Ultrasonographic Evaluation of Salivary Glands for Sjogren’s Syndrome: Diagnostic and Monitoring Insights
Summary
Salivary gland ultrasonography is a promising tool for the diagnosis and evaluation of disease activity and complications in Sjogren’s syndrome (SS). The scanning technique for salivary gland ultrasound examination relevant to SS is described in the article.
Abstract
Sjogren’s syndrome (SS) is a chronic autoimmune condition commonly affecting the exocrine glands, causing oral or ocular dryness and extraglandular manifestations including arthralgia, cytopenia, and lymphoma. The presence of autoantibodies against SSA/Ro, labial salivary gland biopsy, ocular staining, Schirmer’s test, or salivary flow assessment are included in the current classification criteria of SS. However, the availability and invasiveness of these procedures limit their widespread use in clinical settings. Salivary gland ultrasonography (SGUS) is a non-invasive imaging modality for the evaluation of the salivary gland parenchyma and is increasingly utilized to aid diagnosis and monitoring in SS.
This article presents the protocol of SGUS for image acquisition at the parotid and submandibular glands. The objective is to present a standardized, reproducible, and practical approach to diagnostic SGUS for SS in daily clinical settings. Major salivary glands are scanned in a stepwise approach, beginning at the angle of the mandible for the superficial lobe of the parotid gland, followed by the deep lobe below the ramus of the mandible. Submandibular areas are then scanned for the submandibular glands. The steps in obtaining salivary gland images at each anatomical site are explained in the accompanying video. The echogenicity and echotexture at the thyroid gland are taken as a reference. The homogeneity, the presence and distribution of hypoechoic areas within the glands, and the border of the salivary glands are examined. The sizes and features of intra-/peri-glandular lymph nodes are recorded. The most distinctive sonographic feature in SS is glandular heterogeneity with the presence of hypoechoic/hyperechoic areas within the glands.
In summary, while SGUS cannot diagnose SS on its own, it can supplement the current classification criteria of SS and guide the clinical decision for salivary gland biopsy to support the diagnosis of SS in patients with sicca syndrome or suspicious systemic features, combined with autoantibody testing.
Introduction
Sjogren’s syndrome (SS) is an autoimmune disease characterized by lymphocytic infiltration in exocrine glands, including the salivary and lacrimal glands. Xerostomia, keratoconjunctivitis sicca, and extraglandular manifestations, including arthralgia, cytopenia, and lymphoma, are described in patients with SS. SS can be divided into primary and secondary types, in which the latter occurs in association with other connective tissue diseases. In the 2016 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria for primary SS, anti-SSA/Ro positivity and lymphocytic infiltration in labial salivary gland biopsy contributed to the two major criteria, whereas Schirmer’s test, ocular staining score, and unstimulated salivary flow represented the rest of the minor criteria1. The criteria are summarized in Table 1. Labial biopsy is an invasive procedure with potential complications, including sampling error, sensory loss, and hematoma formation2. Ocular staining tests and salivary flow assessment require specialized settings and might not be widely available.
Salivary gland ultrasonography (SGUS) is a non-invasive imaging modality that provides a detailed examination of the superficial salivary gland structure, which is commonly affected in patients with SS. Lymphocytic infiltration and inflammation in the salivary glands often lead to fatty infiltration, fibrosis, and loss of parenchyma, resulting in parenchymal inhomogeneity detected in SGUS3. The Outcome Measures in Rheumatology Clinical Trials (OMERACT) working group derived an ultrasound scoring system from grades 0 to 3 to semi-quantify inhomogeneity and the presence of hypoechoic areas within the parotid or submandibular glands4. A cutoff of grade 2 or above in at least one gland was found to correlate with positive labial biopsies, sialometry, and positive autoantibodies5. It demonstrated excellent specificity and good sensitivity in fulfilling the 2016 ACR/EULAR classification criteria for primary SS when given similar weight as the minor criteria. This protocol presents a standardized and practical approach for diagnosing SGUS in SS in clinical settings.
Protocol
The protocol demonstrated a clinical protocol for conduction of SGUS and the demonstration of scanning is performed on a de-identified healthy volunteer. Ethical approval is not required as the study is beyond the common rule and FDA definition of human research subject. Verbal consent from patients was obtained for taking clinical photos and their publication.
1. Transducer and machine settings
- Select a high-frequency linear transducer of 10 MHz (see the Table of Materials).
- Set to B mode.
- Use the highest frequency of the probe.
- Adjust the depth (3 cm) and gain (50%-60%).
NOTE: The depth is usually 1 cm deeper than the target structure. Auto-gain function is available for some ultrasound consoles. - For a right-handed examiner, place the ultrasound machine next to the patient's right shoulder.
2. Patient preparation
- Position the patient in the supine position with the neck slightly extended. Use a pillow to support the patient's head.
