The Active Place Avoidance (APA) Test, an Effective, Versatile and Repeatable Spatial Learning Task for Mice
Summary
Here, we present a protocol for the active place avoidance test, a hippocampus-dependent spatial learning paradigm designed for rodents. Altering key parameters allows for re-testing of animals before and after treatments or over time.
Abstract
Hippocampus-dependent spatial learning in rodents has been tested using a variety of methods. These include the Morris water maze (MWM), Y-maze, and novel object location (NOL) tasks. More recently, the active place avoidance (APA) task has been developed as an alternative to these more traditional approaches. In the APA task, mice must use spatial cues placed around a rotating arena to avoid a stationary shock zone. Due to the multiple parameters that can be adjusted, the APA task has been demonstrated to be a very versatile approach. It lends itself to being used longitudinally and repeatedly for the same cohort of mice. Here, we provide a detailed protocol to successfully conduct the APA task. We also highlight alternative APA approaches that can be used to examine different components of spatial learning. We describe the data collection and analysis processes. Critical steps during the APA task are discussed to increase the likelihood of successfully conducting the test. The APA task has several advantages over more traditional spatial navigation tests. It is appropriate to use with aged mice or those with disease phenotypes such as Alzheimer’s disease. The complexity of the task can be easily altered, allowing a wide range of mouse strains to be tested. Further, the APA task is suitable for testing animals that have undergone surgery or experimental interventions that may have affected motor or neural function, such as stroke or traumatic brain injury.
Introduction
Active place avoidance (APA) is an effective tool to test hippocampus-dependent spatial learning in rodents1,2,3,4. During the APA task, the animal is placed on a rotating arena and is required to use visual cues to orientate themselves and avoid an aversive shock zone5. The rotation of the arena ensures that the mouse is unable to use an idiothetic approach for navigation, nor can scent marks be used, as these cues rotate on the platform while the shock zone remains stationary5. Altering the speed and direction of the arena, as well as the location of the shock zone and visual cues, allows for the re-testing of mice multiple times6,7,8. The APA offers several distinct advantages compared to the Morris water maze (MWM), one of the most widely used spatial learning tests. Importantly, mice have an aversion to swimming and find the MWM task extremely stressful9. Further, aged mice have been reported to float during the MWM task10, making it unsuitable as a spatial learning task in many instances. Further, as the MWM task requires a hidden, submerged platform for the mice to locate during testing. This necessitates the water to be opaque, which is typically achieved via the addition of white paint. Tracking and analysis of animals during behavioral tasks requires sufficient contrast between subject and surrounds, excluding certain mouse strains such as Swiss or BALB/c from being tested in the MWM. In the APA task, this issue is circumvented through the addition of black plastic under the grid.
Multiple APA paradigms have been designed to test spatial learning, demonstrating its utility as an effective behavioral tool. For example, acquisition, retention, and consolidation of spatial learning is typically accomplished by daily testing of animals that can range from 3-5 days6,7,11,12. Memory and learning are quantified by comparing the number of shocks received each acquisition day. Time to first entrance and the maximum time avoiding the shock zone are also important parameters that can be used to determine changes in learning ability during the task. Alternatively, spatial working memory can be tested by conducting a single, 30 min APA session2,13 where spatial learning is measured as within-session changes by comparing performance, such as shock number, in 5 min bins.
In this article, we describe the APA task and highlight the key features that must be considered when conducting this spatial learning test.
Protocol
All animal procedures were approved by The University of Queensland Animal Ethics Committee under the guidelines of the National Health and Medical Research Council of Australia (approval number: QBI/189/15).
1. APA room setup
NOTE: The APA apparatus comprises an elevated arena with a metal grid floor enclosed by a 32 cm-high transparent circular boundary. The metal bars are evenly spaced (0.5 cm apart) and have 0.3 cm diameter.
- Ensure the APA apparatus is within the camera frame mounted on the ceiling. Track the mouse using commercially available animal tracking software.
