5), 1627-1644 (2019).
- Virdis, C., Sumby, K., Bartowsky, E., Jiranek, V. Lactic acid bacteria in wine: Technological advances and evaluation of their functional role. Frontiers in Microbiology. 11, 3192 (2021).
- Burns, T. R., Osborne, J. P. Impact of malolactic fermentation on the color and color stability of Pinot noir and Merlot Wine. American Journal of Enology and Viticulture. 64, 370-377 (2013).
- Piao, H., et al. Insights into the bacterial community and its temporal succession during the fermentation of wine grapes. Frontier of Microbiology. 6, 809 (2015).
- Figdor, D., Gukabivale, K. Survival against the odds: Microbiology of root canals associated with post-treatment disease Endodontic Topics. 18, 62-77 (2011).
- Bokulich, N. A., Joseph, C. M. L., Greg Allen, G., Benson, A. K., Mills, D. A. Next-generation sequencing reveals significant bacterial diversity of botrytized wine. Plos ONE. 7 (5), e36357 (2012).
- Berbegal, C., et al. A metagenomic-based approach for the characterization of bacterial diversity associated with spontaneous malolactic fermentations in wine. International Journal of Molecular Science. 20, 3980 (2019).
- Leveau, J. H., Tech, J. J. Grapevine microbiomics: bacterial diversity on grape leaves and berries revealed by high-throughput sequence analysis of 16S rRNA amplicons. International Symposium on Biological Control of Postharvest Diseases: Challenges and Opportunities. 905, 31-42 (2010).
- Rubiola, S., Macori, G., Civera, T., Fanning, S., Mitchell, M., Chiesa, F. Comparison between full-length 16s RNA metabarcoding and whole metagenome sequencing suggests the use of either is suitable for large-scale microbiome studies. Foodborne Pathogens and Disease. 19, 495-504 (2022).
- Bokulich, N. A., et al. Associations among wine grape microbiome, metabolome, and fermentation behavior suggest microbial contribution to regional wine characteristics. Mbio. 7 (3), e00631-e00716 (2016).
- Siren, K., et al. Multi-omics and potential applications in wine production. Current Opinion in Biotechnology. 56, 172-178 (2019).
- Kidd, S. P., Bastian, S. E. P., Eisenhofer, R., Welsh, B. L. Monitoring the viable grapevine microbiome to enhance the quality of wild wines. Microbiology Australia. 44 (1), 13-17 (2023).
- Lorenz, P., Eck, J. Metagenomics and industrial applications. Nature Reviews Microbiology. 3, 510-516 (2005).
- Tosi, E., Azzolini, M., Guzzo, F., Zapparoli, G. Evidence of different fermentation behaviours of two indigenous strains of Saccharomyces cerevisiae and Saccharomyces uvarum isolated from Amarone wine. Journal of Applied Microbiology. 107 (1), 210-218 (2009).
- Stefanini, I., Cavalier, D. Metagenomic approaches to investigate the contribution of the vineyard environment to the quality of wine fermentation: potentials and difficulties. Frontiers in Microbiology. 9, 991 (2018).
- Caporaso, J. G., et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 5, 335-336 (2010).
- Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J. A., Holmes, S. P. DADA2: High-resolution sample inference from Illumina amplicon data. Nature Methods. 13, 581-583 (2016).
- Quast, C., et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Research. 41 (Database issue), D590-D596 (2013).
- Anderson, M. J. A new method for non-parametric multivariate analysis of variance. Austral Ecology. 26 (1), 32-46 (2001).
- Bolyen, E., et al. interactive, scalable and extensible microbiome data science using QIIME 2. Nature Biotechnology. 37, 852-857 (2019).
- Fadiji, A. E., Babalola, O. O. Metagenomics methods for the study of plant-associated microbial communities: A review. Journal of Microbiological Methods. 170, 105860 (2020).
- van Hijum, S. A., Vaughan, E. E., Vogel, R. F. Application of state-of-art sequencing technologies to indigenous food fermentations. Current Opinion in Biotechnology. 24, 178-186 (2013).
- Víquez-R, L., Fleischer, R., Wilhelm, K., Tschapka, M., Sommer, S. Jumping the green wall: The use of PNA-DNA clamps to enhance microbiome sampling depth in wildlife microbiome research. Ecology and Evolution. 10 (20), 11779-11786 (2020).
- Bukin, Y. S., Galachyants, P., Yu, I. V., Morozov, S. V., Bukin, A. S., Zakharenko, T. I., Zemskaya, The effect of 16S rRNA region choice on bacterial community metabarcoding results. Scientific Data. 6, 190007 (2019).