- Remove any clothing or jewelry around the neck that may obstruct the ultrasound transducer.
3. Scanning of the thyroid gland
- Orient the probe with the marker pointing towards the patient's right side for transverse images. For longitudinal scanning, orient the marker at the cephalic side.
- Apply gel to the probe surface.
- Place the probe below the thyroid cartilage for scanning of the thyroid gland. The right and left lobes of the thyroid gland are connected by the isthmus. Save the images and take the echogenicity of the thyroid gland as a reference (Figure 1).
- If there is concomitant thyroid disease, compare the echogenicity to that of the surrounding muscles. Normal salivary glands are usually more echogenic than muscle.
4. Scanning of the parotid gland
- Turn the patient's head to the opposite side of the gland being examined.
- Place the transducer longitudinally along the anterior border of the parotid gland, just anterior to the ear and parallel to the tragus, moving from the superior to the inferior pole of the gland (Figure 2).
- Place the probe transversely at the angle of the mandible to obtain the transverse scan. Ideally, the parotid gland is located between the mastoid and mandible ramus in transverse view. Scan the parotid gland transversely from the superior to the inferior pole (Figure 3).
- Slide the probe below the ramus of the mandible to visualize the deep part of the parotid gland, which could be partially obscured by the mandible. Place the probe between the mastoid process and the angle of the mandible.
- Save and label the images in both the longitudinal and transverse views.
- Assess the size of the parotid gland.
- Compare the echogenicity with that of the thyroid gland. The echogenicity of the normal parotid gland is similar to that of the thyroid gland.
- The retromandibular vein and external carotid artery divide the superficial and deep parotid lobes. Turn on the color doppler to differentiate blood vessels from the dilated intraglandular duct.
- Capture the degree of inhomogeneity, the clearness of the gland margin, and the presence of hypo-/an-echoic areas within the glands.
- Note the presence of focal lesions and document the size, location, shape, and echogenicity. Turn on the color doppler to look for vascularity.
- Score the degree of sonographic involvement on a scale of 0-3 as defined: Grade 0: normal, homogenous parenchyma; grade 1: mild inhomogeneity without hypo-/an-echoic areas; grade 2: moderate inhomogeneity with the presence of scattered hypo-/an-echoic areas; grade 3: Diffuse inhomogeneity with the presence of hypo-/an-echoic areas in the entire gland4.
- The presence of lymph nodes within the parotid gland could be normal and common, especially when located in the superficial lobe. Measure the diameter of the lymph nodes. Evaluate the shape, echogenicity, and preservation of fatty hilum.
- Repeat the scanning at the contralateral parotid gland. Compare the size and echotexture between the two parotid glands.
5. Scanning of the submandibular gland
- Position the patient in the supine position with the head maximally tilted to the back.
- To obtain the transverse view of the submandibular gland, position the transducer just below the angle of the mandible at the submandibular area, which is bounded by the mandible, mylohyoid muscles, and the anterior belly of the digastric muscles. Examine the entire submandibular gland from anterior to posterior (Figure 4).
- Obtain the longitudinal view by placing the probe medial to the horizontal body of the mandible. Visualize the facial artery in this plane (Figure 5).
- Save and label the images in both transverse and longitudinal views of the submandibular gland.
- Assess the size of the submandibular gland.
- Examine the echogenicity of the submandibular gland, which is usually less echogenic than the parotid gland.
- Assess the degree of inhomogeneity, the clearness of the gland margin, and the presence of hypo-/an-echoic areas within the glands. Note the presence of focal lesions.
- Grade the degree of inhomogeneity using the OMERACT score: Grade 0: normal parenchyma; Grade 1: minimal change with mild inhomogeneity without hypo-/an-echoic areas; Grade 2: moderate change with moderate inhomogeneity with focal hypo-/an-echoic areas; Grade 3: severe change with diffuse inhomogeneity with hypo-/an-echoic areas occupying all the surface of the gland. If the evaluation of the gland is not possible due to qualitative parameters, the fatty gland refers to grade 1, and the fibrous gland indistinguishable from adjacent tissue corresponds to grade 34.
- Measure the size of the submandibular lymph nodes. Evaluate the shape, echogenicity, and preservation of fatty hilum.
- Repeat the scanning at the contralateral submandibular gland. Compare the size and echotexture between the two submandibular glands.
6. Documentation
- Document the sonographic involvement on a scale of 0-3 at all four major salivary glands. A Score of ≥2 at any gland is considered compatible with SS.
- Document the presence of lymph nodes if any. Record the diameter, shape, and whether fatty hilum is preserved.
- Document the presence of focal abnormalities if any.
