Membraneless Hydrogen Peroxide Fuel Cells as a Promising Clean Energy Source
Summary
This protocol introduces the design and evaluation of innovative three-dimensional electrodes for hydrogen peroxide fuel cells, utilizing Au-electroplated carbon fiber cloth and Ni-foam electrodes. The research findings highlight hydrogen peroxide’s potential as a promising candidate for sustainable energy technologies.
Abstract
In an in-depth investigation of membraneless hydrogen peroxide-based fuel cells (H2O2 FCs), hydrogen peroxide (H2O2), a carbon-neutral compound, is demonstrated to undergo electrochemical decomposition to produce H2O, O2, and electrical energy. The unique redox properties of H2O2 position it as a viable candidate for sustainable energy applications. The proposed membraneless design addresses the limitations of conventional fuel cells, including fabrication complexities and design challenges. A novel three-dimensional electrode, synthesized via electroplating techniques, is introduced. Constructed from Au-electroplated carbon fiber cloth combined with Ni-foam, this electrode showcases enhanced electrochemical reaction kinetics, leading to an increased power density for H2O2 FCs. The performance of fuel cells is intricately linked to the pH levels of the electrolyte solution. Beyond FC applications, such electrodes hold potential in portable energy systems and as high surface area catalysts. This study emphasizes the significance of electrode engineering in optimizing the potential of H2O2 as an environmentally friendly energy source.
Introduction
A fuel cell is an electrochemical device that utilizes fuel and oxidant to convert chemicals into electrical energy. FCs have higher energy conversion efficiency than traditional combustion engines since they are not bound by the Carnot Cycle1. By utilizing fuels such as hydrogen (H2)2, borohydride-hydrogen (NaBH4)3, and ammonia (NH3)4, FCs have become a promising energy source that is environmentally clean and can achieve high performance, offering significant potential to reduce human dependence on fossil fuels. However, FC technology faces specific challenges. One prevalent issue is the internal role of a proton exchange membrane (PEM) in the FC system, which acts as a safeguard against internal short circuits. The integration of an electrolytic membrane contributes to increased fabrication costs, internal circuit resistance, and architectural complexity5. Moreover, transforming single-compartment FCs into multi-stack arrays introduces additional complications due to the intricate process of integrating flow channels, electrodes, and plates to enhance power and current outputs5.
Over the past decades, concerted efforts have been made to address these membrane-related challenges and streamline the FC system. Notably, the emergence of membraneless FC configurations using laminar co-flows at low Reynold numbers has offered an innovative solution. In such setups, the interface between two flows functions as a "virtual" proton-conducting membrane6. Laminar flow-based FCs (LFFCs) have been widely studied, leveraging the benefits of microfluidics7,8,9,10. However, LFFCs require stringent conditions, including high energy input for pumping laminar fuels/oxidants, mitigation of reactant crossover in fluidic streams, and optimization of hydrodynamic parameters.
Recently, H2O2 has gained interest as a potential fuel and oxidant due to its carbon-neutral nature, yielding water (H2O) and oxygen (O2) during electrooxidation and electroreduction processes at electrodes11,12. H2O2 can be mass-produced using a two-electron reduction process or by a two-electron oxidation process from water12. Subsequently, in contrast to other gaseous fuels, liquid H2O2 fuel can be integrated into existing gasoline infrastructure 5. Besides, the H2O2 disproportionation reaction makes it possible to serve H2O2 as both fuel and oxidant. Figure 1A shows a schematic structure of a facile H2O2 FC's architecture. In comparison to traditional FCs2,3,4, the H2O2 FC utilizes the advantages of device "simplicity." Yamasaki et al. demonstrated membraneless H2O2 FCs, playing the role of both fuel and oxidant. The described mechanism of electrical energy generation has inspired research communities to continue this research direction6. Subsequently, electrooxidation and electroreduction mechanisms using H2O2 as a fuel and oxidant have been represented by the following reactions13,14
In the acidic media:
Anode: H2O2 → O2 + 2H+ + 2e–; Ea1 = 0.68 V vs. SHE
Cathode: H2O2 + 2H+ + 2e– → 2H2O; Ea2 = 1.77 V vs. SHE
Total: 2 H2O2 → 2H2O + O2
In the basic media:
H2O2 + OH– → HO2– + H2O
Anode: HO2– + OH– → O2 + H2O + 2e–; Eb1 = 0.15 V vs. SHE
Cathode: HO2– + H2O + 2e– → 3OH–; Eb2 = 0.87 V vs. SHE
Total: 2 H2O2 → 2H2O + O2
Figure 1B illustrates the working principle of H2O2 FCs. H2O2 donates electrons at the anode and accepts electrons at the cathode. Electron transfer between the anode and cathode occurs through an external circuit, resulting in the generation of electricity. The theoretical open circuit potential (OCP) of H2O2 FC is 1.09 V in acidic media and 0.62 V in basic media13. However, numerous experimental results have shown lower values, reaching up to 0.75 V in acidic media and 0.35 V in basic media, compared to the theoretical OCP. This observation can be attributed to the presence of a mixed potential13. Additionally, the power and current output of H2O2 FCs cannot compete with the mentioned FCs2,3,4due to the limited catalytic selectivity of the electrodes. Nevertheless, it is noteworthy that current H2O2 FC technology can outperform H2, NaBH4, and NH3 FCs in terms of overall cost, as shown in Table 1. Thus, the enhanced catalytic selectivity of electrodes for H2O2 electrooxidation and electroreduction remains a significant challenge for these devices.
