Tracking Sugar-Elicited Local Searching Behavior in Drosophila
Summary
This protocol describes a behavioral assay for recording sugar-elicited search behavior using Drosophila melanogaster. The assay can be utilized to study feeding and foraging-related behaviors, as well as the underlying neuronal mechanisms.
Abstract
Foraging behavior is essential for the survival of organisms as it enables them to locate and acquire essential food resources. In Drosophila, hunger triggers a distinct search behavior following the consumption of small quantities of a sugar solution. This report presents a simple experimental setup to study sugar-elicited search behavior with the aim of uncovering the underlying mechanisms. Minute quantities of concentrated sugar solution elicit sustained searching behavior in flies. The involvement of path integration in this behavior has been established, as flies utilize their trajectory to return to the sugar location. The most recent findings provide evidence of temporal modulation in the initiation and intensity of the search behavior after sugar intake. We have also used this setup for artificial activation of specific taste-receptor neurons in the pharynx, which elicits the search behavior. The Drosophila neurogenetic toolkit offers a diverse array of tools and techniques that can be combined with the sugar-elicited search behavior paradigm to study the neural and genetic mechanisms underlying foraging. Understanding the neural basis of hunger-driven searching behavior in flies contributes to the field of neurobiology as a whole, offering insights into the regulatory mechanisms that govern feeding behaviors not only in other organisms but also in humans.
Introduction
Food-seeking and foraging behavior is a fundamental survival strategy exhibited by organisms across taxa. Two types of foraging behaviors have been identified in insects: hunger-induced search for food and post-meal local search1. When hungry, insects rely on sensory cues to locate food sources. Upon encountering and consuming a small food patch, they initiate a local search behavior characterized by convoluted paths and circling around the food location.
Sugar-elicited search behavior, a particular form of local search, was first studied over 60 years ago, by American biologist Vincent Dethier in blowflies2. When starved, flies are presented with a small amount of sugar such that it does not satiate them, they begin a local search. Typical search behavior is characterized by a highly tortuous walk with low locomotory and high turning rate and making returns to the location of the sugar drop. Subsequent studies had investigated this behavior in house flies and fruit flies3,4. The initiation, intensity, and duration of the search is regulated by the internal state of the animal (e.g., deprivation and motivation) as well as external factors such as resource availability and quality1,5,6.
Advancements in tracking technologies have provided researchers with valuable tools to capture and analyze behavior within controlled arenas. Here, we present a behavioral paradigm for tracking freely walking flies after sugar ingestion. This simple setup enables the study of sugar-elicited search behavior in Drosophila by capturing and analyzing the fly's motion in response to concentrated sugar solution provided in an arena. Employing advanced tracking technology and data analysis techniques, the locomotor patterns, spatial exploration, and response dynamics to sugar stimuli have been successfully quantified.
Using this assay, it has been experimentally demonstrated that the sugar-elicited search involves the use of path integration and can be spatially-temporally separated from sugar intake7,8. Furthermore, it has been shown that the behavior can be triggered by the activation of pharyngeal taste neurons9. Recent results show that sugar stimulus is not an innate releasing mechanism but also modulatory, and controls the initiation of the behavior temporally8. Using this paradigm, we have also studied this behavior in honey bees (Apis mellifera)7,8.
The eventual aim of this research is to unravel neural circuits and novel genetic components involved in regulating search behavior through targeted genetic manipulations and neuroimaging techniques. Food search behaviors have proven to be highly effective experimental paradigms for studying navigation and spatial memory in insects. These behaviors provide a unique opportunity to investigate the sensory perception, decision-making processes, and motor coordination involved in the search for rewarding food sources in flies. Additionally, the findings from these studies have broader implications for understanding feeding behaviors in other organisms, including humans, as many fundamental genetic and neural mechanisms are evolutionarily conserved. Dysregulation of feeding behaviors is associated with various neurological and metabolic disorders10. Therefore, the neural and genetic mechanisms underlying search behavior in flies may offer new avenues for understanding and potentially addressing these complex human health challenges.
