Green Synthesis, Characterization, Encapsulation, and Measurement of the Release Potential of Novel Alkali Lignin Micro-/Submicron Particles
Summary
We describe novel, simple methodologies of synthesis and characterization of biocompatible lignin micro- and submicron particles. These formulations provide a facile approach for the utilization of the heteropolymer, as well as an alternative for the rational design of multifunctional carrier matrices with potential applicability in biomedicine, pharmaceutical technology, and the food industry.
Abstract
The applicability of biopolymer micro-/nano- technology in human, veterinary medicine, pharmaceutical, and food technology is rapidly growing due to the great potential of biopolymer-based particles as effective carrier systems. The use of lignin as a basic heteropolymer biomatrix for the design of innovative micro-/submicron formulations allows the achievement of increased biocompatibility and offers various active functional groups presenting opportunities for customization of the physicochemical properties and bioactivities of the formulations for diverse applications. The aim of the present study was to develop a simple and ecofriendly methodology for the synthesis of lignin particles with micro- and submicron size; to evaluate their physicochemical, spectral, and structural characteristics; and to examine their capacity for encapsulation of biologically active molecules and potential for in vitro release of bioflavonoids in simulated gastrointestinal media. The presented methodologies apply cheap and green solvents; easy, straightforward, quick, and sensitive processes requiring little equipment, non-toxic substances, and simple methods for their characterization, the determination of encapsulation capacity towards the poorly water-soluble bioactive compounds morin and quercetin, and the in vitro release potential of the lignin matrices.
Introduction
Nowadays inclination towards biopolymers such as cellulose, chitosan, collagen, dextran, gelatin, and lignin as precursors for the design of micro-/submicron carriers with customizable size, physicochemical properties, and biofunctionalities has increased in the biomedical, pharmaceutical, and food technology industries due to their applicability in tissue engineering, 3D bioprinting, in vitro disease modeling platforms, packaging industry, emulsion preparation, and nutrient delivery among others1,2,3.
Novel studies highlight the aspects of lignin-based hydrogels as well as micro- and nano- formulations4 as advantageous vehicles used for food packaging materials5, energy storage6, cosmetics7, thermal/light stabilizers, reinforced materials, and drug-carrier matrices8 for the delivery of hydrophobic molecules, improvement of UV barriers9, as reinforcing agents in nanocomposites, and as an alternative to inorganic nanoparticles due to some recent safety issues10,11,12. The reason behind this tendency is the biocompatibility, biodegradability, and non-toxicity of the natural hetero biopolymer, as well as its proven bioactivities of lignin-antioxidant potential and radical scavenging, anti-proliferative, and antimicrobial activities13,14,15,16,17.
Scientific literature reports various methods for synthesis (self-assembly, anti-solvent precipitation, acid precipitation, and solvent shifting)18 and characterization of lignin-based micro-/nano- scaled formulations, including the application of expensive or harmful solvents such as tetrahydrofuran (THF), dimethyl sulfoxide (DMSO), N,N-dimethylformamide (DMF), and acetone, and complicated, indirect, and tedious processes that use a lot of equipment and toxic substances12,19,20.
To overcome the latter disadvantages, the following protocols present novel methodologies for the synthesis of lignin-based micro-/submicron particles using cheap and green solvents; easy, straightforward, quick, and sensitive processes requiring little equipment, non-toxic substances, and simple methods for their characterization and the determination of encapsulation capacity towards poorly water-soluble bioactive compounds and in vitro release potential of the lignin matrices. The presented lab-scale production methods are advantageous for the manufacture of functional lignin carriers with tunable sizes, high encapsulation capacity, and sustainable in vitro release behavior utilizing simple characterization procedures and eco-friendly chemicals that can find application in various areas of biomedical sciences and food technology. Two flavonoids were applied as target molecules encapsulated into the lignin particles: morin-into the microparticles, and quercetin-into the submicron particles. The difference in the structures of both flavonoids Is only the position of the second -OH group in the B-aromatic ring: the -OH group is on the 2' position in morin and on the 3' position in quercetin, thus both organic compounds are positional isomers. The latter fact presumes similar behavior of both bioactive natural compounds in the processes of encapsulation and/or release.
Protocol
1. Synthesis of lignin microparticles
- Prepare a 50 mg/mL alkali lignin aqueous solution by dissolving 2.5 g of alkali lignin in 50 mL of ultrapure water on a magnetic stirrer.
- Prepare 1% Tween 80 solution by dissolving 1 mL of Tween 80 in 100 mL of ultrapure water.
- Prepare a 2 M solution of HNO3 by diluting 6.65 mL of 67% HNO3 (density = 1.413 g/mL) with ultrapure water to a final volume of 50 mL.
- Slowly add 15 mL of the 1% Tween 80 solution to 50 mL of the 50 mg/mL alkali lignin solution.
- Agitate the mixture on a magnetic stirrer at 500 rpm for 10 min so that the surfactant becomes well dispersed.
- Add 20 mL of 2 M HNO3 dropwise with a syringe at a flow rate of approximately 150 µL/s to the mixture.
- Continue stirring the mixture for 30 min when the dark brown solution is transformed into a light brown suspension of microparticles.
- Transfer the suspension into 1.5-2 mL test tubes and centrifuge for 30 min at 15,000 × g in an ultracentrifuge at 10 °C.
- Collect the supernatant for further analyses and rinse the microparticles with ultrapure water.
- Repeat the rinsing/ultracentrifugation procedures 3x.
- Dip the container with the microparticles in an ice bath before the ultrasonic homogenization.
- Homogenize the microparticles for 4 min at an intensity of 93% on an ultrasound homogenizer.
- Lyophilize the microparticles at a temperature of -64 °C in a freeze dryer and store them in an exicator for further use.