In this study, we introduce a three-dimensional porous structure electrode to improve the interaction between the electrode and H2O2 fuel, aiming to increase the reaction rate and enhance power and current output. We also investigate the impact of solution pH and H2O2 concentration on the FC's performance. The electrode pair used in this study comprises a gold-electroplated carbon fiber cloth and nickel foam. Structural characterization is conducted using X-ray Diffraction (XRD) and Scanning Electron Microscopy (SEM), with Open Circuit Potential (OCP), polarization, and power output curves serving as the primary parameters for FC testing.
Protocol
1. Pre-processing of materials
NOTE: Ni-foam (commercially available, see Table of Materials) with 25 mm x 25 mm x 1.5 mm is used for the H2O2 FC's anode.
- Immerse the Ni-foam sample into alcohol and deionized (DI) water, sonicate for three times, 5 min in solvent and water. Subsequently, place the Ni-foam on a clean glass substrate.
- Utilize the carbon fiber cloth (see Table of Materials) as the cathode substrate. Cut the carbon cloth into 25 mm x 25 mm square pieces using scissors.
- Immerse the carbon cloth sample into acetone, 75% alcohol, DI water, and sonicate three times for 5 min, respectively. Then, flush the carbon cloth with DI water to remove residues of alcohol. Place the carbon cloth on a glass substrate.
NOTE: Based on the discussed research results15,16, Au as the cathode and Ni as the anode have been chosen as catalysts for H2O2 FCs. Metals like Pt, Pd, Ni, Au, and Ag have specific catalytical selectivity towards H2O2 oxidation or reduction reaction, making them suitable electrode materials. The Au@carbon fiber electrode offers a combination of electrocatalytic activity, stability, and enhanced conductivity, making it a suitable choice for membraneless hydrogen peroxide fuel cells.
2. Electroplating of Au on a carbon cloth
- Prepare reagents for electroplating as given by the following: chloroauric acid (HAuCl4), potassium chloride (KCl), hydrochloric acid (HCl), and DI water (see Table of Materials).
- Prepare 80 mL solutions (based on the volume of the beaker) in a clean beaker with 0.005 M HAuCl4, 0.1 M KCl, and 0.01 M HCl. Seal the opening and stir the solution for 15 min.
- Prepare the electroplating material carbon cloth, and the plating solution. The electroplating process is run by Electrochemical Station (ES) (see Table of Materials).
NOTE: Three electrode method is selected here for plating: carbon cloth as the Working Electrode (WE), graphite rod as the Counter Electrode (CE), and Ag/AgCl (saturated 1 M KCl solution) as the Reference Electrode (RE). - Ensure each electrode is clamping the correct object. Immerse electrodes into the plating solution.
- Start the ES. Set the program to the Chronoamperometry Method, as shown in Figure 1C. Ensure a single depositing circle is as follows: working potential 0.1 V for 0.1 s and resting potential 0.2 V for 0.2 s. As the result, the AuCl4– ion diffuses uniformly around the WE.
- Set Electroplating Circles at 800, 1600, 2400, and 3200 circles. Run the program.
NOTE: Typically, the Chronoamperometry method program in ES cannot achieve 1600 cycles. Alternatively, Multi-Potential Steps program of ES can also be used for the electroplating method, the same selections as the Chronoamperometry method (see manufacturer's instructions).
- Set Electroplating Circles at 800, 1600, 2400, and 3200 circles. Run the program.
- After electroplating, close the ES, pack the reagents, and collect Au electroplated carbon fiber cloth (Au@CF).
- Immerse the Au@CF into the DI water three times to remove the solution residues. Place it on a glass substrate for drying in the air.
- Cut the un-plated part of the Au@CF caused by the clamps to prevent part of CF from contacting solutions.
- Measure the size of Au@CF (a: length, b: width) with a ruler for calculating current/power densities.
3. Performance characterization of an FC
- Prepare solutions with two concentrations, one solution for pH gradient (1 mol H2O2, pH = 1, 3, 5, 7, 9, 11, 13), while the other one for H2O2 (CHP) gradient (pH = 1, CHP = 0.25 mol, 0.5 mol, 1 mol, 2 mol).
- Characterize the FC performance by ES with two electrodes for OCP and three electrodes for the polarization and power output curves (steps 3.3-3.6).
- Re-wash Ni-foam and Au@CF again with DI water two times. Place them aside for standby.
- Obtain OCP data during the testing of an FC: OCP is an essential parameter in the FC performance.
- Use Ni-foam as both RE and CE, and Au@CF as WE. Add the solution to the test beaker. Connect electrodes to the ES. Turn on the ES.
- Set the program to Open Circuit Potential – Time Method; Run Time: 400 s, Sample Interval: 0.1 s, High E Limit: 1 V, Low E Limit: -1 V. Run the program.