- The APA arena generally rotates at 1 rpm speed, and a pre-designated 60° stationary shock zone is set within the rotating arena. When the mouse enters the shock zone, deliver a mild foot shock of 0.5 mA (60 Hz, 500 ms).
- Ensure that the location of the shock zone remains constant during testing and is set electronically within the experimental setup. The rotating arena carries the mouse into the shock zone unless the mouse actively moves to avoid it.
- Place four novel visual cues on four different room walls at the same height as the rotating platform, typically 30-50 cm away from the arena. Ensure the cues are neutral colors, such as black-and-white symbols or shapes printed on A3 paper and laminated for easy cleaning (Figure 1A).
- Ensure that the light intensity of the room is between 30-70 Lux. Increasing light intensity induces anxiety-like behavior and reduces exploration.
- Before starting, open the Tracker program and select the APA task.
- In the Options for Tracker 2D, select the Experiment tab. Here, ensure that Place Avoidance- One Frame- Position Only is selected. This will allow configuring the parameters required. Save the configuration file and adjust as required.
- In the Experiment tab set the experiment duration in the Experiment Time box. A typical experiment duration is 600 s or 10 min.
- Make sure the Enable Timer is selected. Change the shock parameters in the Timer region as discussed above.
- Enter the common experimental details in the space provided in the Room Frame region of the Experiment tab. For example, ensure that the Default Output File name is populated with the date, a simple experimental identifier, and the day of testing. Finish the name with an underscore "_" to allow the addition of a unique mouse ID during the experiment.
- In the Room Frame region is also the Targets tab. Click the Edit button to provide the ability to ensure the entire arena is included in the region of interest. Then, select Arc to provide the adjustable parameters for the size and location of the shock target zone (Figure 1B).
- Open the Tracking tab to adjust the parameters to ensure successful mice tracking. The contrast box has either Dark or Light options to allow for both dark (e.g., C57Bl/6) or light (e.g., BALB/c) mice. This creates an effective contrast between the background and the mice. When using albino strains of mice, place a piece of black plastic under the arena to allow for this contrast to be achieved (Figure 2).
- Set the mice size and area ranges in this region. Set these parameters to effectively recognize the mouse when in the arena. Alternatively, set these after pressing the From Calibrator button.
- Select the From Calibrator button to ensure the arena is completely in the region of interest mask.
- Start the arena in this tab to make sure that when the arena rotates, the arena remains in the mask. This tab is also critical to select the appropriate contrast threshold. Move the red line in the Threshold pane to adjust the contrast threshold.
NOTE: Figure 3A shows an optimal threshold selection, as evidenced by a solid orange region and a blue "X" where the mouse is located. A poor threshold is shown in Figure 3B and shows only speckled orange and no "X".
- Start the arena in this tab to make sure that when the arena rotates, the arena remains in the mask. This tab is also critical to select the appropriate contrast threshold. Move the red line in the Threshold pane to adjust the contrast threshold.
- Use the Devices tab and set the rotation direction and speed of the arena using the velocity button. Select both positive and negative velocities, representing clockwise and anti-clockwise rotations. Set the shock intensity in the Current Source section. The most common setting for mice is 1 rpm rotation and a 0.5 mA shock.
- Alter how or when to deliver the shocks within the Current Source tab.
- Ensure the Current mode is selected to Track Dependent. This will provide an electric shock when the mouse moves into the shock zone.
- Select Time to give shocks at a time interval set by the user. Use previously recorded tracks to shock a mouse by selecting From File. This is to provide a yoked control mouse subjected to an identical number of shocks at the same duration and intensity independent of spatial learning.
NOTE: The File Output and Window tabs allow data and video files to be saved in a specific directory. The From Image button within the File Output tab also allows the region of interest to capture the entire arena to be selected.
- Retreat behind the curtain and begin the trial. The experimenter's presence close to the arena and any unnecessary noise may affect animal performance.