Protocol
Adult male flies of the Drosophila melanogaster Canton-S (CS) wild-type strain were used for the present study.
1. Experimental preparation
- Fly rearing
- Collect adult male flies emerging within a 12 h period and maintain them on standard fly media (prepared in house) for 48 h at 25 °C with 75% relative humidity in a 12 h light/dark cycle.
NOTE: The Drosophila media composition used was (for 1 L of media) corn flour (80 g), D-glucose (20 g), sugar (40 g), agar (8 g), yeast powder (15 g), propionic acid (4 mL), methyl 4-hydroxybenzoate (1.25 g in 3 mL of ethanol), and orthophosphoric acid (600 µL) (see Table of Materials). The initial feeding period ensures that the flies have access to sufficient food and nutrients before the starvation phase. While both male and female flies elicit the search behavior, but starvation time is more consistent among male flies. Additionally, female flies change their feeding preference after mating11.
- Collect adult male flies emerging within a 12 h period and maintain them on standard fly media (prepared in house) for 48 h at 25 °C with 75% relative humidity in a 12 h light/dark cycle.
- Starvation procedure
- After the feeding period, starve the flies of food but with access to water.
NOTE: To standardize the hunger state across trials and strains, it is suggested to determine the duration of 90% survival of the population under food-starved conditions. Based on this result, CS flies were starved for 28 h (Figure 1A) as circadian rhythms and other factors can impact the search behavior12. Circadian changes of fly searching activities at different times of a day were also measured (Figure 1B). We noticed reduced relative search activity at night compared to light phase in flies. - Calculate the food-starvation tolerance by depriving two- day old flies of food. Place 15-20 flies in a vial with soaked tissue paper at the bottom. This serves as a substrate and ensure that the flies have access to water throughout the starvation period.
- Count the number of flies during the food-starvation period at regular intervals of 1 h. The duration at which 90% of the starved flies survived was used as the starvation period (Figure 1A). Use multiple replicates (3-4 for each strain) to minimize the impact of individual variations and provide a more reliable assessment of starvation tolerance.
NOTE: The food-starvation tolerance of each strain was determined in order to establish a standardized hunger state among different strains and experiments. By counting the surviving flies at these intervals, one can monitor the survival rate over time and determine how long each strain could tolerate food deprivation before succumbing to starvation.
- After the feeding period, starve the flies of food but with access to water.
- Procedure for recording the behavior
- Transfer individual starved flies in small tubes (Figure 2A). Do this in batches of 5-6 flies to minimize isolation time. By testing single flies, isolating each fly, any observed changes or actions can be attributed to the specific fly being observed, rather than being influenced by interactions with other flies.
- Use 90 mm Petri dishes as an arena for behavioral assays (bigger petri dishes can be used. There was no difference in behavior in Petri dishes with larger diameter). Illuminate the arena from the bottom by a panel of surface-mounted cool white LEDs (Figure 2B,C). To maintain a uniform visual environment and minimize external distraction, surround the experimental arena by a white polyvinyl chloride pipe (51.5 mm height, 114 mm inner diameter).
NOTE: This pipe acts as a barrier, preventing any visual stimuli from outside the arena from influencing the fly's behavior. By reducing external distractions, one can focus solely on the fly's interaction with the food source, maintaining consistency throughout the experiment. - Use light intensity of 320 lux at the center of the arena.
- Position 0.2 µL sugar solution in the center of the arena. 500 mM sucrose solution was used in the reported experiments but this can be varied. Introduce the fly to the arena using a 2 mL micro centrifuge tube (inner diameter 8.7 mm, length reduced to 5 mm by stuffing cotton at the bottom; Figure 2A) housing a single fly inverted over the sugar drop.
- Once the fly starts ingesting the droplet, remove the fly container, giving the fly unrestricted access to the food source.
- Film the 2D position of the arena with an overhead camera.