2. Synthesis of lignin submicron particles
- Prepare a 5 mg/mL alkali lignin aqueous solution by dissolving 125 mg of alkali lignin in 25 mL of ultrapure water on a magnetic stirrer.
- Slowly add 1 mL of 96% EtOH to the alkali lignin solution.
- Agitate the mixture on a magnetic stirrer at 500 rpm for 3 min.
- Prepare 50 mL of a 1% solution of citric acid by dissolving 0.5 g of citric acid in ultrapure water to a final volume of 50 mL.
- Add 7 mL of 1% citric acid dropwise with a syringe at a flow rate of approximately 4 mL/min to the mixture.
- Continue stirring the mixture for 10 min when the brown clear solution will transform into a cloudy light brown suspension of submicron particles.
- Transfer the suspension into test tubes and centrifuge for 30 min at 15,000 × g in an ultracentrifuge at 10 °C.
- Collect the supernatant for further analyses and rinse the microparticles with ultrapure water.
- Repeat the rinsing/ultracentrifugation procedures 3x.
- Dip the container with the microparticles in an ice bath before the ultrasonic homogenization.
- Homogenize the microparticles ultrasonically for two cycles of 4 min each at an intensity of 96% in an ultrasound homogenizer.
- Cool the containers for 1 min after the first cycle.
- Lyophilize the microparticles at a temperature of -64 °C in a freeze dryer and store them in an exicator for further use.
3. Synthesis of natural flavonoid-encapsulated lignin micro-/submicron particles
- Repeat steps 1.1-1.5 for the microparticles.
- Weigh 0.08 g of morin, dissolve it in 1 mL of EtOH, and add this ethanolic solution to the mixture.
- Agitate the mixture on a magnetic stirrer at 500 rpm for 20 min.
- Add 20 mL of 2 N HNO3 dropwise with a syringe at a flow rate of approximately 150 µL/s to the mixture.
- Continue stirring the mixture for 60 min.
- Repeat steps 1.8-1.13.
- Repeat step 2.1 for the submicron particles.
- Weight 0.04 g of quercetin, dissolve it in 1 mL EtOH and add this ethanolic solution to the alkali lignin aqueous solution.
- Agitate the mixture on a magnetic stirrer at 500 rpm for 10 min.
- Repeat steps 2.4-2.13.
4. Determination of the encapsulation efficiency of lignin micro-/sumicro- particles
- Calculate the content of the added bioactive substance during the procedure for the synthesis of both types of flavonoid-encapsulated lignin particles.
- Determine spectrophotometrically the absorption of the flavonoid in the supernatant obtained during steps 1.9 and 2.8 after diluting it with 96% EtOH.
- Calculate the concentration of the non-entrapped morin/quercetin using the calibration curves of the flavonoids.
- Calculate the encapsulation efficiency (EE, %) of the lignin microparticles towards the natural flavonoids by using equation (1):
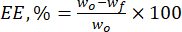 (1)
(1)
Where wo is the total quantity of the bioactive substance added (mg) and wf is the quantity of the free non-entrapped flavonoid (mg). - Calculate the drug loading capacity (DLC, %)-an important parameter representing the amount of drug in the particles per unit weight of the carrier system-by using eq. (2):
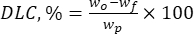 (2)
(2)
Where wp is the total quantity (yield) of lignin micro-/submicron particles obtained after lyophilization (mg).
5. Characterization of lignin micro- and submicron particles
- Determination of particle number, size, and size distribution
- Assess the particle size and particle size distribution of the samples using an automatic-cell counter with the option for bead count. Add with a micropipette 1 µL of the lignin/flavonoid micro-/submicron particles suspension in ultrapure water in the well of the counting slide required for the operation.
- Wait for the number of particles in 1 mL of the suspension, as well as their number and distribution by size to be shown in the display of the automatic cell counter.
NOTE: The apparatus allows the storage of the data on a USB flash. The automatic cell counter special software allows further processing of the saved digital and photo files.
- Determination of the content of surface acidic/basic groups of lignin particles by potentiometric titration
- Weight 0.04 g of unloaded/flavonoid-encapsulated lignin particles.
- Transfer them to an Erlenmeyer flask, add 10 mL of 0.1 M HCl, and place the flask on a magnetic stirrer at 250 rpm.
- Fill a 50 mL burette with a 0.1 M standard solution of the titrant NaOH.
- Measure the initial pH of the solution in the Erlenmeyer flask with a bench pH meter before starting the titration.
- Start the titration and measure the pH of the analyzed solution after each 0.5 mL added portion of the titrant.
- Store the experimental data in a table containing the volume of the titrant applied and the corresponding value of pH.
- Stop the titration when an approximately constant value of the pH is reached by increasing the volume of the titrant solution.
- Plot the experimental data in the form of zero-, first- and second-derivative differential titration curves.
- Determine the equivalent points and the corresponding equivalent volumes of the titrants used.
- Calculate the contents of the acidic Aa and Ab basic groups on the surface of unloaded and flavonoid-loaded lignin particles by using equations (3) and (4):
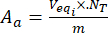 , mgeq/g (3)
, mgeq/g (3)
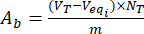 mgeq/g (4)
mgeq/g (4)
Where Veqi is the equivalent volume (mL); NT the normality of the titrant (mgeqv/mL); VT the volume of the titrant used for the determination procedure (mL); m the weight of the analyzed sample (g).
- Determination of the pH point of zero charge (pHPZC) of lignin-based particles by the solid addition method.
- Prepare 60 mL of 0.1 M aqueous solution of NaCl.
- Add 9 mL of the 0.1 M NaCl solution in each of five stoppered conical flasks and adjust the pH to pHi = 2, 4, 7, 10, and 12 (where i = 1-5 denote the number of the corresponding solution), respectively by the addition of either 0.1 M HCl or 0.1 M NaOH. Adjust the total volume of the solution in each flask to 10 mL exactly by adding NaCl solution of the same strength.