- Ensure that any noise and odor are limited during the trial, which can provide the mouse another cue, affecting their performance. Examples to minimise this include ensuring a closed clinical waste bin, using rooms removed from noisy lab spaces, and thoroughly cleaning the equipment between mice. Researchers may consider using the white noise generator to mask unrelated external noises.
- Let the home cage bedding remain the same throughout the behavioral testing period, as this may provide new stimulation and affect the behavior.
- To avoid diurnal variations, conduct testing at a consistent time each day.
2. Habituation to experimenter handling
- Handle each mouse daily for 30 s to 1 min for at least 2-3 days before the testing. Animal handling significantly reduces stress and anxiety-related behavior during testing.
- Use the same lab coat and avoid wearing strong deodorants, colognes, or perfume during the habituation and testing.
3. Habituation to the APA arena (1 day)
- Bring the mouse into the anteroom or testing room for habituation. Leave the mouse to habituate for a minimum of 30 min. Set the light intensity in the anteroom or testing room before mice are brought in to habituate.
- Set up the Tracker software.
- Create an experiment-specific folder. Depending on the experimental paradigm, have separate folders for each day or trial. Set up experiment configurations as described above and save these configurations to use in the future.
- Before starting a trial, open the saved configuration by clicking the File tab, then click the Save symbol, add a unique mouse ID in the newly opened window, and run the trial by pressing the Play tab.
- Habituate the mouse to the APA apparatus by exposing it to the rotating arena for 5 min without delivering shocks.
- Remove the mouse from the home cage by lifting it from the tail's base and gently placing it onto the gloved hand. Transport the mouse to the APA apparatus and place it away from the shock zone, facing the wall.
- Retreat behind the curtain and begin the trial.
- At the end of the testing, remove the mouse and return to the home cage.
- Collect all urine and scat, and clean the grid thoroughly with 80% (v/v) ethanol.
- Repeat steps 3.4-3.7 for all mice.
4. Acquisition training using APA (1-6 days)
- Set the room lighting to identical conditions as on the habituation day.
- Bring the mouse into the anteroom or testing room and allow it to habituate for a minimum of 30 min.
- Set up the Tracker software as described above.
- Set the duration of the trial.
- Ensure the current source is on and set (i.e., 0.5 mA).
- Place the mouse on the arena away from the shock zone and facing the wall.
- Retreat behind the curtain and begin the trial by pressing the Play button. Monitor the mouse on the computer screen and intervene if required. For example, the mouse is not receiving shocks or appears overly stressed, as evidenced by excessive jumping or vocalizing.
- At the end of the testing, remove the mouse and return to the home cage.
NOTE: Make sure mice are receiving and reacting to the shocks. Mice respond to the shock by rearing back and vocalizing. If this is not the case, they may not be receiving the shock. This could be due to scat on the grid or due to inadequate tracking. Therefore, cleaning the grid after each trial and optimizing the mouse tracking, as discussed above, is essential.
5. Reversal acquisition training (Optional, 1-6 days)
- In the reversal task, reposition the shock zone to a new location, generally 180° from the previous position. Assess the mouse's ability to flexibly learn a novel shock zone location. The room cues are typically not changed during reversal learning.
- Repeat steps 3.4-3.7 for all mice.
6. Probe trial (Optional, 1 day)
- In the probe trial, measure the time to the first entrance and/or the maximum time avoiding the shock zone.
NOTE: This indicates memory consolidation after the acquisition phase. A well-trained mouse will avoid entering the shock zone for a prolonged period (>60 secs), showing evidence of spatial learning. - Set the room light intensity as on the acquisition training day.
- Habituate the mouse in the testing room or anteroom for 30 min.
- Set up the Tracker software.
- Set the trial duration to the same time as the testing period previously conducted (for example, 10 min or 30 min, depending on trial parameters).
- Do not deliver shocks for this trial.
- Place the mouse on the opposite side of the aversive shock zone, facing the wall.