NOTE: Flea3 (Point Grey, 1214 mm lens, see Table of Materials) was used for the present study and recorded at 40 frames per second (fps). However, any camera that provides good contrast with the background can be used. One can record at 30-60 fps, depending on the nature of the experiment. Record in .avi format since it is compatible with the tracking software. - Record the trial until the time the fly escaped from the arena. The flies were freely walking and there was no lid on the arena.
- By allowing the fly to decide when to stop searching, fly away, or walk to the periphery of the arena, observe the fly's natural behavior and food search strategy.Wipe the Petri dishes with 70% ethanol between the trials and dry them completely or else use a new Petri dish.
NOTE: It is important to conduct all experiments between 2 h and 6 h after lights are on when the flies exhibit consistently high activity levels. This time frame ensures that the flies are in an active state, maximizing the chances of observing their natural food searching behavior and reducing the impact of other factors that may affect their behavior, such as circadian rhythms. The behavioral set-up was housed inside a temperature and humidity- controlled room. The arena was placed on a vibration- free table. This experimental setup involved isolating and testing individual flies for their response to sugar. A sugar drop was provided to the starved flies and their behavior was video recorded (Figure 2B,C, Video 1). The experiments were conducted during a specific time frame when the flies exhibited consistent high activity levels.
- Analyze the trajectories following step 2 to determine the search behavior.
2. Analysis of trajectories for local search
- Analyze the recorded videos using Ctrax software11 (see Table of Materials).
NOTE: The software tracks and converts the fly's position in the video into x, y coordinates, enabling precise tracking and analysis of its movements. Refer to Supplementary File 1 for details on how to use Ctrax. - Divide the trajectories into two phases: the initial feeding phase and the search phase. Define the end of feeding and start of walking as flies moving at a speed >4 mm s-1 in three consecutive frames.
NOTE: After the feeding was over, the rest of the trajectory was used as the search response of the flies. This study used VirtualDub (see Table of Materials) to remove the feeding phase from the videos before tracking. - Quantify the search by the following parameters:
- Path length: This parameter represents the distance walked by the fly from its starting point during the food search (in mm).
- Stay time: The time spent by the flies walking during the search (in s). It indicates the duration of the search and fly's persistence in searching for food.
- Meander: Calculate this as a ratio by dividing the beeline (the distance between the first and the last point of the path) of the path to the total path length, and subtracting from 1. High values of meander indicate more tortuosity in the trajectory.
- Number of returns: Use the developed algorithm to identify and count the number of returns using two concentric circles.
NOTE: An inner circle indicating the origin of search, Rin (2.5 mm) and the outer circle indicating the minimum distance Rout (4 mm) that the fly had to move away from the origin. A return was defined as a movement out of the outer circle (Rout) and then coming back into the inner circle (Rin). - Activity rate: Expressed as a percentage, calculate the activity rate by determining the time during food searching when the fly's walking speed exceeds 2 mm/s. This parameter reflects the fly's level of activity and engagement in the food search. It distinguishes active foragers from less active individuals.
NOTE: MATLAB and Python were used in the present study for further analysis of the trajectories. The scripts can be accessed here: https://github.com/eagermeagre/sugar_elicited_search. The analysis procedure involves using the Ctrax software to track the fly's movements and determine parameters such as the duration of food search, total path length, search time, meander, number of returns and activity rate. These parameters provide valuable insights into the fly's behavior and foraging efficiency during the recorded food searching experiments.
Representative Results
The flies needed to be starved for the period estimated by the food-starvation tolerance and the response to sugar was individually tested (Figure 1A and Figure 2A). The behavior was recorded inside a temperature and humidity-controlled room. 0.2 µL of 500 mM sucrose solution was used for the reported experiments. The sugar drop was positioned in the center of the arena and the flies were introduced to the sugar (Figure 2B). The behavior was recorded until flies escaped from the arena. The videos were analyzed to extract x,y coordinates and trajectories of the flies. Several parameters were used to quantify the behavioral response: path length, stay time, meander, number of returns and activity rate.