- Add 40 mg of dry lignin particles (unloaded, flavonoid-loaded micro-/submicron) to each flask and cap the flasks securely.
- Secure the flasks upright on an orbital shaker and keep them shaking for 24 h.
- Allow equilibration for 30 min and subsequently measure the final pH (pHf) of the supernatants in each flask.
- Plot pHf values against the corresponding initial pH values (pHi).
- The point of zero charge (pHPZC) is defined as the pH value at which the curve ΔpH versus pHi intersects the straight line with coordinates (pHi; pHi).
- Determination of total phenolic content (TPC) of lignin particles
NOTE: The total phenolic content (TPC) of the micro-/submicron lignin particles is determined via a modified Folin-Ciocalteu colorimetric method.- Mix 200 µL of an aqueous suspension of particles with a concentration of 500 µg/mL with 600 µL of ultrapure water and 200 µL of Folin-Ciocalteu reagent (1:1, v/v).
- After 5 min, add 1.0 mL of 8% Na2CO3 and 1.0 mL of Milli-Q water to the mixture and incubate it in the dark at 40 °C for 30 min in a water bath with intermittent agitation.
- Centrifuge the suspension at 5,300 × g for 2 min.
- Prepare a blank containing no particles.
- Transfer 3.5 mL of the supernatant in a 10 mm quartz cuvette and measure the absorbance on a UV/Vis spectrophotometer in the visible region at 760 nm against the blank.
- Prepare a calibration curve of the standard gallic acid following steps 5.3.1-5.3.5; only instead of 200 µL of the lignin particle suspension, use the ethanolic solution of gallic acid with initial concentrations of 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 150, and 200 µg/mL.
- Express the experimental data of the microparticles as mg of gallic acid equivalents in milligrams per gram of dry sample (mg GAE/g).
- Calculate TPC using equation (5):
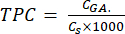 mg GAE/g (5)
mg GAE/g (5)
Where CGA is the concentration of the sample equivalent to the concentration of the standard gallic acid obtained from the calibration plot of the acid (µg GA/mL); Cs is the concentration of the sample, which is equal to the dry sample mass divided by the volume of the solvent (µg/mL).
6. Determination of the in vitro release capacity of lignin particles
- Prepare 250 mL of simulated enzyme-free gastric medium by adjusting the pH of standard PBS solution with 0.1 M HCl to pH = 1.2.
- Prepare 250 mL of each of the two simulated intestinal fluid solutions by adjusting the pH of the standard PBS solution with 0.1 M NaOH/0.1 M HCl to pH = 6.8 and 7.4, respectively.
- Add 25 mg of flavonoid-encapsulated micro-/submicron particles to 50 mL of the simulated enzyme-free gastric medium in a glass batch reactor supplied with a mechanical stirrer and place it in a thermal water bath at a constant temperature of T = 37 ± 0.2 oC.
- Dip the stirrer to a depth of 2/3 of the liquid volume to ensure full mixing of the solid and liquid phases and ensure maximal mass transfer without stagnant zones.
- Take out 1 mL of sample from the reactor every 10 min up to the 90th min and immediately pipette 1 mL of fresh simulated fluid solution into the reactor to prevent change of the total volume and to ensure sink conditions.
- Repeat the same procedure including steps 6.3-6.6 with both simulated intestinal fluid solutions with pH = 6.8 and 7.4, respectively, for 200 min.
- Perform analogous experiments with unloaded lignin particles in the three simulated media and use the samples as blanks for zeroing the spectrophotometer.
- Determine the absorption of the samples spectrophotometrically after filtering the samples and diluting them with 96% EtOH against the blank samples from step 6.7 and calculate the corresponding flavonoid concentration using the corresponding calibration curves of morin obtained at pH = 1.2, 6.8, and 7.4, respectively.
- Calculate the cumulative release (CR) of the bioflavonoids by using equation (6) in µg/mL and the cumulative release percentage (CRP) by equation (7):
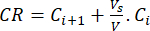 (6)
(6)
Where Ci and Ci+1 are the concentrations of morin/quercetin in the ith and (i+1)th samples (µg/mL); Vs the sample volume taken from the batch reactor (mL); V the total volume of the simulated media (mL).
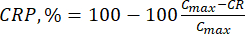 (7)
(7)
Where Cmax is the maximum concentration of the biologically active compound in the carrier (µg/mL).
7. Statistical analyses
- Express the experimental data as means ± standard deviations (SD) of three independent measurements.
- Determine the statistical significance of the experimental results by performing the ANOVA test as the post hoc test. Consider a value of p < 0.05 statistically significant.
Representative Results
An anti-solvent precipitation technique was executed to produce alkali lignin micro-/submicron particles. An aqueous solution of diluted inorganic acid-nitric acid/organic acid-citric acid was dispersed into an alkali lignin aqueous solution, enriched with an eco-friendly surfactant/ethanol, which resulted in the gradual precipitation of the biopolymer solute and, after sonication, a suspension of compact micro-/submicron particles was finally produced (Figure 1).
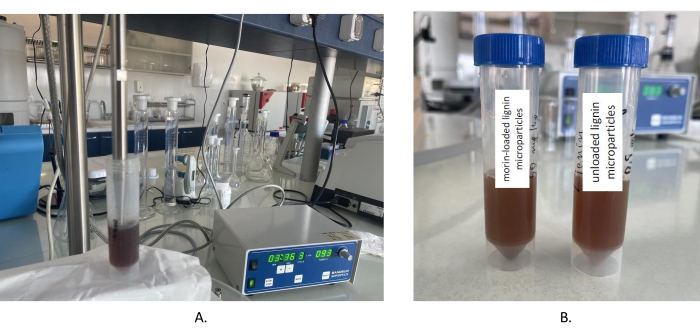
Figure 1: Homogenization of lignin particles. (A) Ultrasonic homogenization of the synthesized lignin submicron particles; (B) Homogenized morin-loaded and unloaded lignin microparticles. Please click here to view a larger version of this figure.