NOTE: It often takes time for the FC output to stabilize. Run measurements until stable FC results are obtained. - Measure the data. Close the program. Wash the beaker and electrodes. Add other solutions for specific tests.
- Test output performance of FC based on OCP data. Here, only original Linear Sweep Voltammetry (LSV) curve data is required. Further output data can be calculated from the LSV curve.
- Re-wash Ni-foam and Au@CF with DI water (repeat two times). Use Ni-foam as RE and CE, Au@CF as WE. Add the solution to the test beaker.
- Set the program to LSV, OCP as Initial E, 0 V as Final E, scan rate as 0.01 V/s, corresponding to the conditions of open circuit (OCP) and short circuit (0 V). Run the program.
- Collect the data, close the program, wash the beaker and electrodes, and add other required solutions for specific tests.
- Wash the electrodes after experiments and store them on a glass.
NOTE: Experiment data can be stored in EXCEL format.
4. Structural characterization of electrodes
NOTE: XRD is a facile and reliable method to analyze samples. XRD is taken to detect elements of the electrodes, such as electroplated Au on the carbon cloth. XRD tests are done before and after FC characterization to analyze potential corrosion and degradation of electrodes. For example, Au particles' can detach from CF, and nickel corrosion may occur in acidic solutions5.
- Wash the electrodes with DI water (two times) and dry them in the air at room temperature.
- Scrape off metals on the electrodes with tweezers. Collect the metal powder and place it in a container.
- Perform XRD tests17on the metal powder samples.
- Take SEM to characterize the morphology of the electrodes and investigate infiltration and electroplating between the gold and carbon fiber cloth. In addition, characterize the corrosion of nickel by SEM.
5. Data processing and power output calculation
- All data can be analyzed in EXCEL. Use Excel or Origin to analyze data and plot experimental graphs.
- Use the OCP data to characterize the selectivity of electrodes, e.g., using a table or a line figure. Use average potential for table legends. Typically, a line figure is used to demonstrate the stability of the FC.
- Use the LSV data to characterize the output performance of FC. There are two columns of data in the EXCEL file. Typically, one data set shows potential (U), and the other is recorded current (I). Calculate the power output using the following equation: P = U × I
NOTE: A high current (I) value shows a satisfactory performance of the FC. For example, a large electrode surface area results in higher currents. A normalized parameter referred to FCs' performance is current density (ID), which is equal to the current divided by the surface area (A) of the electrodes: ID = I/A - Subsequently, calculate the power density (PD) as: PD = U × ID
NOTE:It is essential to take the absolute value, as preliminary data values may be negative due to the direction of the current, which is not desirable during measurements. - Comparing parameters using U, ID, and PD within a single figure is straightforward. Assign ID to the x-axis, U to the left y-axis, and PD to the right y-axis.
Representative Results
Electroplating results
Figure 2 shows the electroplating results. Figure 2A indicates the X-ray diffraction result. Figure 2B,C are the micrographs. Figure 2D,E are SEM results. The effective deposition of gold (Au) on the carbon fiber cloth (CF) was first confirmed using the physical change of color in the carbon fiber cloth from black to golden yellow, as shown in Figure 2B,C. Further verification is achieved by the X-ray diffraction analysis, which showed clear peaks at 38.2°, 44.4°, 64.6° and 77.6° corresponding to the (111), (200), (220), and (311) crystal planes of face-centered cubic (fcc) Au (PDF card no. 04-0784), thereby confirming successful electroplating of Au onto the CF substrate.
The Au content in the Au@carbon fiber electrode can be estimated as follows: starting with 1 g of HAuCl4; it can be utilized for approximately 15 electroplating sessions. The gold content (mAu) from 1 g of HAuCl4 is calculated using the formula:
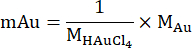
Accodring to estimation mAu = 0.58 g. When divided among the 15 sessions, each electroplating solution contains the following weight of Au3+ ions:
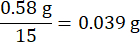
However, it is essential to note that not all Au3+ ions in the solution are deposited onto the carbon fiber cloth during the electroplating process. Therefore, each carbon fiber cloth contains less than 0.039 g of Au.
SEM images in Figure 2D-Iindicated a uniform dispersion of Au nanoparticles across the carbon fiber cloth. Remarkably, the original three-dimensional porous structure of the CF remained well-preserved post-electroplating. This preservation is crucial, as it provides a large surface area for interaction with the H2O2 fuel, thereby improving the reaction rate and power output.
Figure 3 illustrates Au@CFs subjected to varying numbers of electroplating cycles. Figure 3A highlights the color progression of the Au electroplated carbon fiber cloth. The color transition from the original black of the CF to the gold of the Au, which intensified from light to dark, suggests an increase in the volume of Au deposited on the CF as the number of electroplating cycles increased. Further support for this conclusion comes from the SEM images, Figure 3B–E, which reveal the evolving distribution of Au across the carbon fiber cloth.