Sugar intake in starved flies leads to a local search with a meandering path and loops (Figure 3A,C). As a negative control, starved flies were recorded that were not presented with sugar8. These flies when introduced to and empty arena, did not initiate a search and escaped the arena (Figure 3B,D). This group is referred as unfed flies. The parameters of search: path length, stay time, meander and number of returns were significantly lower in unfed flies compared to the flies that were given a sugar drop (Figure 4A–D).
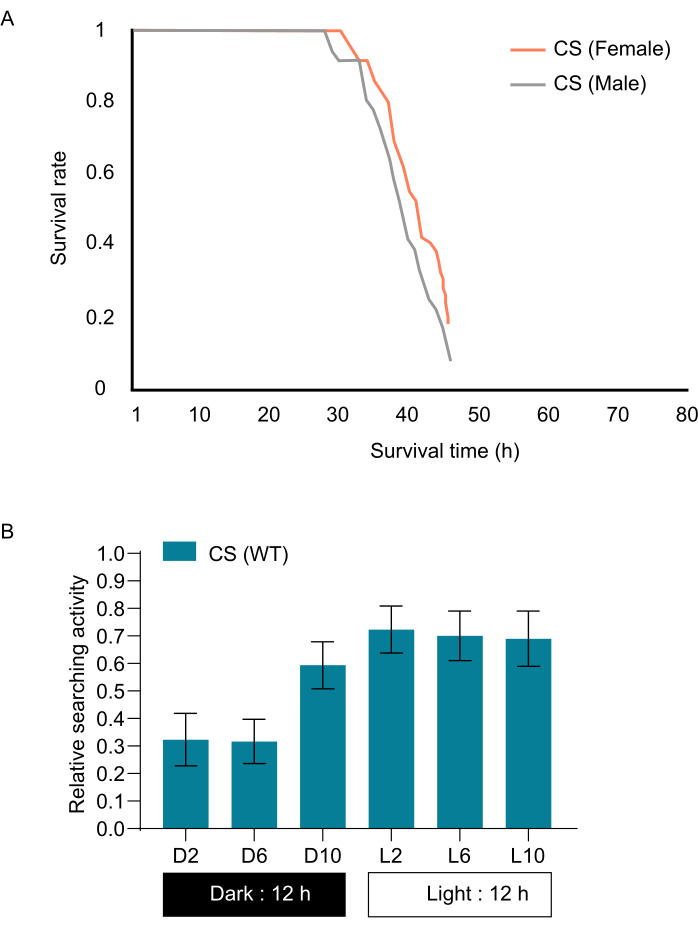
Figure 1: Starvation curves of wildtype strain CS. (A) Starvation curve showing survival rate for wild-type CS male and female flies. (B) Circadian changes of fly searching activities at different times of a day. Error bars represent S.E.M. (n = 10). Please click here to view a larger version of this figure.
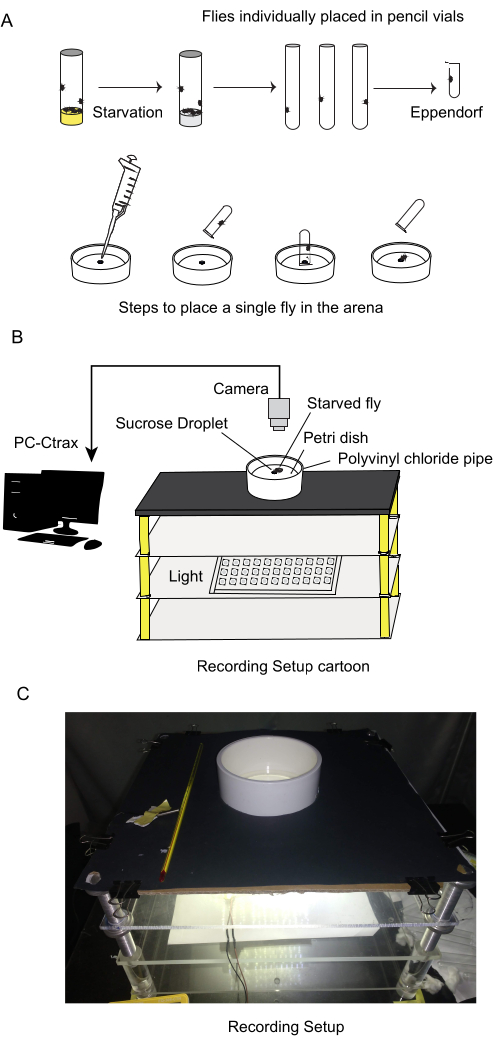
Figure 2: Experimental setup including starvation tolerance calculation, behavior recording procedures, and trajectory analysis. (A) Food-starvation tolerance was determined to establish a standardized hunger state. For behavior recording, individual flies were isolated in small tubes before the experiment. The behavioral arena, a 90 mm Petri dish, was uniformly illuminated from the bottom. A drop of sugar solution was placed in the center and flies were given unrestricted access to the food source. (B) The behavior was recorded until flies escaped from the arena. Video analysis used Ctrax software for tracking and MATLAB/Python for trajectory analysis. (C) Photograph of the experimental setup. Please click here to view a larger version of this figure.