The size, number, and size distribution of the unloaded and morin-encapsulated lignin microcarriers were determined (Figure 2). The experimental data proved higher concentration, 1 × 107 particles/mL (2,037 particles/µL), and higher average size, 6.1 µm, of the bioflavonoid-loaded microcarriers (Figure 2B) than the unloaded ones with a concentration of 7.4 × 106 particles/mL (1,474 particles/µL) and average size of 5.7 µm (Figure 2A). The percent size distribution of both types of particles within the size range 3-6 µm was 75.2% for the unloaded and 69.3% for the morin-encapsulated microcarriers and 20.2% and 25.2%, respectively, within the range of 7-10 µm. The quantity, concentration, and flow rate of the antisolvent, nitric acid, are essential for the size of the particles. The higher concentration and greater amount of the acid leads to larger particles, while the higher flow rate provokes aggregation of the suspension.
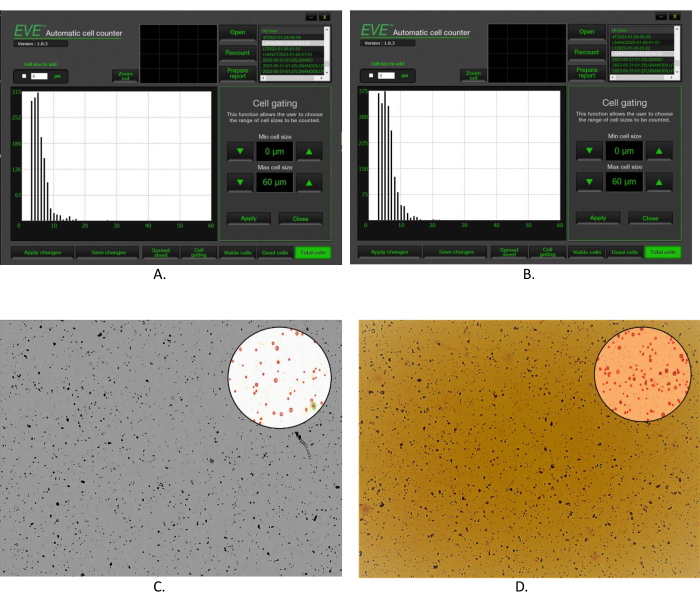
Figure 2: Particle size distribution. (A) Actual size distribution ofunloaded lignin microparticles in 1 µL of suspension-software of the particle counter; (B) actual size distribution of morin-encapsulated alkali lignin microparticles in 1 µL of suspension-software of the particle counter. (C) Particle counter microscopic photo of the distribution of the unloaded lignin microparticles; (D) particle counter microscopic photo of the distribution of the morin-encapsulated alkali lignin microparticles. Please click here to view a larger version of this figure.
Figure 3 presents the UV/Vis absorption spectra of ethanol morin solutions, alkali lignin aqueous solutions, and the mixtures containing morin and lignin with different initial concentrations. Obviously, the absorption peaks of pure lignin and the bioflavonoid do not coincide and the heteropolymer does not exert any disruptive influence during the applied spectrophotometric method for the determination of morin concentration in the liquid phase after encapsulation of the flavonoid into the polymer microcarriers and during the in vitro release experiments. The maximum absorption of morin in the two-component mixture shifted to a higher wavelength, from λmax = 359 nm to λmax = 395 nm, as a result of the increased pH of the medium due to the presence of alkali lignin. The latter deviation of the absorption maximum in the visible area provoked the necessity for the design of calibration curves of morin at various pH values of the medium (Figure 4A). The three standard curves characterized by very strong linear correlations were proven by the high values of the regression coefficients (R2 > 0.99) within the morin concentration range Co = 2.5-100 µg/mL. Similarly, the three standard curves of quercetin in the three simulated physiological compartments, presented in Figure 4B, showed high linearity within the same concentration range.
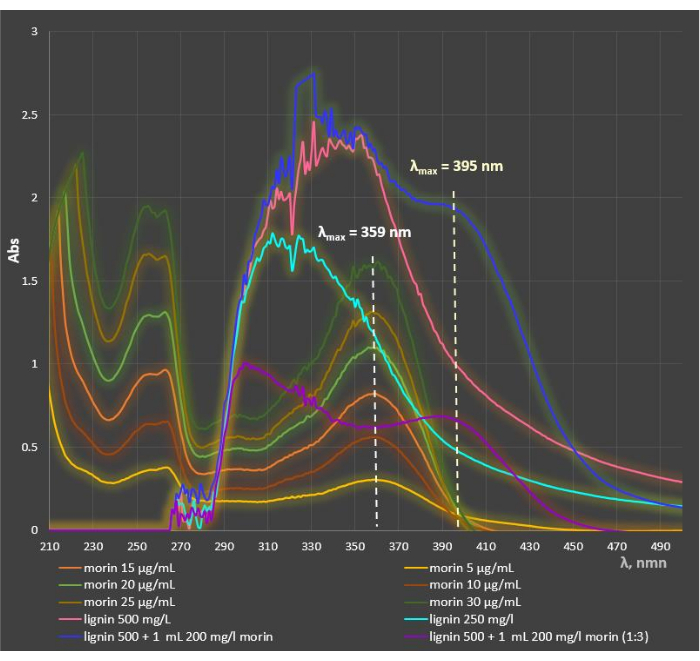
Figure 3: Comparison of the UV/Vis spectra of ethanolic solutions of morin, alkali lignin aqueous solutions, and mixtures containing morin and lignin with different initial concentrations. The spectra of pure lignin and morin do not coincide and the heteropolymer does not exert any disruptive influence. The addition of lignin to morin leads to shifting of the maximum absorption of morin to a higher wavelength, from λmax = 359 nm to λmax = 395 nm. Please click here to view a larger version of this figure.