H2O2 FC performance results
OCP of the FCs was measured for different solution pHs and H2O2 concentrations, which helps interpret the electrochemical properties of H2O2 FCs. The OCP's explicit dependency on both pH and H2O2 concentration was observed. The highest OCP was achieved at a pH of 1 and an H2O2 concentration of 0.25 mol. The OCP findings related to pH agree with previous studies indicating that H2O2 disproportionation and FC performance are highly pH-dependent18. A low pH helps stabilize H2O2 solutions, as H2O2 can be seen as a weak acid. The substantial chemical decomposition of H2O2 in basic solutions with pH above 11 can be clearly observed. Besides, according to Nernst Equation18, OCP typically increases as the concentration of electrolytes increases. However, in contrast to other FCs, the OCP of H2O2 FCs decreases as H2O2 concentration increases, especially using Au electrodes19. It is hypothesized that this observation is due to the mixed potential or limited electrode selectivities. A higher concentration leads to a higher chemical decomposition rate, thus decreasing the OCP. Nonetheless, further research is required to investigate this observation in more detail.
Polarization and power output curves have been obtained using specific solutions and electroplating circle conditions. The maximum power output was obtained at the optimal conditions using specific electroplating circle conditions (pH 1, H2O2 concentration 1 mol, electroplating circle 3200). Using optimal solution conditions, the maximum power output was obtained at the optimal conditions (pH 1, H2O2 concentration 2 mol). This maximum power output value is significantly higher than those obtained with the non-optimal conditions, further highlighting the importance of optimization of electroplating circles and solution conditions to achieve high-performance H2O2 FCs.
The H2O2 FCs exhibit satisfactory stability using optimal conditions during continuous 400 s measurements.
XRD analysis17 of the Ni-foam and Au@CF electrodes (before and after the FC performance tests) showed negligible changes, indicating satisfactory corrosion resistance of the electrodes using optimal operation conditions. Following the outlined protocol, observed results are illustrated and show the enhanced performance of the H2O2 FC with the three-dimensional porous electrode.
Positive result:
As depicted in Figure 4A, an increase in power and current densities was observed with a higher number of electroplating cycles. This observation is attributed to more Au being plated onto the CF with larger electroplating cycles, which provides a more active material for facilitating electrochemical reactions. However, the FC performance enhancement does not increase proportionally with the number of electroplating cycles. A modest improvement of approximately 20% in current and power densities was recorded when the electroplating cycles doubled from 800 to 1600. It suggested that excessive electroplating may lead to the formation of a layer that encapsulates the nano Au, thereby decreasing the mass transport efficiency. This finding can provide valuable insights into the electroplating method of catalyst fabrication.
Figure 4B illustrates the durability of the open-circuit potential (OCP) of the H2O2 FC at different concentrations in solutions with a pH of 1. The data indicates that the electrode can operate continuously for at least 400 s without significant degradation, showcasing the robustness of electrodes fabricated through electroplating.
Interestingly, the OCP of the H2O2 FC surpassed the theoretical value in both acidic and basic media, suggesting that the designed electrode effectively catalyzes reactions within the FC. The power and current output of the FC were found to increase with H2O2 concentration, reaching a peak at a specific pH. This observation confirms the effectiveness of the electrode and highlights the influence of solution pH and H2O2 concentration on FC performance.
Figure 4C presents the FC's polarization and power density curves under optimal conditions. A peak power density of approximately 0.8 W m-2 was achieved at an H2O2 concentration of 2 M and a pH of 1, demonstrating the efficiency of the three-dimensional porous electrode in facilitating the FC's reactions and boosting power output.
Negative result:
On the other hand, suboptimal results were observed at excessively high H2O2 concentrations. As shown in Figure 4C, an increase in the H2O2 concentration beyond an optimal point does not result ina corresponding proportional increase in performance, underlining the existence of an ideal H2O2 concentration for FC operation to balance fuel usage and performance output. For instance, the maximum power density increased by about 44% from 0.56 W m-2 to 0.81 W m-2 when the H2O2 concentration increased eight times from 0.25 M to 2 M.
Moreover, the FC performance was found to be sensitive to the solution pH. Under extreme acidic and basic conditions (pH values below 3 and above 11), the power output of the FC was relatively high, as seen in Figure 4D.
These results elucidate how various parameters influence the performance of H2O2 FCs. With further optimization of the chemical composition and the development of more selective electrocatalysts, this promising and environmentally friendly energy device can find practical applications. Table 1 shows the competitive fuel costs and energy generated on the FC systems20,21,22.
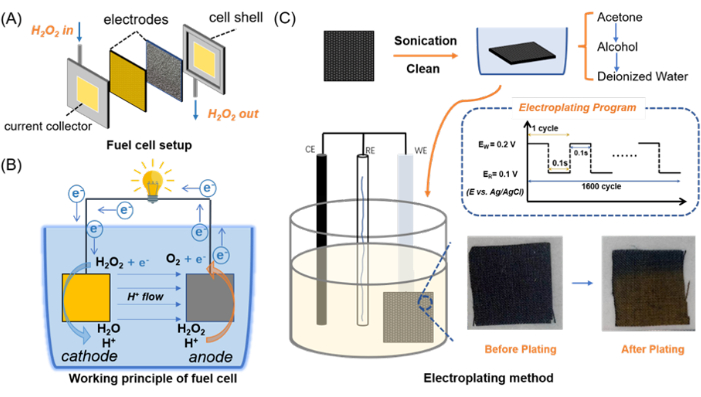
Figure 1: Schematic representation of this study. (A) Schematic integration of a single membraneless FC. (B) A working principle of the H2O2 FC. (C) The process of electroplating method with pre-processing on carbon fiber cloth, electroplating solution of 5 mM HAuCl4, 1 M HCl, and electroplating program. Please click here to view a larger version of this figure.