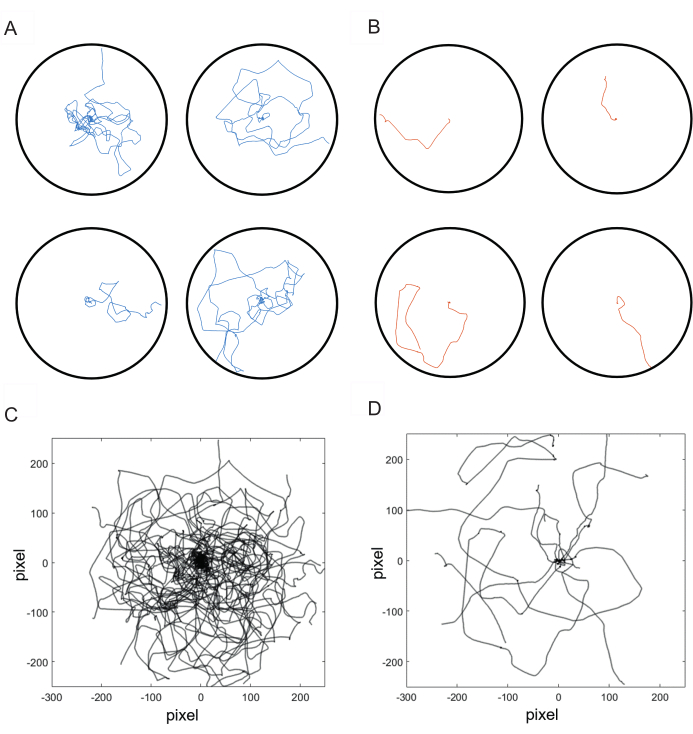
Figure 3: Necessity of the sugar intake for initiating local search. (A) Individual trajectories of flies fed 500 mM, 0.2 µL sugar solution. (B) Individual trajectories of flies that were given no sugar. (C) Overlay of search trajectories flies from the control group (n = 11). (D) Overlay of the paths of the flies (n = 11) which were given no sugar. All the trajectories are normalized to the starting point of walking. This figure is adapted from Shakeel and Brockmann8. Please click here to view a larger version of this figure.