Representative Results
Here we described the interpretation of salivary gland ultrasound images to aid diagnosis of SS. The anatomical annotation at the scanning sites is summarized in Figure 1, Figure 2, and Figure 3. The echotexture of the thyroid gland is taken as the reference. Normal parotid glands should appear homogeneous with clear demarcation with overlying tissues and muscles. The echotexture of normal parotid glands is comparable to that of the thyroid gland. The retromandibular vein and external carotid artery are landmarks for dividing the superficial and deep lobes of the parotid gland. Normal submandibular glands demonstrate a clear demarcation with the overlying structures and are usually less echogenic and finer than the thyroid and parotid glands. The facial artery can be visualized along the submandibular parenchyma.
The homogeneity and the presence of anechoic/hypoechoic areas and hyperechoic bands within the salivary gland parenchyma are recorded. Hypo-/an-echoic areas are defined as small areas within the SG that generate few or no echoes and are non-compressible by the probe with the absence of blood flow on the color doppler. The OMERACT Ultrasound Working Group proposed a semi-quantitative scoring system of 0 to 3 through Delphi exercise. A score of ≥2 at any one gland is considered compatible with SS5. Figure 4 and Figure 5 show representative images from OMERACT grades 1 to 3. Hyperechoic bands might be present in SS because of fatty infiltration or fibrous tissue.
In addition to the homogeneity, the presence of lymph nodes within the parotid and submandibular glands should be recorded. Normal lymph nodes have a cutoff size of 9 mm and are usually round or oval with the preservation of hyperechoic fatty hilum6. Figure 6 shows a submandibular gland lymph node with preserved fatty hilum. The presence of lymph nodes within the parotid gland can be physiological, whereas lymph nodes are not present in normal submandibular glands. Features suspicious of malignant lymph nodes include a larger size, round shape, loss of fatty hilum, intranodal necrosis, and peripheral vascularity.
Extranodal lymphoma of the major salivary glands is a serious complication in SS, and SGUS can identify suspicious features to guide further investigation such as biopsy. The presence of focal lesions in patients with OMERACT scores 2 or 3 should raise the concern of lymphoma, especially when additional suspicious features, including the presence of septation, posterior acoustic enhancement, and hypervascularity.
Other common pathological conditions that can be identified on SGUS include sialolithiasis, sialadenitis, mucocele, and salivary gland tumors. In contrast to SS, these conditions more commonly affect the unilateral salivary gland. Sialolithiasis appears as a hyperechoic lesion within the salivary gland or duct and might accompany a dilated intraglandular duct or acoustic shadowing. Sialadenitis is often associated with hyperechogenicity, heterogeneity, and glandular edema. Salivary gland tumor usually appears as a hypoechoic focal lesion on SGUS while an indistinct border and irregular lesion might suggest a malignant lesion. Therefore, it is important to complement the SGUS findings with comprehensive history taking, physical examinations, and relevant investigations for accurate diagnosis.
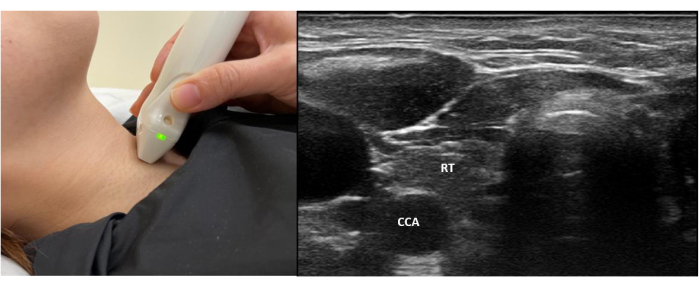
Figure 1: Probe position and standard view of the normal thyroid gland. This shows the placement of the probe at the central neck region to obtain a standard view of the normal thyroid gland for reference of echogenicity. Abbreviations: RT = Right lobe of Thyroid gland; CCA = Common carotid artery. Please click here to view a larger version of this figure.
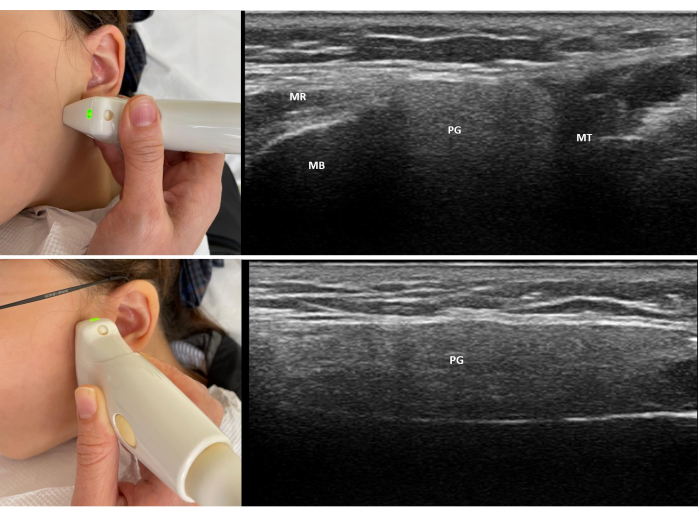
Figure 2: Probe position and standard view of scanning of the left parotid gland. (A) The top image shows the placement of the probe anterior to the ear and parallel to the tragus to obtain a standard view of the left parotid gland. (B) The bottom image shows the placement of the probe at the angle of the mandible to obtain the transverse view of the left parotid gland. Abbreviations: PG = parotid gland; MB = Mandible; MT = Mastoid; MR = Masseter. Please click here to view a larger version of this figure.