- Start the trial and retreat behind the curtain.
- Make sure the mouse is tracked efficiently.
- Monitor the mouse on the computer screen and stop the trial when it enters the shock zone. Some researchers prefer to continue the trial for 5 min to see if the mouse continues to return to the shock zone.
- Gently pick up the mouse and return to the home cage.
- Ensure all urine and scat are collected, and the grid is thoroughly cleaned with 80% (v/v) ethanol.
7. Track analysis
NOTE: The performance of the task can be achieved via different tracking software. Below is how the included software is used to determine the performance during the APA task. In this instance, the data is analyzed using the Track Analysis program.
- To analyze the data, open the Track Analysis program and select Avoidance from the dropdown menu in the main window.
- Click on Add Task to upload the data files saved during the acquisition phase in a new window. In Group Name, create a group to analyze, e.g., Day 1 or time of analysis.
- Click the Output Directory to select the location to save the analyzed data.
- Add the files to be analyzed by clicking on the Add Files tab and selecting the files from the local drive.
- Set the time to be analyzed by clicking the Set Time tab. This provides the ability to define the period which will be analyzed, i.e., 0 to 600 s. Alternatively, analyze the data in bins, i.e., 60 s.
- Once all tracks are added, click the Analysis tab and select Run Analysis to analyze the data. The analysis will produce several folders. The data for analysis will be in the TBLfiles folder. Open these data files in a spreadsheet and use them for further analysis, i.e., pairwise comparison or repeated measures ANOVA.
NOTE: The analysis will also produce other folders, including PS files that will have a single-page description of the mice during testing, showing a trace map and where the shocks were received.
Representative Results
Mice with intact spatial learning ability will show a decrease in the number of shocks during successive acquisition trials (Figure 4A). Similarly, the maximum time avoiding the shock zone will increase as the mouse learns to successfully navigate away from the shock zone (Figure 4B). However, mice that are unable to learn an effective avoidance strategy will show a constant number of shocks for each acquisition trial (Figure 4A). Often, mice that fail to identify the shock zone will receive multiple shocks during each entrance into the zone. Trace maps are useful to provide examples of mice that learn to avoid the shock zone (Figure 4C) and those that are unable to avoid the shock zone (Figure 4D). In both instances, these trace maps represent the final day of acquisition. The mouse in Figure 4C received only 2 shocks, as represented by the two circles. Also, note that the trace map shows the mouse spending most of the time on the opposite side of the shock zone which is represented by the red wedge. Conversely, the mouse in Figure 4D received more shocks, and the trace map reveals a disordered pattern. Examples of mice that are unable to successfully learn to avoid the shock zone are those that have reduced hippocampal neurogenesis either due to older age, as shown by the 18-month-old mice (Figure 4A,B– modified from Blackmore et al., 20217), chemical ablation of immature neurons6 or hippocampal lesions (see Codd et al., 2020)8.
It is important to distinguish between an unsuccessful trial due to the mouse failing to learn as opposed to a failure in the set-up of the equipment. The two most common causes for poor results due to equipment failure are poor tracking of the mouse (Figure 5A) or the mouse not receiving a shock. Poor tracking can prevent the mouse from receiving a shock when it is in the shock zone. Alternatively, poor tracking can inaccurately induce a shock when the mouse is not in the zone. In both instances, this will prevent the mouse from developing an effective avoidance strategy. Poor tracking can be solved by adjusting the threshold in the "From Calibrator" tab. Poor tracking is typically defined as more than 1000 bad frames during a 10-min period and occurs very rarely. Poor tracking can become an issue with aged mice, where alopecia can develop. When receiving a shock, the mouse will react either by tensing or, on occasion, vocalizing. The mouse will typically move, if even slightly, and can be seen on the live tracking software. When the mouse stays perfectly still within the shock zone, a clear line of shocks will be shown (Figure 5B). This may be due to the shock box not being turned on or scat stuck between the bars, reducing the amplitude of the shock being delivered to the animal.