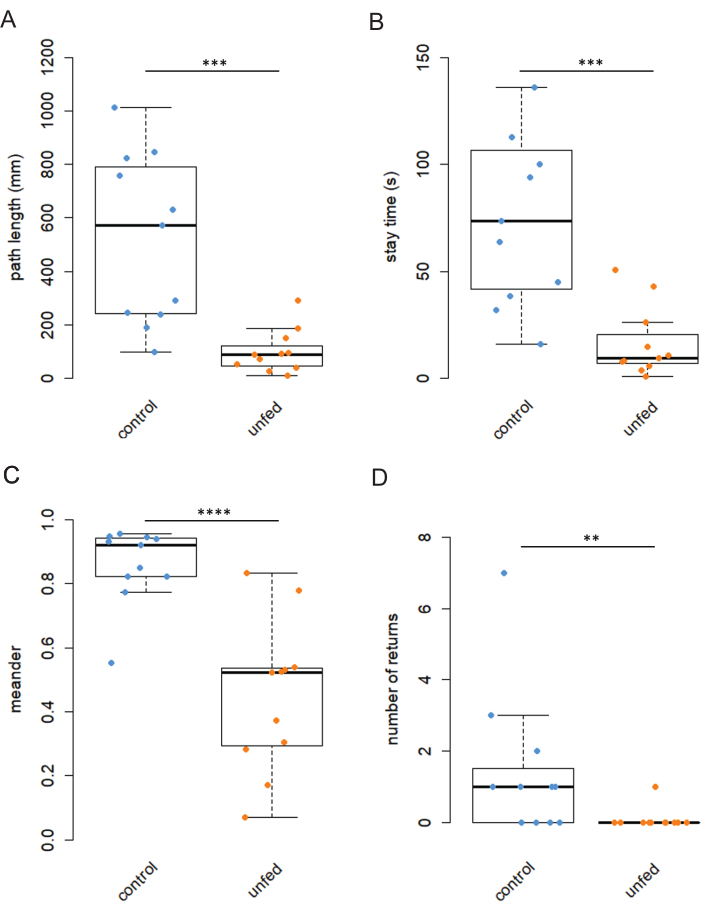
Figure 4: Reduction of behavioral parameters in unfed flies. (A–D) Path length, stay time, meander and number of returns were smaller for hungry flies that were not given sugar reward compared to control flies that were given stimulated with sugar. **p < 0.001, ***p < 0.0001, ****p < 0.00001, Wilcoxon Rank Sum Test. This figure is adapted from Shakeel and Brockmann8. Please click here to view a larger version of this figure.
Video 1: Search behavior of a fly with real-time trajectory of the path. Please click here to download this Video.
Supplementary File 1: Step-wise instructions for tracking the video file using CTRAX. Please click here to download this File.
Discussion
The current study introduces a straightforward paradigm for investigating sugar-elicited search behavior in Drosophila, first described by Dethier2. This innate behavior allows the flies to engage in a local search for additional food resources following the encounter of a food reward. The most crucial aspect of the experimental protocol involves appropriately motivating the flies. Firstly, the flies must be in a state of hunger, having been deprived of food while still having access to water, to ensure sugar ingestion. In order to have the uniform hunger state across experimental trials the duration at which 90% of the population survives was used as the starvation period. Crucially, the induction of a search response post-feeding necessitates the provision of a food stimulus that is of sufficient quality but not enough to fully satiate the flies. Therefore, standardizing the concentration and amount of sugar, and starvation duration might be time-consuming but is imperative for robust and reliable behavior.
In this study, a 500 mM, 0.2 µL sucrose solution was employed as the stimulus for starved flies. Sugar intake evokes a characteristic local search behavior characterized by increased turning behavior and frequent returns to the location of the sugar drop (Video 1). Conversely, hungry flies that are not provided with any sugar fail to exhibit a search response. Notably, all the behavior-related parameters including path length, stay time, meander, and number of returns were significantly lower in unfed flies. We have previously demonstrated that the ingestion of water alone does not elicit a search response9.
This setup offers a cost-effective and low-maintenance approach for studying this innate behavior. While a backlit arena is used in this study, top lighting can also be employed as long as there is sufficient contrast between the fly and the background. The tracking software used relies on the detection of fly movement against a static background13. The camera and resolution settings can be adjusted based on the specific scale of behavior under investigation. Importantly, this methodology enables the study of various components of foraging behavior, including sensory cue attention during foraging, food- engagement and feeding, locomotor control of search, and decision-making processes associated with exploitation and exploration, among others. Moreover, this paradigm facilitates the investigation of local search, a behavior that is commonly observed across diverse taxa in various ecological contexts6. Studying this behavior in Drosophila, opens avenues for scientific inquiry aimed at understanding the neural pathways involved in foraging. We have studied local search in honey bees and have shown that the behavior bears resemblance to flies7,8.