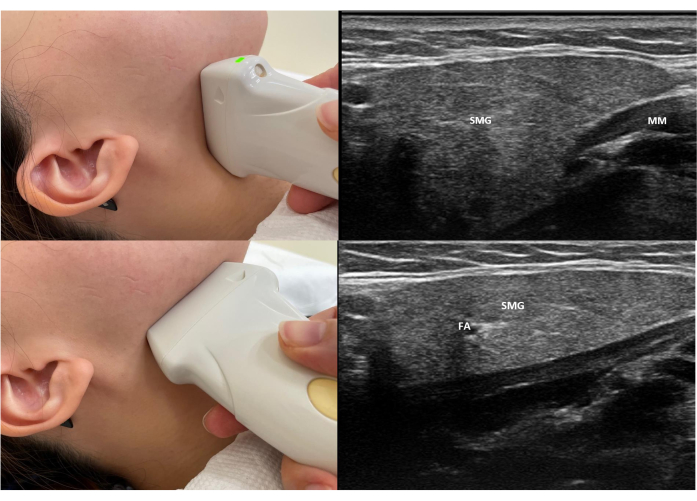
Figure 3: Probe position and standard view of scanning of the right submandibular gland. (A) The top image shows the position of the probe placement and the standard view of a transverse scan of the right submandibular scan. (B) The bottom image shows the position of the probe placement and the standard view of the longitudinal gland of the right submandibular scan. Abbreviations: SMG = submandibular gland; FA = facial artery; SC = Subcutaneous tissue; MM = mylohyoid muscle. Please click here to view a larger version of this figure.
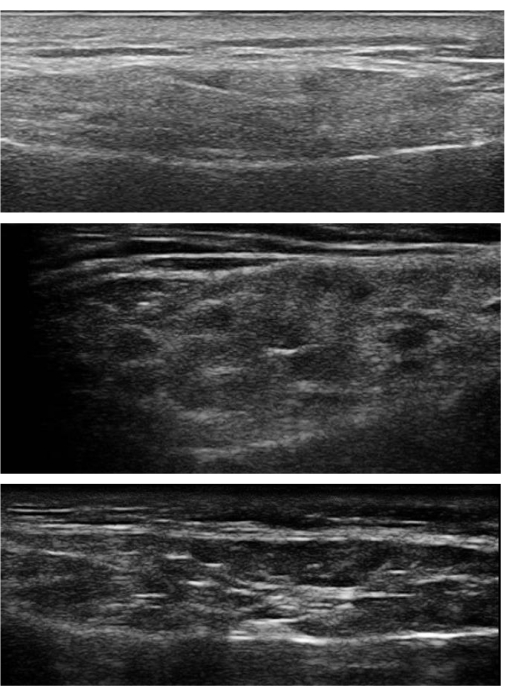
Figure 4: Grade 1 to 3 changes in the parotid glands. (A) Top image: Grade 1 changes in the right parotid gland. Mild inhomogeneity without hypo-/an-echoic area was observed. (B) Middle image: Grade 2 changes in the right parotid gland. There was moderate inhomogeneity with the presence of focal hypo-/an-echoic areas. Normal parenchyma was still observed in between the hypoechoic areas. (C) Bottom image: Grade 3 changes in the left parotid gland. Severe and diffuse inhomogeneity with the presence of hypo-/an-echoic areas in the entire gland. There were also hyperechoic bands and indistinct posterior glandular margins. Please click here to view a larger version of this figure.