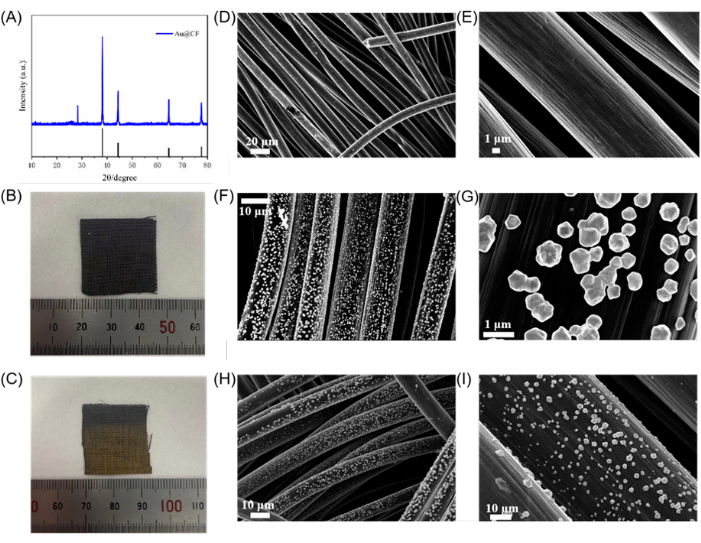
Figure 2: Structural characterization of 1600 circles electroplated Au@CF. (A) XRD result of Au@CF. (B) Micrograph of CF before electroplating. (C) Micrograph of CF after Au depositing. (D,E); (F,G); and (H,I) are SEM results for CF before electroplating, after electroplating, and after performance tests, respectively. Scale bars: (D), 20 µm; (E,G), 1 µm; (F,H,I), 10 µm. Please click here to view a larger version of this figure.
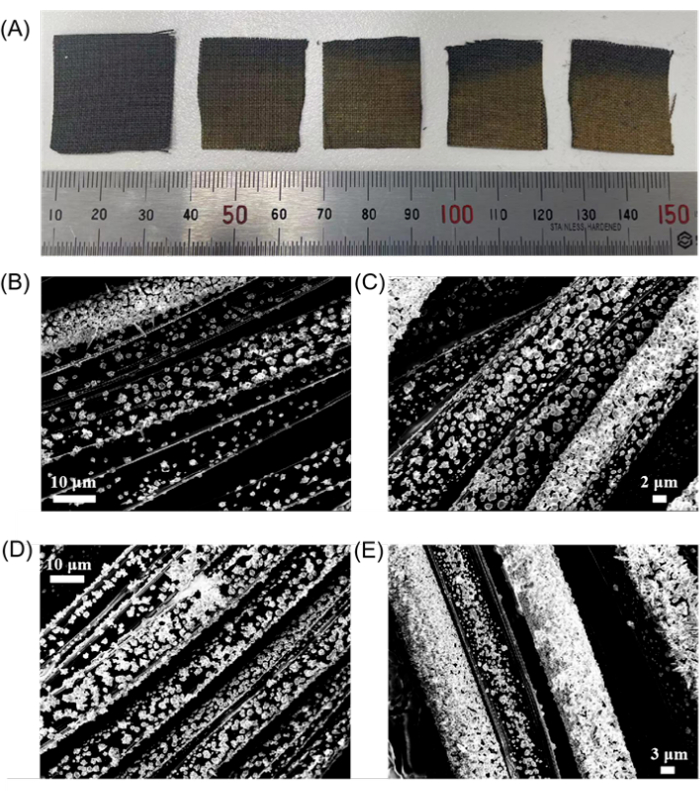
Figure 3: Micrograph and SEM results of Au@CF under different electroplating circles. (A) From left to right are 0, 800, 1600, 2400, and 3200 electroplating circles Au@CF, respectively. (B–E) are SEM images of Au@CF under 800, 1600, 2400, and 3200 electroplating circles, respectively. Scale bars: (B,D), 10 µm; (C), 2 µm; (E), 3 µm. Please click here to view a larger version of this figure.