Recent studies have demonstrated that local search behavior can be triggered by optogenetic activation of various sugar sensory neurons in flies14,15,16. However, it remains unclear to what extent the local searches observed in these studies accurately represent the natural behavior of flies in response to actual sugar intake. Feeding behavior is tightly regulated in flies, and these findings indicate that the activation of pharyngeal sugar receptors initiates the search behavior. Tarsal taste sensilla are responsible for detecting sugar and inducing the proboscis extension reflex, while pharyngeal taste neurons determine whether feeding should proceed17,18. Once ingested, the sugar solution travels through the esophagus to the proventriculus and enters the crop, with its expansion monitored by a recurrent nerve19. Additionally, it is worth noting that some of the aforementioned studies involved the harnessing or confinement of flies, whereas this method allows the animals to freely walk throughout the experiment. The flies in our experiments were sufficiently motivated to stay and search within the arena without the imposition of a lid.
Understanding the intricate interplay between neural pathways, genetic factors, and environmental cues that govern search behavior in flies can shed light on the fundamental principles of information processing, learning, and memory formation. Furthermore, dysregulation of foraging behavior has been implicated in various human disorders, including eating disorders and obesity. The extensive array of neurogenetic tools available in Drosophila provides a valuable resource for investigating the sugar-elicited search behavior and unravelling the neural and genetic mechanisms underlying foraging. In combination with optogenetic manipulation and functional imaging, this paradigm presents a powerful and promising approach20,21,22. However, modifying the setup for real-time manipulation of neuronal activity with optogenetics might be challenging. To monitor the neuronal activity in the brain while a fly is performing the searching behavior a different set-up will be needed such as the tethered fly on a tread ball. Many aspects of foraging behavior, such as feeding regulation and decision-making processes, are highly conserved across species. Therefore, insights gained from studying the neural mechanisms of foraging in flies can provide valuable insights into similar processes in other organisms, including humans.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
We thank Ravikumar Boyapati for helping with setting up the arena. This work is funded by Wellcome trust DBT Intermediate India Alliance grant (Grant number IA/I/15/2/502074) to P.K. M.S. was funded by a fellowship by the Indian Council of Medical Research (ICMR). A.B. was funded by NCBS-TIFR institutional funds (No. 12P4167) and the Department of Atomic Energy, Government of India (No. 12-R& D-TFR-5.04-0800 and 12-R& D-TFR-5.04-0900).
Materials
| 2 mL Eppendorf tube | Sigma Aldrich | BR780546 | Used to introduce the fly to the sugar drop |
| Agar | SRL | 9002-18-0 | |
| Azure lens | https://www.rmaelectronics.com/azure-photonics-azure-1214mm/ | ||
| Camera | Logicool, Japan | ||
| Corn flour | locally available | ||
| Ctrax software | https://ctrax.sourceforge.net/ | ||
| D-glucose | SRL | 50-99-7 | |
| Flea3 | Sony | https://www.flir.com/products/flea3-usb3/?vertical=machine+vision&segment=iis | |
| glass tube | Borosil | Used to house the flies individually | |
| Kimwipe | Kimberly-Clark | 34155 | Used to provide access to water for flies during food starvation |
| LED light panel | custom-made in the workshop | ||
| Light Meter | TENMARS | TM-203 | |
| Methyl 4-hydroxybenzoate | Fisher Scientific | 99-76-3 | |
| Orthophosphotic acid | SRL | 7664-38-2 | |
| Petri dish (90 mm) | Tarsons | 460090 | |
| Propionic acid | SRL | 79-09-4 | |
| Sucrose | Qualigens | Q28105 | |
| Sugar | locally available | ||
| VirtualDub | https://www.virtualdub.org/ | ||
| White polyvinyl chloride pipe (67 mm inner diameter × 100 mm height) | custom-made in the workshop | ||
| Yeast powder | SRL | REF-34266 |
Referenzen
- Jander, R. Ecological aspects of spatial orientation. Annu Rev Ecol Evol Syst. 6 (1), 171-188 (1975).