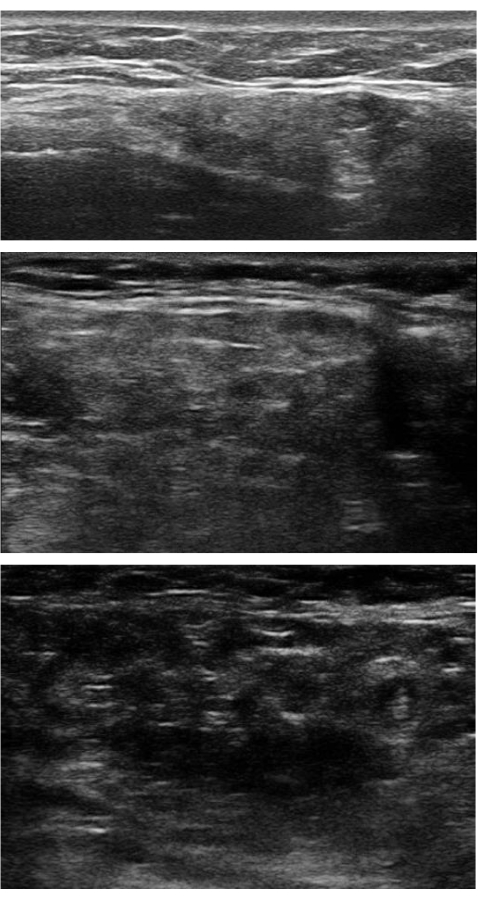
Figure 5: Grade 1 to 3 changes in the submandibular glands. (A) Top image: Grade 1 changes in the right submandibular gland. There was mild inhomogeneity without hypoechoic areas. (B) Middle image: Grade 2 changes in the right submandibular gland. The gland appeared moderately inhomogeneous with the presence of focal hypoechoic areas. (C) Bottom image: Grade 3 changes at the left submandibular gland. The hypo-/an-echoic areas occupied the entire gland. Please click here to view a larger version of this figure.
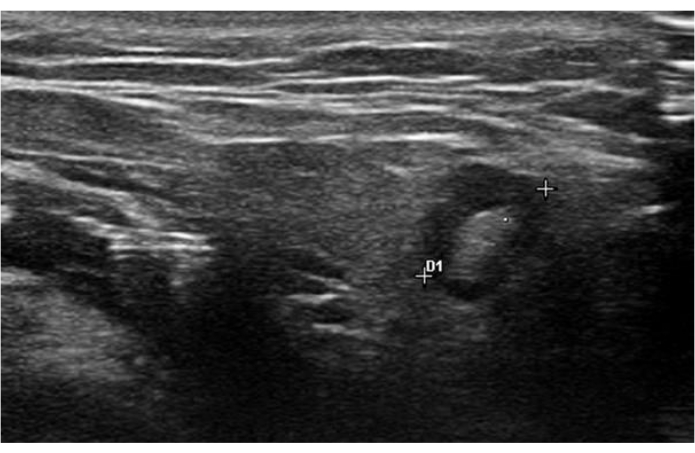
Figure 6: A submandibular lymph node with preserved fatty hilum. The diameter is 7.5 mm. Please click here to view a larger version of this figure.
Table 1: 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren's syndrome. The classification criteria for primary SS consisted of two major and three minor items. A score of 4 or above classified a patient with SS. Please click here to download this Table.
Table 2: Summary of studies on the reliability and diagnostic performance of SGUS using the OMERACT scoring system. Abbreviations: SGUS = Salivary gland ultrasonography; SS = Sjogren's Syndrome; PG = Parotid gland; SMG = submandibular gland. Please click here to download this Table.
Discussion
Ultrasonography of the major salivary glands is a non-invasive and accessible imaging modality with high spatial resolution. The procedure of performing SGUS at the major salivary glands for patients with suspected SS is described in this article to facilitate reproducible and standardized examination. Parotid and submandibular glands are superficially located, which permits detailed examination by high-frequency ultrasound. SGUS is useful in evaluating various clinical conditions, including SS, sialadenitis, sialolithiasis, and salivary gland tumors; however, the main scope of this article is focused on the description of the SGUS scanning technique and relevant results in the context of SS. We describe a stepwise approach to diagnostic SGUS with video guidance for patients with clinical suspicion of SS, such as oral or ocular dryness, systemic features from the EULAR Sjogren's syndrome disease activity index (ESSDAI), and positive anti-SSA autoantibody.
Normal salivary glands appear homogeneous with well-defined margins. In patients with SS, common sonographic findings include inhomogeneity with the presence of hypo-/an-echoic areas within the glandular surface. The emergence of hypo-/an-echoic areas was believed to represent peripheral duct inflammation, lymphocytic infiltration, and intraglandular ductal dilatation. In the early stages of SS, glandular enlargement with inhomogeneity is common. Dilated intraglandular ducts, known as sialectasis, are usually bilateral and symmetrical in SS. Progressive glandular distortion, cystic changes, and sialectasis might emerge as the disease progresses. In the late stages of SS, glandular atrophy, fibrotic changes, and severe inhomogeneity are often observed. Fibrotic major SGs would appear as hyperechogenic when compared to the thyroid gland. The SG margin might appear indistinct and ill-defined in SS and in severe cases, the border between SG and surrounding tissue is not visible. Diffuse punctate calcification over bilateral parotid glands can be observed in SS but computed tomography scans usually appreciate calcifications in greater detail.