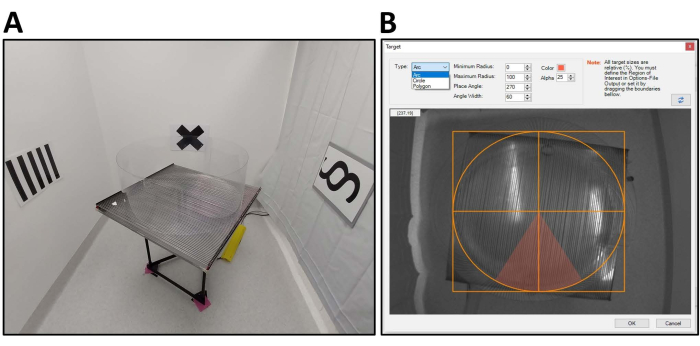
Figure 1: APA apparatus, behavior room, and shock zone setting. (A) An example of the testing arena and room setup. APA apparatus is elevated and placed in the center of the room, surrounded by novel visual cues. Black and white visual cues are used at the same height as the platform. (B). The Target function within the Experiment tab allows the masking of the entire arena and creates a location of the shock zone. A shock zone, represented by the red wedge, has been created at 270° in this example. Please click here to view a larger version of this figure.

Figure 2: APA setup for albino mouse strains. The APA arena can be set up for albino strains of mice, such as BALB/c, by selecting the Light option in the Tracking tab and creating a black arena background. An albino mouse on a black background achieves high contrast and offers better mouse tracking. Please click here to view a larger version of this figure.
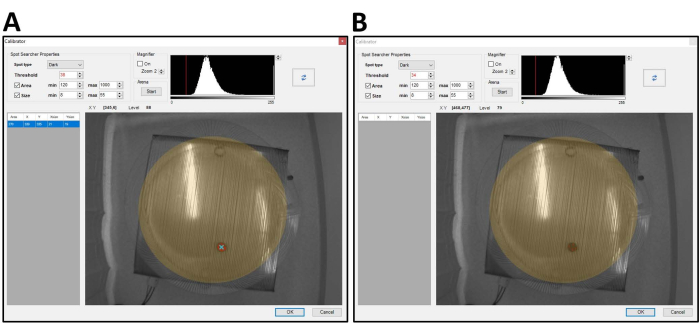
Figure 3: Adjusting the threshold for mouse tracking is essential. The threshold must be appropriately adjusted to ensure good animal tracking during the trial. The threshold is adjusted by moving the red line in the threshold pane in the From Calibrator tab. (A) An example of a good threshold selection with a solid orange region and a blue X on the object. (B) A poor threshold with speckled orange. Poor tracking leads to the loss of an animal in the arena or prevents the mouse from receiving a shock when it is in the shock zone. Please click here to view a larger version of this figure.