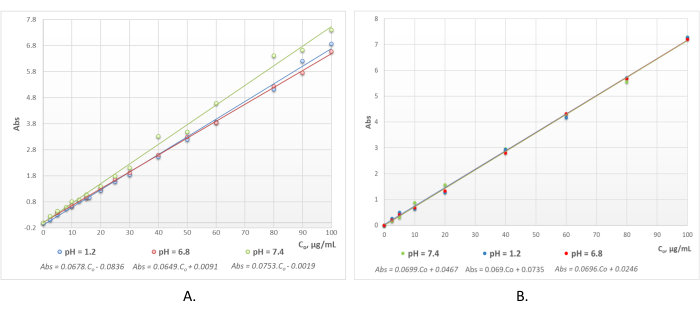
Figure 4: Calibration curves of ethanolic flavonoid solutions. (A) Morin and (B) quercetin within the concentration range Co = 2.5-100 µg/mL at pH = 1.2 (in blue) (corresponding to simulated gastric fluid), pH = 6.8 (in red) (corresponding to simulated small intestinal fluid), and pH = 7.4 (in green) (corresponding to simulated colon fluid). Please click here to view a larger version of this figure.
The relative concentration of acidic and basic active sites/functional groups on the surface of the unloaded and loaded alkali lignin particles was determined by potentiometric titration. The calculations were based on the equivalent titrant volumes determined by the second derivative differential titration curves (Figure 5). The values of the determined pKa, concentrations of acidic (strong, weak, total) functional groups, and pH and pHpzc of the micro- and submicron particles are presented in Table 1.
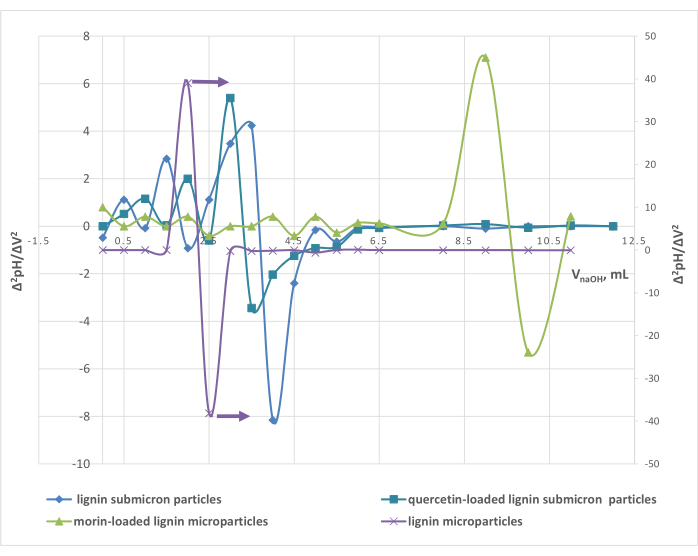
Figure 5: Second derivative differential potentiometric titration curves of the unloaded and loaded lignin micro-/submicron particles. Please click here to view a larger version of this figure.
| Parameter | lignin microparticles | morin-encapsulated lignin microparticles | lignin submicron particles | quercetin-encapsulated lignin submicron particles |
| Veq., mL | 10.5 | 2.75 | 2.25 4.3 |
2.75 3.75 |
| pKa | 11.1 | 10.8 | 3.0 8.0 |
4.2 7.0 |
| Aa (strong), mgeq/g | 26.25 | 6.88 | 16.38 | 16.3 |
| Aa (weak), mgeq/g | 11.25 | 13.13 | 11.25 | 13.13 |
| Aa (total), mgeq/g | 37.5 | 20 | 27.63 | 29.43 |
| pH (aqueous suspension) | 4.45 | 4.1 | 4.54 | 4.13 |
| pHpzc | 2.3 | 2.0 | 3.8 | 3.0 |
Table 1: Values of the equivalent volume of titrant (Veq), the negative base -10 logarithm of the acid dissociation constant (pKa), concentrations of acidic (strong, weak, total) functional groups (Aa, mgeq/g), pH and point of zero charge (pHpzc) of the unloaded and loaded lignin micro- and submicron particles. The micro- and submicron, unloaded and flavonoid-loaded lignin particles are negatively charged because their pH > pHpzc.
The total phenolic content (TPC), determined by a modified Folin-Ciocalteu colorimetric method and calculated as gallic acid equivalents, was 78.2 mg GAE/g of the unloaded lignin particles, while the value of TPC of the morin-encapsulated microcarriers with the same concentration was 2.3 times higher (183.43 mg GAE/g). The latter indicates that the hetero biopolymer particles are enriched with additional phenolic groups due to the incorporation of the flavonoid molecules. The flavonoid encapsulation efficiency was: 98.1% for morin and 97.6% for quercetin. The drug encapsulation capacities were 28.2% for the morin-loaded microparticles and 39.0% for the quercetin-encapsulated submicron particles.
The in vitro cumulative release of morin and quercetin was investigated in simulated gastrointestinal enzyme-free media: gastric, small intestinal, and colon fluids at pH = 1.2, 6.8, and 7.4, respectively (Figure 6). The highest release efficiency of approximately 24% was achieved after 30-40 min at pH = 6.8. According to the experimental results, the quantity of the released flavonoid in the simulated small intestinal medium prevailed twice this released in the simulated colon environment and three times the release efficiency determined in the stomach. The highest extent of quercetin release established in SIF at pH = 7.4 on the 70th– 90th min was 34%, which surpassed the cumulative release of the flavonoid in SGF (pH = 1.2) and SIF (pH = 6.8) at 23.5% and 18%, respectfully.