Various novel scoring systems were developed to standardize SGUS with a meta-analysis showing sensitivity ranging from 45.8% to 91.6% and specificity from 73% to 98.1%7. In 1992, De Vita et al. proposed a scoring system to score the degree of glandular inhomogeneity ranging from 0 to 6 and demonstrated sensitivity and specificity of 88.8% and 84.6%, respectively, in diagnosing primary SS8. The addition of other scoring items to improve the diagnostic accuracy was evaluated in subsequent studies. Hočevar et al. described a novel SGUS scoring system ranging from 0 to 48 to measure five distinctive sonographic features of SS: 1. Homogeneity; 2. Hypoechogenic areas; 3. Hyperechogenic reflections; 4. Clearness of salivary gland borders and 5. Parenchymal echogenicity3. A cut-off US score of 17 provided a sensitivity of 58.8% and specificity of 98.7%. Subsequently, Cornec et al. evaluated the accuracy of SGUS for diagnosis of primary SS using echo-structure, gland size, and blood flow to the parotid gland determined by doppler waveform analysis. The echo-structure of each gland was graded on a scale of 0 to 4 by the presence and size of hypoechogenic areas, the presence of hyperechogenic bands, and calcifications9. A cutoff of grade 2, which was defined as the presence of multiple hypoechoic areas of <2 mm and hyperechoic bands, demonstrated a sensitivity of 62.8% and a specificity of 95.0%. Glandular size and blood flow were found to have poor diagnostic performance.
While these SGUS scoring systems comprehensively reviewed different sonographic features in SS, the variability and diversity of the grading systems might impose hurdles in the widespread application of SGUS in diagnosis and research in SS, especially for rheumatologists who are less experienced in performing US examinations. Among different sonographic features, glandular inhomogeneity was associated with satisfactory inter-reader reliability10. The OMERACT group therefore proposed a semi-quantitative scoring system on a scale of 0-3 to measure the degree of parenchymal inhomogeneity at the major salivary glands, with grade 0 representing normal parenchyma and grade 3 representing diffuse parenchymal involvement with extensive hypoechoic areas. The presence of focal hypoechoic areas differentiated grade 2 from grade 1 involvement in this OMERACT grey-scale scoring system. This scoring system demonstrated substantial intra- and interrater reliability in a validation study conducted by Finzel et al.11. A subsequent cohort study was conducted by Fana et al. to evaluate the cut-off value of the OMERACT scoring system in diagnosing SS5. A score of 2 or more at ≥1 major salivary gland was associated with positive autoantibodies, positive labial biopsy, sialometry, and positive Schirmer's test. A cutoff score of 2 at ≥1 major salivary gland had a sensitivity of 72% and specificity of 91% in diagnosing primary SS. The concordance between SGUS and minor salivary gland biopsy was 78% in a Spanish study12. SGUS demonstrated a higher sensitivity (90% vs. 76%) but lower specificity (67% vs. 90%) than minor salivary gland biopsy in fulfilling the ACR/EULAR classification criteria for primary SS. The OMERACT grey-scale scoring system was adopted in reporting SGUS findings in this study. The diagnostic accuracy of using the OMERACT scoring system was further evaluated by several study groups13,14,15. Robin et al. performed SGUS in 72 subjects and confirmed that SGUS demonstrated an area under the curve (AUC) of 0.751 in diagnosing SS13. The utility of the OMERACT scoring system was also validated in Asian populations. A multicenter study in China conducted SGUS with the OMERACT scoring system in 246 primary SS patients and 167 controls and found that SGUS had a sensitivity of 78% and specificity of 91.6% for diagnosing SS14. Table 2 summarizes the results on the reliability and diagnostic accuracy of the OMERACT scoring system in SS.
Incorporating SGUS as part of the 2016 ACR/EULAR classification criteria in primary SS was assessed in the UTOPIA study. Using over 500 realistic vignettes from data of patients with suspected primary SS, both SGUS findings and criteria-based diagnosis were found to be independently associated with an expert diagnosis of primary SS (p < 0.001)16. The addition of SGUS to the 2016 ACR/EULAR criteria improved sensitivity from 90.2% to 95.6% while maintaining similar specificity. This strategy was further validated in a Dutch cohort involving 234 primary SS patients with all ACR/EULAR items and SGUS performed. The addition of SGUS as a minor criterion to the criteria showed a comparable AUC to the original ACR/EULAR classification criteria using labial gland biopsy (0.966 vs. 0.965)17. Of the 134 patients included in the study by Fana et al., the incorporation of SGUS as a minor criterion with other clinical features could eliminate the need for invasive minor salivary gland biopsy in 46% of patients5. In patients suspected of SS, we proposed to perform serology tests for anti-SSA antibody and SGUS as initial investigations. A diagnosis of primary SS is substantiated if both tests are positive, and this potentially would reduce the number of patients requiring invasive minor salivary gland biopsy.