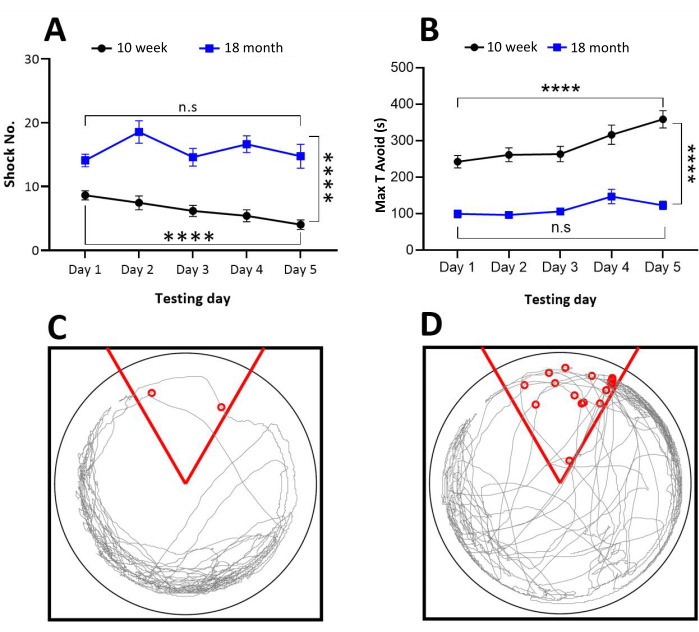
Figure 4: Comparison of performance between young (10 weeks) and older (18 months) mice on a 5-day learning paradigm and Trace maps. (A) The 10-week-old mice received significantly fewer shocks compared to the 18-month-old mice during 5 days of testing; note that the difference in the number of shocks received was minimal on the first day of testing between the groups, but young mice with intact memory learned to avoid the entry into shock zone more quickly than the older group. (B) Maximum time avoidance was computed as the maximum time spent avoiding the shock during the 10 min trial. The younger mice rapidly learned to avoid entry into the shock zone compared with older mice, suggesting the young mice are learning effectively. (C) The mouse in this trace map received only two shocks, as represented by the two circles in this acquisition trial. This mouse also spent more time in the arena opposite the shock zone, which is represented by the red wedge. (D) This mouse received more shocks and spent more time close to the shock zone, suggesting that spatial learning was not achieved in this mouse. Two-way, repeat measure ANOVA with Bonferroni post hoc tests were used to test significance. ****p<0.0001. Panels A and B were modified from Blackmore et al.7. Please click here to view a larger version of this figure.
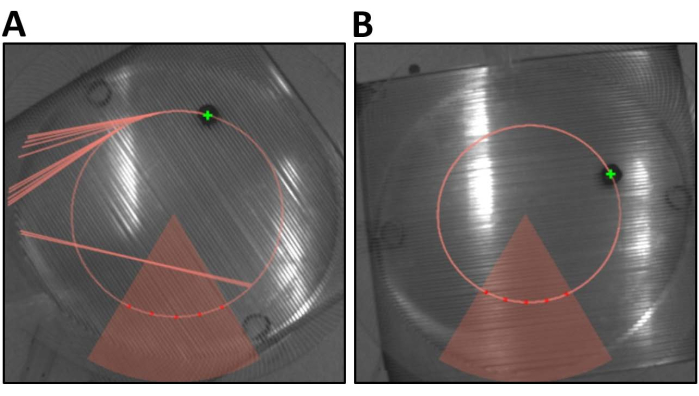
Figure 5: Trace maps provide important information for each mouse during each trial. (A) Note the straight lines that are present in this example of tracking. This is due to the tracking software incorrectly identifying a mouse during the task. (B) An example of good tracking during the trial. Please click here to view a larger version of this figure.
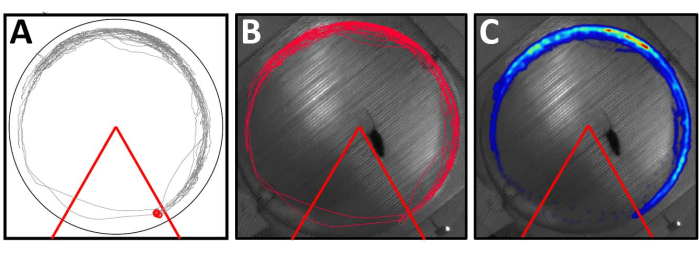
Figure 6: Track visualization and heatmap on different animal tracking programs. Both (A) Program 1 and (B) Program 2 detect the animal's location and movement to create track plots to visually inspect if the animal learns the task or the effect of experimental treatment. Both programs show identical track plots from an animal who learned the task efficiently. (C) A heatmap can also be created, which facilitates the identification of hotspots and clustering of the data points. Please click here to view a larger version of this figure.