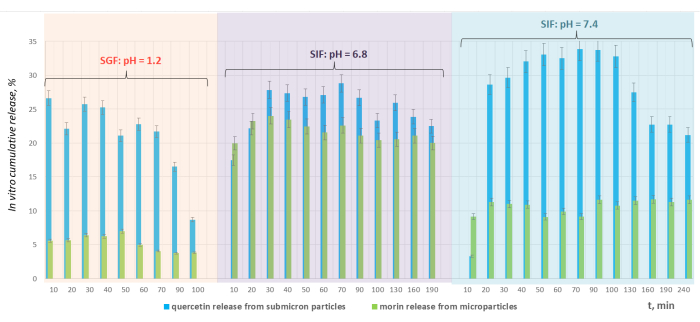
Figure 6: Comparative analyses of the cumulative in vitro release efficiency of morin and quercetin from lignin micro- and submicron particles in simulated physiological media. The highest extent of morin release was achieved in simulated small intestinal medium. The highest release efficiency of quercetin was registered in simulated colon fluid. Please click here to view a larger version of this figure.
Discussion
Among the main critical issues of modern synthesis methodologies for the design of drug-carrier formulations based on biopolymers is the application of hazardous organic reagents – volatile and flammable solvents, such as tetrahydrofuran, acetone, methanol, and even DMSO in high concentrations – which limits their applicability in biomedicine, pharmaceutical industry, and food technology due to the manifestation of possible toxic effects20,21,22,23,24. Another crucial point is the involvement of complicated chemical reactions (e.g., esterification, polymerization) or expensive apparatus during the synthesis procedure. Both techniques presented in the current manuscript overcome the latter limitations by the implication of alternative solvents (water) and non-toxic compounds such as surfactants (Tween 80) and cross-linking agents (ethanol, citric acid), classifying them as "green" synthesis methods. Furthermore, the methodologies offer a solution satisfying the critical necessity and urge for the development of inexpensive, eco-friendly, sustainable procedures for the design of lignin particles, serving as biodegradable, bioactive, and biocompatible carrier templates of physiologically active substances25.
To obtain lignin particles with the desired size, two production conditions were chosen: one with high lignin concentration (50 g/L) and nitric acid as an anti-solvent agent and another with lower lignin concentration (5 g/L), ethanol as an antisolvent, and citric acid playing a double role of antisolvent and cross-linking agent simultaneously, since these were the two variables influencing the size of lignin particles. The flow rate during both procedures was kept low to provide smaller particles and to prevent their aggregation. There are some critical points that have to be considered regarding the choice of the inorganic and organic acids for the synthesis protocols.
Nitric acid was chosen because it is a strong inorganic acid, which offers a high extent of precipitation of alkali lignin, and by controlling the rate of its addition particles within the desired size range can be obtained. Moreover, it is expected that the addition of HNO3 could provide modification of the heteropolymer particles due to probable chemical changes of lignin structure associated with: processes of nitration-substitution reactions of H-atoms in the benzene rings with -NO2 groups; esterification of aliphatic -OH groups and the formation of ester functional groups; and/or oxidation of phenolic -OH and -OCH3 groups resulting in the formation of quinone structures. Concerning the role of the precipitant and lignin concentration for the size of the synthesized particles, on the one hand, the higher initial lignin concentration combined with the addition of the strong nitric acid (pKa = -1.4) and the limited solubility of the alkali heteropolymer in the inorganic acid led to the production of particles within the micrometer range. On the other hand, the addition of ethanol to the aqueous solution of alkali lignin with the lower concentration provokes the formation of a fine suspension due to the partial solubility of alkali lignin in the alcohol. Moreover, the subsequent addition of citric acid led to the production of particles within the nanometer range because the organic acid is weaker (pKa1 = 3.13) than nitric acid, consequently offering lower precipitation extend.
Some basic properties of nanosized pharmaceuticals are drug circulation, drug release from dosage forms at specific sites, and absorption through biological membranes. These properties are considerably influenced by some physical and chemical characteristics of the nanoparticle carriers and by the encapsulated drug molecules.
The physicochemical characteristics of biopolymer carriers: concentration of surface-active acidic and basic groups, point of zero charge (pHPZC), size, particle size distribution, as well as the spectral characteristics of the particles before and after the incorporation of the bioactive substance, are essential parameters that must be taken into account when evaluating the functional groups, reactivity, stability, and homogeneity of particles10.
Particle size, particle size distribution, charge, and morphology are among the major factors that impact these evaluations. Particle size impacts their stability, reactivity, and drug release behavior26. Smaller particles offer a larger mass-transfer area, which leads to a higher drug-release rate. In contrast, the smaller mass-transfer surface area of larger particles results in a lower rate of drug diffusion inside these particles.
The application of titrimetric methods as basic techniques for the determination of acidic and basic sites and functional groups present on solid surfaces is constantly expanding. The main advantages of potentiometric titration include time- and labor-saving, high precision, and the elimination of reference standards and expensive apparatus. The method was applied in the present study as it allows the characterization of biopolymer particles by qualitative and semiquantitative determination of the nature and number of active sites present on the surface of loaded and unloaded biopolymer carriers27.