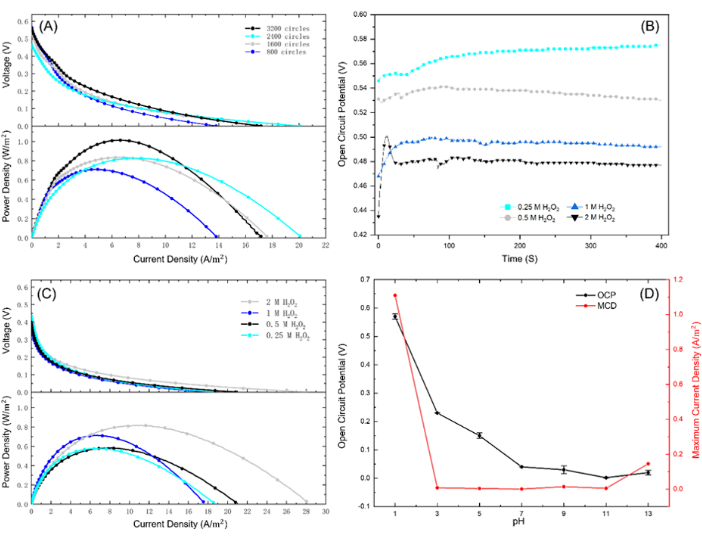
Figure 4: Performance characterization of the FC. (A) Polarization (left) and power output (right) curves using different electroplating circles (800, 1600, 2400, 3200 circles) in pH = 1, CHP = 1 M solutions. (B) OCP test under different H2O2 concentrations (0.25 M, 0.5 M, 1 M, 2 M H2O2) in pH = 1 solutions and with 1600 circles electroplated Au@CF for 400 s. (C) Polarization (left) and power output (right) curve under different H2O2 concentrations (0.25 M, 0.5 M, 1 M, 2M) in pH = 1 solutions and with 1600 circles electroplated Au@CF. (D) OCP and maximum current density curves under different pH conditions ranging from pH = 1 to 13 in 1 M H2O2 solutions and with 1600 Au@CF electroplated circles. Please click here to view a larger version of this figure.
| Fuels | Free energy (kJ/mol) | Fuel cost | Energy cost($/kW) | Reference |
| Hydrogen | -237 | 6.9 $/kg | 200 | 20 |
| NaBH4 | -1273 | 55 $/kg | 10.2 | 21 |
| H2O2 | -120 | 1.8 $/ton (bulk) | 1.84 | 22 |
Table 1: Competitive costs of fuels and the cost of energy generated on the FC systems.
Discussion
Several parameters significantly influence the performance of a membraneless hydrogen peroxide fuel cell beyond solution pH and H2O2 concentration. The choice of electrode material dictates electrocatalytic activity and stability, while the electrode's surface area can enhance reaction sites. Operating temperature affects reaction kinetics, and the flow rate of reactants can determine the mixing efficiency of fuel and oxidant. The concentration of any catalyst used is pivotal for reaction rates, and impurities can inhibit or poison the catalyst. The fuel cell's design, including electrode spacing and flow channel geometry, impacts mass transport and kinetics. The type and concentration of the electrolyte influence ionic conductivity, and the external circuit's resistance and design can alter operational parameters like current and power density. Additionally, the concentration of oxygen, a byproduct of H2O2 decomposition in the presence of specific catalysts, can also play a role in the cell's performance. Optimizing these parameters is essential for the efficiency and durability of membraneless hydrogen peroxide fuel cells7,8,9,10.
The investigation was concentrated on the fabrication of a three-dimensional porous electrode tailored for hydrogen peroxide fuel cells (H2O2 FCs), and the enhancement of the fuel cell's performance through the regulation of solution parameters11,12. The successful electroplating of Au onto CF was confirmed using XRD analysis and SEM imaging. The resultant three-dimensional porous Au@CF electrode exhibited robust performance in H2O2 FCs under optimal solution conditions (pH 1, H2O2 concentration 2 M), and an optimal performance ratio was achieved at 1600 electroplating cycles, as indicated by OCP, power output, and superior stability.
These results carry pivotal implications for designing and developing efficient electrodes for H2O2 FCs. The three-dimensional porous structure is poised to maximize the contact area with the H2O2 fuel, potentially enhancing the electrochemical reaction rate and boosting the power output of the FCs. Additionally, the remarkable corrosion resistance of the Au@CF electrode suggests that the chosen materials have the potential for long-term, stable operation of H2O2 FCs.
Certain critical elements require meticulous consideration to further enhance the performance of H2O2 FCs with a three-dimensional porous structure electrode. Foremost among these is the fabrication of the porous electrode. Proper surface treatment and regulation of the pore size distribution are of utmost importance, as these factors directly impact the electrocatalytic efficiency of the electrode. Furthermore, the management of the H2O2 concentration and solution pH is critical. Performance of the FC can be diminished due to excessive or insufficient H2O2 concentration, as observed in the experiments, and a similar trend was observed when the solution pH deviated from the optimal range.
To improve the performance of the H2O2 FC under optimal conditions, certain modifications may be warranted. For example, the electrode surface treatment process can be optimized to achieve a more diverse range of pore sizes, which may enhance the reaction kinetics. When the expected power output is not achieved, specific research steps may be needed, which can involve fine-tuning the H2O2 concentration, adjusting the pH, and contemplating other parameters, including temperature and pressure, which can impact the performance of FCs.
While the successful development of the novel electrode and the promising results from the FCs tests pave the way for potential applications of H2O2 in clean energy technology, further research is warranted to refine the electrode's design, selectivity, and surface area toward H2O2 FC practical applications.