Discussion
In conclusion, the active place avoidance test is an effective spatial learning task that can be used on a variety of mouse strains and experimental conditions. The APA task overcomes limitations associated with other spatial learning paradigms14, such as the MWM, which is stressful for the mice as measured by cortisol levels9. The MWM is also unsuitable for aged mice, where they have been reported to float during the task10. Although other spatial learning tests, such as the Barnes maze and novel object location test, are less stressful, they are limited by how often repeat testing can be conducted on the same cohort of mice. Therefore, the main advantage of the APA task is that it can be used multiple times as several parameters can be adjusted to maintain novelty. Indeed, we have used the APA task up to 5 times on the same cohort of mice to examine the effect of hippocampal ablation and the subsequent effect of exercise8. In each instance, the parameters, including the arena rotation, shock zone, and spatial cues, were altered between tests. This was effective in ensuring that the mice used spatial navigation cues to re-learn the task as evidenced by the control animals starting with a high number of shocks and then decreasing during subsequent test days for each testing period8. Typically, at the end of a 5-day test paradigm, we consider that any animal that received more than 10 shocks on the final day or has a maximum avoidance of less than 60 s has not learned the paradigm.
Beyond the ability to easily modify settings to allow multiple rounds of spatial testing, the APA task ensures that mice must use spatial navigation to effectively avoid the shock zone. For example, animals must use external cues to locate and avoid entering the stationary shock zone by navigating away from it5. As the arena is rotating, animals are not able to use an idiothetic approach for navigation, nor can they use exteroceptive cues such as odor because these cues rotate with the arena while the shock zone and spatial cues remain stationary5.
It is also important to ensure that mice are appropriately habituated to the researcher and APA arena. The intensity of foot shock also needs to be optimized, as both too low and too high shock intensity can compromise mice's ability to learn and perform the task5. The shock intensity is typically set to 0.5 mA and should not exceed 0.7 mA. For animals that have increased anxiety-like behavior, consider reducing both light intensity and foot shock intensity. Increased anxiety during the APA task can present as either excessive jumping, uncontrolled running within the arena, or prolonged freezing. The protocol described here used a shock intensity of 0.5 mA, the same intensity that has previously been used with BALB/c, which is known to have higher anxiety-like behavior15.
Here, we describe the animal tracking software supplied by the company that provided the active place avoidance rig used. Alternative video tracking software is also suitable for analyzing behavioral performance. These programs can also accurately measure and analyze mouse performance during APA tasks. These programs allow the creation of several zones and locations within the APA arena to assess behavior. The arena setting for an APA consists of one triangular shock zone, where the number of entrances, time to first enter, and time spent in the shock zone are measured. Additional zones can also be added within the arena. For example, we can add a central zone or a zone opposite the shock zone to measure the time spent, and distance traveled in those zones as an animal strategy to avoid the aversive zone. These programs track the mouse center of mass, which is then saved and displayed above the reference frame for visual inspection (Figure 6A,B). Finally, it is also possible to create a density heatmap for individual and group performance (Figure 6C).
When conducting the APA task, there are potential issues that need to be addressed. On occasion, mice will need to be excluded from the analysis due to non-responsiveness to the shock zone. As always, exclusion should only be considered when they meet pre-defined outlier conditions, for example, falling outside 2 standard deviations from the mean. Complex behavioral tasks such as the APA typically require high N values of animals. We suggest conducting a power analysis to calculate the appropriate sample size before conducting APA. This will depend on the strain used and treatment groups. From experience, we find that an n value of 10 or more for each group provides sufficient power when conducting APA experiments. The main issue with this task is ensuring high-quality tracking of the mouse during the task. The habituation stage of the task should be used to confirm that this is occurring. Mice not responding to a shock is often due to scat between the grid bars. It is, therefore, essential to clean the rig after every animal and remove any scat or urine. This will also reduce the stress for the animals that follow. The APA task typically involves a 5-day paradigm, which may present some limitations for studies involving interventions effective less than 5 days; however, short-term memory or spatial learning acquisition can still be assessed for such studies using the 30 min, single-session approach.