The surface charge of biological and medical micro-/nano- carriers plays an important role in cellular uptake28. The pHPZC corresponds to zero surface charge density, that is, to equivalent amounts of negative and positive charges developed by proton equilibria. The determination of these values provides information on the specificity of adsorption29. However, as the parameter isoelectric point represents only the external surface charges of particles in suspension, while the point of zero charge varies in response to the total net surface charge (external and internal) of the particles, the pHpzc protocol was applied for the first time in the present study as a simple and effective method for characterization of biopolymer drug-carriers. According to the concept of pHpzc, at pH above the pHpzc, the surface of the biopolymer particles is predominantly negatively charged, while a net positive charge is observed when the pH of the suspension is below the pHpzc. From the experimental data presented in Table 1, it could be concluded that the micro- and submicron, unloaded and flavonoid-loaded lignin particles are negatively charged because their pH > pHpzc.
The efficiency of flavonoid loading is influenced by encapsulation efficiency and drug loading capacity. Encapsulation efficiency (E, %) is defined as the ratio of the amount of the drug incorporated in the particles to the overall amount in the formulation. The encapsulation efficiency is influenced by drug characteristics, the solvent, and the carrier30.
The efficient delivery of a physiologically active substance, however, depends on the manner and extent to which its molecules are released from the carrier matrix. Thus, it is very important to consider the drug release mechanism and the release rate31,32,33. By elucidating the in vitro release mechanism of bioflavonoids from their biopolymeric micro-/nano-carriers, one can simulate and predict the behavior of the flavonoid and the carrier in a real physiological medium and optimize the design of pharmaceutical formulations with improved bioavailability. The experimental in vitro results obtained in this study are useful for clinical practice as they prove that due to the lower extent of morin/quercetin release from lignin submicron and micro- particles in the gastric environment, the innovative biopolymer particles are suitable for oral administration due to the lower risk of gastric irritation as compared to direct oral administration of the bioactive substances. The innovative biopolymer microparticles are suitable for oral administration due to the lower risk of gastric irritation as compared to direct oral administration of the bioactive substances. Moreover, the submicron particles, due to their small size and significant release potential, could be applied as injectable formulations. In addition, the novel lignin micro- and submicron carriers offer an opportunity to overcome the limitations reported by other scientists related to difficulties associated with the oral administration of high doses of certain bioflavonoids, resulting from their tendency to form saturated solutions in the intestinal tract, which in turn hinders the dissolution process and their efficient resorption.
The ease of synthesis, the biocompatibility of the resulting particles, as well as the possibility for customization of the current protocol, represent the major advantages of the presented methodology. The size of the particles is optimal for their intended applications, offering enough surface area available for attachments of therapeutics and targeting moieties, which in turn does not require more particles to be administered to achieve target dosage requirements. The use of lignin as the basic heteropolymer matrix for the synthesis of innovative particles allows for increased biocompatibility and offers various active functional groups presenting opportunities for customization of the particles for diverse applications.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This study was supported by the Bulgarian Scientific Fund under Contract № KΠ-06 H59/3 and by Scientific Project No. 07/2023 FVM, Trakia University.
Materials
| automatic-cell counter | EVE, NanoEnTek | ||
| Citric acid | Sigma | 251275 | ACS reagent, ≥99.5% |
| digital water bath | Memmert | ||
| Eppendorf tubes, 1.5-2 mL | |||
| Ethanol | Sigma | 34852-M | absolute, suitable for HPLC, ≥99.8% |
| Folin–Ciocalteu’s phenol reagent | Sigma | F9252 | |
| freeze dryer | Biobase | ||
| gallic acid | Sigma- | BCBW7577 | monohydrate |
| HCl | Sigma | 258148 | ACS reagent, 37% |
| HNO3 | Sigma | 438073 | ACS reagent, 70% |
| lignin, alkali | Sigma | 370959 | |
| morin | Sigma | PHL82601 | |
| NaCl | Sigma | S9888 | ACS reagent, ≥99.0% |
| Na2CO3 | Sigma | 223530 | powder, ≥99.5%, ACS reagent |
| NaOH | Sigma | 655104 | reagent grade, 97%, powder |
| orbital shaker | IKA | KS 130 basic | |
| pH-meter | Consort | ||
| phosphate-buffered saline (PBS) | Sigma | RNBH7571 | |
| Quercetin hydrate | Sigma | STBG3815V | |
| statistical software for Excel | Microsoft Corporation | XLSTAT Version 2022.4.5. | |
| Tween 80 | Sigma | P8074 | BioXtra, viscous liquid |
| ultracentrifuge | Hermle | Z 326 K | |
| Ultrapure water system | Adrona | INTEGRITY+ | |
| ultrasound homogenizer | Bandelin Sonopuls | HD 2070 | |
| UV/Vis spectrophotometer | Hach-Lange | DR 5000 |
Referenzen
- Yu, X., et al. Lignin nanoparticles with high phenolic content as efficient antioxidant and sun-blocker for food and cosmetics. ACS Sustainable Chem. Eng. 11 (10), 4082-4092 (2023).
- Boarino, A., Klok, H. -. A. Opportunities and challenges for lignin valorization in food packaging, antimicrobial, and agricultural applications. Biomacromolecules. 24 (3), 1065-1077 (2023).
- Aadil, K., Barapatre, A., Jha, H. Synthesis and characterization of Acacia lignin-gelatin film for its possible application in food packaging. Bioresour. Bioprocess. 3 (27), 1-11 (2016).
- Sharma, S., et al. Valorization of lignin into nanoparticles and nanogel: characterization and application. Bioresour. Technol. Reports. 18, 101041 (2022).
- Zadeh, E. M., O’Keefe, S. F., Kim, Y. -. T. Utilization of lignin in biopolymeric packaging films. ACS Omega. 3 (7), 7388-7398 (2018).
- Beaucamp, A., et al. Lignin for energy applications – state of the art, life cycle, technoeconomic analysis and future trends (Critical Review). Green Chem. 24, 8193-8226 (2022).
- Antunes, F., et al. From sugarcane to skin: Lignin as a multifunctional ingredient for cosmetic application. Int J Biol Macromol. 234, 123592 (2023).