Other than supporting the diagnosis of primary SS, SGUS is also useful to rule out SS in patients with negative anti-SSA antibody. In the original 2016 ACR/EULAR classification criteria, patients with negative anti-SSA antibody often require minor salivary gland biopsy to exclude the possibility of SS. Mossels et al. have demonstrated good agreement between parotid and labial gland biopsies with SGUS findings18. Among patients with positive anti-SSA antibody and positive SGUS, 78% and 94% had positive parotid and labial gland biopsies, respectively. Alternatively, 89%-98% of patients with both negative SGUS and absent anti-SSA antibodies did not fulfill the ACR/EULAR classification criteria. Subsequently, in a French cohort of 337 patients presenting with sicca syndrome, SGUS abnormalities were significantly more common in patients with SS than patients with non-Sjogren's dry syndrome (NSDS)19. Only 8% and 5% of patients with NSDS had an SGUS score of ≥2 or ≥3, respectively, compared to 51% and 43% in patients diagnosed with SS (both p < 0.0001). The authors concluded that SS is very unlikely in anti-SSA negative patients with an SGUS score of <2 and minor salivary gland biopsy could be avoided.
There are ongoing efforts to incorporate SGUS into the assessment of disease activity in SS. A pathological SGUS score of ≥2 was found to correlate with the glandular domain in ESSDAI and SS Disease Activity Index20. In another Taiwanese study, there was a higher prevalence of rheumatoid factor positivity, the elevation of immunoglobulin G level, and elevated interleukin-25 titer in patients with an SGUS score of ≥221. Apart from gray-scale ultrasound, the OMERACT ultrasound subgroup recently published a reliability study on color doppler ultrasound scoring to evaluate salivary gland vascularity as a surrogate marker of glandular inflammation22. Further studies to validate this approach in a larger sample size with longitudinal measurements are needed before incorporating color doppler ultrasound into standard SGUS examination.
SGUS is also valuable to investigate glandular swelling in SS. It was reported that around 5% of patients SS developed B cell lymphoma, with non-Hodgkin lymphoma of the mucosa-associated lymphoid tissue being the most common histological type as a sequala of chronic inflammation at the salivary glands23,24. The sonographic features of lymphoma of the major salivary glands in SS were described by Lorenzo et al.25. Twenty-seven primary SS patients underwent SGUS followed by core needle biopsy and 14 cases of lymphoma, in which all were low-grade B-cell non-Hodgkin lymphoma of MALT, were diagnosed. All cases of lymphoma had an OMERACT score of ≥2 on SGUS. Additional sonographic features that were more commonly observed in the lymphoma group included the presence of very hypoechoic focal areas, the presence of septation, hypervascularity, oval shape, and posterior acoustic enhancement. An ultrasound-guided core needle biopsy can be considered in suspicious cases to obtain histological proof of lymphoma.
There are several limitations of SGUS and the first is the limitation of ultrasound beams to penetrate deep tissue. The deep lobe of the parotid gland is often partially obscured by the mandible and pathology in the deep lobe might be missed by SGUS. Second, the accuracy of SGUS is operator-dependent. The use of shear elastography in assessing salivary gland stiffness to circumvent operator dependence in SGUS was recently described, but further validation studies are needed before it can be widely applied26. Lastly, there is limited data in using SGUS for monitoring therapeutic responses in SS currently, and future prospective studies to include serial SGUS assessments in therapeutic trials of SS should be considered.
To conclude, SGUS plays an important role in diagnosing SS and there is growing evidence to support incorporating SGUS into the classification criteria. A standardized approach using the OMERACT scoring system to examine the major salivary glands with ultrasound was described in this article. SGUS is useful and widely validated to supplement the current classification criteria for diagnosis of SS especially in individuals with positive anti-SSA antibodies.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Materials
| Examination couch | / | Quantity: 1 | |
| High frequency linear transducer of at least 10 MHz | SL2325 18-6 MHz linear transducer | Quantity: 1 | |
| Ultrasound console | Esaote my lab seven | Quantity: 1 | |
| Ultrasound gel | / | Quantity: 1 |
Referenzen
- Shiboski, C. H., et al. American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 69 (1), 35-45 (2017).
- Varoni, E., et al. Local complications associated with labial salivary gland biopsy for diagnosis of Sjögren’s Syndrome: A retrospective cohort study. J Clin Exp Dent. , e713-e718 (2020).
- Hočevar, A., et al. Ultrasonographic changes of major salivary glands in primary Sjögren’s syndrome. Diagnostic value of a novel scoring system. Rheumatology. 44 (6), 768-772 (2005).
- Jousse-Joulin, S., et al. Video clip assessment of a salivary gland ultrasound scoring system in Sjögren’s syndrome using consensual definitions: an OMERACT ultrasound working group reliability exercise. Ann Rheum Dis. 78 (7), 967-973 (2019).
- Fana, V., et al. Application of the OMERACT Grey-scale Ultrasound Scoring System for salivary glands in a single-centre cohort of patients with suspected Sjögren’s syndrome. RMD Open. 7 (2), e001516 (2021).