In summary, this article provides a detailed description of how to set up and use the active place avoidance paradigm to test the spatial learning of mice. The ability to change conditions so that multiple mouse strains of varying color can be tested is a distinct advantage over other, more traditional spatial tests such as the MWM. Further, the modification of multiple parameters allows for repeat testing so that the changes in spatial learning can be accurately compared during various experimental paradigms or during physiological aging. In a short period of time, the APA test has been demonstrated to be an accurate and effective alternative for hippocampus-dependent spatial learning. In the future, the APA task can be used as a reliable method for assessing therapeutic or exercise interventions on cognitive and spatial behavior in both wild-type and transgenic mice.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
We thank the Queensland Brain Institute (QBI) Animal Behaviour Facility for the development and maintenance of the apparatus described in this manuscript.
Materials
| Constant Current Source CS02 | BioSignal Group | N/A | Acton, Massachusetts, United States |
| Control Box | BioSignal Group | N/A | Acton, Massachusetts, United States |
| Ethovision | Noldus | version 16 | Wageningen, Netherlands |
| Shock Scrambler | BioSignal Group | N/A | Acton, Massachusetts, United States |
| Track Analysis | BioSignal Group | version 2.2 | Acton, Massachusetts, United States |
| Tracker Programme | BioSignal Group | version: 2.36 | Acton, Massachusetts, United States |
Referenzen
- Cimadevilla, J. M., Fenton, A. A., Bures, J. Functional inactivation of dorsal hippocampus impairs active place avoidance in rats. Neurosci Lett. 285 (1), 53-56 (2000).
- Willis, E. F., Bartlett, P. F., Vukovic, J. Protocol for short- and longer-term spatial learning and memory in mice. Front Behav Neurosci. 11, 197 (2017).
- Blackmore, D. G., Brici, D., Walker, T. L. Protocol for three alternative paradigms to test spatial learning and memory in mice. STAR Protoc. 3 (3), 101500 (2022).
- Pastalkova, E., et al. Storage of spatial information by the maintenance mechanism of LTP. Science. 313 (5790), 1141-1144 (2006).
- Stuchlik, A., et al. Place avoidance tasks as tools in the behavioral neuroscience of learning and memory. Physiol Res. 62 (Suppl 1), S1-S19 (2013).
- Vukovic, J., et al. Immature doublecortin-positive hippocampal neurons are important for learning but not for remembering. J Neurosci. 33 (15), 6603-6613 (2013).
- Blackmore, D. G., et al. An exercise "sweet spot" reverses cognitive deficits of aging by growth-hormone-induced neurogenesis. iScience. 24 (11), 103275 (2021).
- Codd, L. N., Blackmore, D. G., Vukovic, J., Bartlett, P. F. Exercise reverses learning deficits induced by hippocampal injury by promoting neurogenesis. Sci Rep. 10 (1), 19269 (2020).
- Harrison, F. E., Hosseini, A. H., McDonald, M. P. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res. 198 (1), 247-251 (2009).
- van Praag, H., Shubert, T., Zhao, C., Gage, F. H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 25 (38), 8680-8685 (2005).
- Zhou, X. A., et al. Neurogenic-dependent changes in hippocampal circuitry underlie the procognitive effect of exercise in aging mice. iScience. 24 (12), 103450 (2021).
- Leinenga, G., Gotz, J. Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer’s disease mouse model. Sci Transl Med. 7 (278), 278ra33 (2015).
- Willis, E. F., et al. Repopulating microglia promote brain repair in an IL-6-dependent manner. Cell. 180 (5), 833-846 (2020).
- Lesburgueres, E., Sparks, F. T., O’Reilly, K. C., Fenton, A. A. Active place avoidance is no more stressful than unreinforced exploration of a familiar environment. Hippocampus. 26 (12), 1481-1485 (2016).
- Crawley, J. N. Behavioral phenotyping strategies for mutant mice. Neuron. 57 (6), 809-818 (2008).