- Dethier, V. G. Communication by insects: Physiology of dancing. Science. 125 (3243), 331-336 (1957).
- White, J., Tobin, T. R., Bell, W. J. Local search in the housefly Musca domestica after feeding on sucrose. J. Insect Physiol. 30 (6), 477-487 (1984).
- Bell, W. J., Cathy, T., Roggero, R. J., Kipp, L. R., Tobin, T. R. Sucrose stimulated searching behaviour of Drosophila melanogaster in a uniform habitat: modulation by period of deprivation. Animal Behav. 33, 436-448 (1985).
- Dethier, V. G. Microscopic Brains. Science. 143 (3611), 1138-1145 (1964).
- Bell, W. J. Searching behavior patterns in insects. Annu Rev Entomol. 35 (1), 447-467 (1990).
- Brockmann, A., et al. Sugar intake elicits intelligent searching behavior in flies and honey bees. Front Behav Neurosci. 12, 280 (2018).
- Shakeel, M., Brockmann, A. Temporal effects of sugar intake on fly local search and honey bee dance behaviour. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. , (2023).
- Murata, S., Brockmann, A., Tanimura, T. Pharyngeal stimulation with sugar triggers local searching behavior in Drosophila. J Exp Biol. 220 (Pt 8), 3231-3237 (2017).
- Nishijo, H., Ono, T. Neural mechanisms of feeding behavior and its disorders new insights into metabolic syndrome. IntechOpen. , (2021).
- Carvalho, G. B., Kapahi, P., Anderson, D. J., Benzer, S. Allocrine modulation of feeding behavior by the sex peptide of Drosophila. Curr Biol. 16 (7), 692-696 (2006).
- Xu, K., Zheng, X., Sehgal, A. Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila. Cell Metab. 8 (4), 289-300 (2008).
- Branson, K., Robie, A. A., Bender, J., Perona, P., Dickinson, M. H. High-throughput ethomics in large groups of Drosophila. Nat Methods. 6 (6), 451-457 (2009).
- Corfas, R. A., Sharma, T., Dickinson, M. H. Diverse food-sensing neurons trigger idiothetic local search in Drosophila. Curr Biol. 29 (10), 1660-1668.e4 (2019).
- Behbahani, A. H., Palmer, E. H., Corfas, R. A., Dickinson, M. H. Drosophila re-zero their path integrator at the center of a fictive food patch. Curr Biol. 31 (20), 4534-4546.e5 (2021).
- Titova, A. V., et al. Displacement experiments provide evidence for path integration in Drosophila. J Exp Biol. 226 (12), jeb245289 (2023).
- Stocker, R. F. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 275 (1), 3-26 (1994).
- LeDue, E. E., Chen, Y. C., Jung, A. Y., Dahanukar, A., Gordon, M. D. Pharyngeal sense organs drive robust sugar consumption in Drosophila. Nat Commun. 6, 6667 (2015).
- Gelperin, A. Abdominal sensory neurons providing negative feedback to the feeding behavior of the blowfly. Zeitschrift für vergleichende Physiologie. 72 (1), 17-31 (1971).
- Simpson, J. H., Looger, L. L. Functional imaging and optogenetics in Drosophila. Genetik. 208 (4), 1291-1309 (2018).
- DeAngelis, B. D., Zavatone-Veth, J. A., Gonzalez-Suarez, A. D., Clark, D. A. Spatiotemporally precise optogenetic activation of sensory neurons in freely walking Drosophila. Elife. 9, e54183 (2020).
- Grover, D., Katsuki, T., Li, J., Dawkins, T. J., Greenspan, R. J. Imaging brain activity during complex social behaviors in Drosophila with Flyception2. Nat Commun. 11 (1), 623 (2020).