- Ahuja, A. T., Ying, M. Sonographic evaluation of cervical lymph nodes. Am J Roentgenol. 184 (5), 1691-1699 (2005).
- Jousse-Joulin, S., et al. Is salivary gland ultrasonography a useful tool in Sjögren’s syndrome? A systematic review. Rheumatology. 55 (5), 789-800 (2016).
- De Vita, S., et al. Salivary gland echography in primary and secondary Sjögren’s syndrome. Clin Exp Rheumatol. 10 (4), 351-356 (1992).
- Cornec, D., et al. Contribution of salivary gland ultrasonography to the diagnosis of Sjögren’s syndrome: Toward new diagnostic criteria. Arthritis Rheum. 65 (1), 216-225 (2013).
- Milic, V. D., et al. Major salivary gland sonography in Sjögren’s syndrome: diagnostic value of a novel ultrasonography score (0-12) for parenchymal inhomogeneity. Scand J Rheumatol. 39 (2), 160-166 (2010).
- Finzel, S., et al. Patient-based reliability of the Outcome Measures in Rheumatology (OMERACT) ultrasound scoring system for salivary gland assessment in patients with Sjögren’s syndrome. Rheumatology. 60 (5), 2169-2176 (2021).
- Barrio-Nogal, L., et al. Ultrasonography in the diagnosis of suspected primary Sjögren’s syndrome and concordance with salivary gland biopsy: a Spanish single-center study. Clin Rheumatol. 42 (9), 2409-2417 (2023).
- Robin, F., et al. Diagnostic performances of ultrasound evaluation of major salivary glands according to the 2019 Outcome Measures in Rheumatology ultrasound scoring system. Arthritis Care Res. 74 (11), 1924-1932 (2022).
- Zhang, X., et al. Salivary gland ultrasonography in primary Sjögren’s syndrome from diagnosis to clinical stratification: a multicentre study. Arthritis Res Ther. 23 (1), 305 (2021).
- Tang, G., et al. Diagnostic value of ultrasound evaluation of major salivary glands for Sjögren’s syndrome based on the novel OMERACT scoring system. Eur J Radiol. 162, 110765 (2023).
- Jousse-Joulin, S., et al. Weight of salivary gland ultrasonography compared to other items of the 2016 ACR/EULAR classification criteria for Primary Sjögren’s syndrome. J Intern Med. 287 (2), 180-188 (2020).
- van Nimwegen, J. F., et al. Incorporation of salivary gland ultrasonography into the American College of Rheumatology/European League Against Rheumatism Criteria for Primary Sjögren’s Syndrome. Arthritis Care & Res. 72 (4), 583-590 (2020).
- Esther, M., et al. Ultrasonography of major salivary glands compared with parotid and labial gland biopsy and classification criteria in patients with clinically suspected primary Sjögren’s syndrome. Ann Rheum Dis. 76 (11), 1883-1889 (2017).
- Omar Al, T., et al. Normal salivary gland ultrasonography could rule out the diagnosis of Sjögren’s syndrome in anti-SSA-negative patients with sicca syndrome. RMD Open. 7 (1), ee001503 (2021).
- Milic, V., Colic, J., Cirkovic, A., Stanojlovic, S., Damjanov, N. Disease activity and damage in patients with primary Sjogren’s syndrome: Prognostic value of salivary gland ultrasonography. PLOS ONE. 14 (12), e0226498 (2019).
- Chen, Y. F., Hsieh, A. H., Fang, Y. F., Kuo, C. F. Diagnostic evaluation using salivary gland ultrasonography in primary Sjögren’s Syndrome. J Clin Med. 12 (6), 2428 (2023).
- Hočevar, A., et al. Development of a new ultrasound scoring system to evaluate glandular inflammation in Sjögren’s syndrome: an OMERACT reliability exercise. Rheumatology. 61 (8), 3341-3350 (2022).
- Voulgarelis, M., Dafni, U. G., Isenberg, D. A., Moutsopoulos, H. M. Malignant lymphoma in primary Sjögren’s syndrome: A multicenter, retrospective, clinical study by the European concerted action on Sjögren’s syndrome. Arthritis Rheum. 42 (8), 1765-1772 (1999).
- Nocturne, G., Pontarini, E., Bombardieri, M., Mariette, X. Lymphomas complicating primary Sjögren’s syndrome: from autoimmunity to lymphoma. Rheumatology. 60 (8), 3513-3521 (2021).
- Lorenzon, M., et al. Sonographic features of lymphoma of the major salivary glands diagnosed with ultrasound-guided core needle biopsy in Sjögren’s syndrome. Clin Exp Rheumatol. 39 Suppl 133 (6), 175-183 (2021).
- Özer, H., et al. Diagnostic performance of gray-scale ultrasound and shear wave elastography in assessing salivary gland involvement in patients with primary Sjögren’s syndrome. J Clin Ultrasound. 51 (1), 187-194 (2023).