The observed behavior in membraneless H2O2 FCs underscores the intricate interplay between pH dynamics and hydrogen peroxide concentration. The performance of H2O2 FCs is not merely a function of its electrode design but is deeply influenced by the electrolyte's chemical composition. At an optimal pH and H2O2 concentration, the electrochemical reactions are facilitated, leading to efficient electron transfer and enhanced power output. The influence of pH can be attributed to its role in modulating the proton activity and the redox potential of the system. A balanced pH ensures that the electrochemical reactions at both the anode and cathode proceed smoothly without any hindrance13,14. On the other hand, H2O2 concentration directly impacts the availability of reactants. An optimal concentration ensures that there is a steady supply of H2O2 molecules to partake in the redox reactions, thereby generating a consistent flow of electrons. However, any deviation from this equilibrium, either an imbalanced pH or a suboptimal H2O2 concentration, can disrupt the electron flow, leading to diminished performance. It is plausible that at extreme pH levels, the structure or activity of the catalyst might be compromised, or side reactions might become predominant. Similarly, too low or too high H2O2 concentrations might either starve the reaction or lead to its saturation, respectively. In essence, the synergy between pH and peroxide concentration is pivotal in determining the efficiency of H2O2 FCs. Future research might clarify deeper into understanding of these parameters, potentially unlocking even greater performance from these sustainable energy devices.
Critical steps
The process of electroplating gold (Au) onto carbon cloth is a cornerstone in the fabrication of the three-dimensional electrodes. The quality, uniformity, and thickness of the gold layer are paramount as they directly impact the electrode's electrochemical attributes. The chronoamperometry technique, which was chosen for this process, offers the advantage of ensuring a consistent and even deposition of gold onto the cloth. However, a delicate balance must be struck in determining the number of electroplating cycles. Too many cycles lead to an over-coating of gold, which might compromise the inherent porous nature of the carbon fiber cloth, thereby affecting its efficiency. The preparation of the H2O2 solution is another critical step that demands precision. The pH level and concentration of the H2O2 solution are instrumental in determining the FC's performance. The research observed a direct correlation between these parameters and the FC's output, highlighting the importance of meticulous solution preparation.
Modifications and troubleshooting
The introduction of a three-dimensional porous electrode was a significant modification aimed at enhancing the interaction between the electrode and the H2O2 fuel. The primary objective behind this design innovation was to accelerate the electrochemical reaction rate, thereby increasing both power and current outputs. However, while the design is promising, the electroplating process might necessitate further refinements to ensure the electrode properties align with the desired outcomes. The research underscored the FC's performance sensitivity to the H2O2 solution's pH and concentration. Any deviations from the optimal parameters can lead to diminished power outputs or even instability in the FC's operation. As a troubleshooting measure, fine-tuning these parameters can rectify performance issues.
Limitations
Despite the advancements, the current H2O2 FC technology has certain limitations. When juxtaposed with other fuel cell technologies, the H2O2 FCs tend to exhibit lower power and current outputs. A primary reason for this is the limited catalytic selectivity of the electrodes. The H2O2 FC's performance is highly contingent on the solution's pH and H2O2 concentration. This sensitivity implies that there's a relatively narrow window of operation within which the FC can deliver optimal results.
Significance with respect to existing methods
The H2O2 FC's membraneless architecture significantly differs from conventional fuel cells1,2,3,4. This design choice effectively circumvents several challenges that plague traditional FCs, such as the high costs associated with fabrication and the complexities inherent in their design. The introduction of a three-dimensional electrode in this research is a game-changer. By facilitating enhanced electrochemical reaction kinetics, this electrode design holds the promise of achieving higher power densities for H2O2 FCs, setting them apart from conventional designs.
Future applications of the technique
Beyond their application in fuel cells, the three-dimensional electrodes have a broader potential. They can be integrated into portable energy systems or even employed as high surface area catalysts. Such versatility can catalyze the evolution of compact and efficient energy devices suitable for diverse applications. The pioneering work on H2O2 FCs is crucial in the global shift from reliance on fossil fuels to the adoption of more environmentally friendly energy alternatives.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Key Technologies R&D Program of China (2021YFA0715302 and 2021YFE0191800), the National Natural Science Foundation of China (61975035 and 52150610489), and the Science and Technology Commission of Shanghai Municipality (22ZR1405000).
Materials
| Acetone | Merck & Co. Inc. (MRK) | 67-64-1 | solution for pre-process of materials |
| Alcohol | Merck & Co. Inc. (MRK) | 64-17-5 | solution for pre-process of materials |
| Carbon fiber cloth | Soochow Willtek photoelectric materials co.,Ltd. | W0S1011 | substrate material for electroplating method |
| Electrochemistry station | Shanghai Chenhua Instrument Co., Ltd. | CHI600E | device for electroplating method and fuel cell performance characterization |
| Gold chloride trihydrate | Shanghai Aladdin Biochemical Technology Co.,Ltd. | G141105-1g | main solute for electroplating method |
| Hydrochloric acid | Sinopharm Chemical ReagentCo., Ltd | 10011018 | adjustment of solution pH |
| Hydrogen peroxide | Sinopharm Chemical ReagentCo., Ltd | 10011208 | fuel of cell |
| Nickel foam | Willtek photoelectric materials co.ltd(Soochow,China) | KSH-2011 | anode material for hydrogen peroxide fuel cell |
| Potassium chloride | Shanghai Aladdin Biochemical Technology Co.,Ltd. | 10016308 | additives for electroplating method |
| Scanning electron microscope | Carl Zeiss AG | EVO 10 | structural characterization for sample |
| Sodium hydroxide | Sinopharm Chemical ReagentCo., Ltd | 10019718 | adjustment of solution pH |
| X-Ray differaction machine | Bruker Corporation | D8 Advance | structural characterization for sample |
Referenzen
- Sazali, N., Wan Salleh, W. N., Jamaludin, A. S., Mhd Razali, M. N. New perspectives on fuel cell technology: A brief review. Membranes. 10 (5), 99 (2020).