- Garg, J., et al. Applications of lignin nanoparticles for cancer drug delivery: An update. Materials Letters. 311, 131573 (2022).
- Anushikha, K. K. Lignin as a UV blocking, antioxidant, and antimicrobial agent for food packaging applications. Biomass Conv. Bioref. , 1-14 (2023).
- Freitas, F. M. C., et al. synthesis of lignin nano- and micro-particles: Physicochemical characterization, bioactive properties and cytotoxicity assessment. Int J Biol Macromol. 163, 1798-1809 (2020).
- Rismawati, R., Nurdin, I. A., Pradiptha, M. N., Maulidiyah, A., Mubarakati, N. J. Preparation and characterization of lignin nanoparticles from rice straw after biosynthesis using Lactobacillus bulgaricus. Journal of Physics: Conference Series. 9th International Seminar on New Paradigm and Innovation of Natural Sciences and its Application. 1524, 012070 (2020).
- Worku, L. A., et al. Synthesis of lignin nanoparticles from Oxytenanthera abyssinica by nanoprecipitation method followed by ultrasonication for the nanocomposite application. Journal of King Saud University – Science. 35 (7), 102793 (2023).
- Gala Morena, A., Tzanov, T. z. Antibacterial lignin-based nanoparticles and their use in composite materials. Nanoscale Adv. 4, 4447-4469 (2022).
- Ivanova, D., Nikolova, G., Karamalakova, Y., Marutsova, V., Yaneva, Z. Water-soluble alkali lignin as a natural radical scavenger and anticancer alternative. Int J Mol Sci. 24 (16), 12705 (2023).
- Ivanova, D., Toneva, M., Simeonov, E., Antov, G., Yaneva, Z. Newly synthesized lignin microparticles as bioinspired oral drug-delivery vehicles: Flavonoid-carrier potential and in vitro radical-scavenging activity. Pharmaceutics. 15 (4), 1067 (2023).
- Yaneva, Z., et al. Antimicrobial potential of conjugated lignin/morin/chitosan combinations as a function of system complexity. Antibiotics. 11, 650 (2022).
- Handral, H. K., Wyrobnik, T. A., Lam, A. T. -. L. Emerging trends in biodegradable microcarriers for therapeutic applications. Polymers. 15 (6), 1487 (2023).
- Figueiredo, P., Lintinen, K., Hirvonen, J. T., Kostiainen, M. A., Santos, H. A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 93, 233-269 (2018).
- Tang, Q., et al. Lignin-based nanoparticles: a review on their preparations and applications. Polymers. 12 (11), 2471 (2020).
- Zhao, W., Simmons, B., Singh, S., Ragauskas, A., Cheng, G. From lignin association to nano-/micro-particle preparation: extracting higher value of lignin. Green Chemistry. 18 (21), 5693-5700 (2016).
- Stewart, H., Golding, M., Matia-Merino, L., Archer, R., Davies, C. Manufacture of lignin microparticles by anti-solvent precipitation: Effect of preparation temperature and presence of sodium dodecyl sulfate. Food Res Int. 66, 93-99 (2014).
- Beisl, S., Friedl, A., Miltner, A. Lignin from micro- to nanosize: Applications. Int. J. Mol. Sci. 18, 2367 (2017).
- Mishra, P. K., Ekielski, A. A simple method to synthesize lignin nanoparticles. Colloids Interfaces. 3, 52 (2019).
- Qian, Y., Deng, Y., Qiu, X., Li, H., Yang, D. Formation of uniform colloidal spheres from lignin, a renewable resource recovered from pulping spent liquor. Green Chem. 16, 2156-2163 (2014).
- Tardy, B. L., et al. Lignin nano- and microparticles as template for nanostructured materials: formation of hollow metal-phenolic capsules. Green Chem. 20, 1335-1344 (2018).
- Silva, M., et al. Paraquat-loaded alginate/chitosan nanoparticles: preparation, characterization and soil sorption studies. J Haz Mat. 190 (1-3), 366-374 (2011).
- Georgieva, N., Yaneva, Z. Comparative evaluation of natural and acid-modified layered mineral materials as rimifon-carriers using UV/VIS, FTIR, and equilibrium sorption study. Cogent Chem. 1 (1), 1-16 (2015).
- Zhang, P., Chen, D., Li, L., Sun, K. Charge reversal nano-systems for tumor therapy. J Nanobiotechnol. 20, 31 (2022).
- Yaneva, Z. L., Georgieva, N. V. Removal of diazo dye from the aqueous phase by biosorption onto ball-milled maize cob (BMMC) biomass of Zea mays. Maced. J. Chem. Chem. Eng. 32 (1), 133-149 (2013).
- Zatorska, M., et al. Drug-loading capacity of polylactide-based micro- and nanoparticles – Experimental and molecular modeling study. Int J Pharmaceutics. 591, 120031 (2020).
- Yaneva, Z., Georgieva, N., Grumezescu, A. M. Chapter 5 – Physicochemical and morphological characterization of pharmaceutical nanocarriers and mathematical modeling of drug encapsulation/release mass transfer processes. Nanoscale Fabrication, Optimization, Scale-Up and Biological Aspects of Pharmaceutical Nanotechnology. , 173-218 (2018).
- Yaneva, Z., Georgieva, N., Staleva, M. Development of d,l-α-tocopherol acetate/zeolite carrier system: equilibrium study. Monatshefte fur Chemie Chemical Monthly. 147 (7), 1167-1175 (2016).
- Yaneva, Z., Georgieva, N. Study on the physical chemistry, equilibrium, and kinetic mechanism of Azure A biosorption by Zea mays biomass. Journal of Dispersion Science and Technology. 35 (2), 193-204 (2014).