- Singla, M. K., Nijhawan, P., Oberoi, A. S. Hydrogen fuel and fuel cell technology for cleaner future: a review. Environmental Science and Pollution Research International. 28 (13), 15607-15626 (2021).
- Cao, D., Chen, D., Lan, J., Wang, G. An alkaline direct NaBH4-H2O2 fuel cell with high power density. Journal of Power Sources. 190 (2), 346-350 (2009).
- Lan, R., Tao, S. Ammonia as a suitable fuel for fuel cells. Frontiers in Energy Research. 2, 2014 (2014).
- Alias, M. S., Kamarudin, S. K., Zainoodin, A. M., Masdar, M. S. Active direct methanol fuel cell: An overview. International Journal of Hydrogen Energy. 45 (38), 19620-19641 (2020).
- Ferrigno, R., Stroock, A. D., Clark, T. D., Mayer, M., Whitesides, G. M. Membraneless vanadium redox fuel cell using laminar flow. Journal of the American Chemical Society. 124 (44), 12930-12931 (2002).
- Yan, X., Xu, A., Zeng, L., Gao, P., Zhao, T. A paper-based microfluidic fuel cell with hydrogen peroxide as fuel and oxidant. Energy Technology. 6 (1), 140-143 (2018).
- Ha, S. M., Ahn, Y. Laminar flow-based micro fuel cell utilizing grooved electrode surface. Journal of Power Sources. 267, 731-738 (2014).
- Liu, Z., et al. A woven thread-based microfluidic fuel cell with graphite rod electrodes. International Journal of Hydrogen Energy. 43 (49), 22467-22473 (2018).
- Peng, J., Zhang, Z. Y., Niu, H. T. A Three-dimensional two-phase model for a membraneless fuel cell using decomposition of hydrogen peroxide with y-shaped microchannel. Fuel Cells. 12 (6), 1009-1018 (2012).
- Wu, K. H., et al. Highly selective hydrogen peroxide electrosynthesis on carbon: in situ interface engineering with surfactants. Chem. 6 (6), 1443-1458 (2020).
- Yang, Y., et al. A facile microfluidic hydrogen peroxide fuel cell with high performance: electrode interface and power-generation properties. ACS Applied Energy Materials. 1 (10), 5328-5335 (2018).
- An, L., Zhao, T., Yan, X., Zhou, X., Tan, P. The dual role of hydrogen peroxide in fuel cells. Science Bulletin. 60 (1), 55-64 (2015).
- Yamazaki, S. I., et al. A fuel cell with selective electrocatalysts using hydrogen peroxide as both an electron acceptor and a fuel. Journal of Power Sources. 178 (1), 20-25 (2008).
- Sanli, A. E., Aytaç, A. Response to Disselkamp: Direct peroxide/peroxide fuel cell as a novel type fuel cell. International Journal of Hydrogen Energy. 36 (1), 869-875 (2011).
- Gu, L., Nie, L., George H, M. i. l. e. y. Cathode electrocatalyst selection and deposition for a direct borohydride/hydrogen peroxide fuel cell. Journal of Power Sources. 173 (1), 77-85 (2007).
- Yang, F., Cheng, K., Wu, T., Zhang, Y., Yin, J., Wang, G., Cao, D. Preparation of Au nanodendrites supported on carbon fiber cloth and its catalytic performance to H2O2 electroreduction and electrooxidation. RSC Advances. 3 (16), 5483-5490 (2013).
- Vidal-Iglesias, F. J., Solla-Gullón, J., Herrero, E., Rodes, A., Aldaz, A. Do you really understand the electrochemical Nernst equation. Electrocatalysis. 4, 1-9 (2013).
- Jing, X., et al. The open circuit potential of hydrogen peroxide at noble and glassy carbon electrodes in acidic and basic electrolytes. Journal of Electroanalytical Chemistry. 658 (1-2), 46-51 (2011).
- Eaves, S., Eaves, J. A cost comparison of fuel-cell and battery electric vehicles. Journal of Power Sources. 130 (1-2), 208-212 (2004).
- Wee, J. H. Which type of fuel cell is more competitive for portable application: Direct methanol fuel cells or direct borohydride fuel cells. Journal of Power Sources. 161 (1), 1-10 (2006).
- Muthukumar, P., Groll, M. Erratum to "Metal hydride based heating and cooling systems: a review". International Journal of Hydrogen Energy. 35 (16), 8816-8829 (2010).

