Point-of-Care Kidney and Genitourinary Ultrasound in Adults: Image Acquisition
Summary
Point-of-care ultrasound (POCUS) of the renal and genitourinary (renal-GU) system can help to screen for certain causes of kidney dysfunction. However, despite its clinical utility, renal-GU POCUS remains underutilized due to a lack of training among clinicians. To address this gap, this article describes renal-GU image acquisition and interpretation.
Abstract
A range of conditions involving the kidneys and urinary bladder can cause organ-threatening complications that are preventable if diagnosed promptly with diagnostic imaging. Common imaging modalities include either computed tomography or diagnostic ultrasound. Traditionally, ultrasound of the kidney-genitourinary system has required consultative teams consisting of a sonographer performing image acquisition and a radiologist performing image interpretation. However, diagnostic point-of-care ultrasound (POCUS) has recently emerged as a useful tool to troubleshoot acute kidney injury at the bedside. Studies have shown that non-radiologists can be trained to perform diagnostic POCUS of the kidneys and bladder with high accuracy for a set number of important conditions. Currently, diagnostic POCUS of the kidney-genitourinary system remains underused in actual clinical practice. This is likely because image acquisition for this organ system is unfamiliar to most clinicians in specialties that encounter acute kidney injury, including primary care, emergency medicine, intensive care, anesthesiology, nephrology, and urology. To address this multi-specialty educational gap, this narrative review was developed by a multi-disciplinary group to provide a specialty-agnostic framework for kidney-genitourinary POCUS image acquisition: indications/contraindications, patient positioning, transducer selection, acquisition sequence, and exam limitations. Finally, we describe foundational concepts in kidney-genitourinary ultrasound image interpretation, including key abnormal findings that every bedside clinician performing this modality should know.
Introduction
Acute kidney injury (AKI), resulting from a variety of etiologies, is a frequent medical diagnosis identified in hospitalized patients. AKI precipitates an abrupt decrease in kidney function that leads to an accumulation of extracellular fluid, urea, and other nitrogenous waste products, along with the dysregulation of electrolytes. Moreover, the diagnosis of AKI portends worse short-term and long-term outcomes and is associated with the consumption of greater healthcare resources1. According to the United States Renal Data System (USRDS), among Medicare Fee-For-Service beneficiaries in 2020, the rate of hospitalization with AKI was 62 admissions per 1000 patient years2. Furthermore, a systematic review of 154 studies that adopted the Kidney Disease Improving Global Outcomes (KDIGO) AKI diagnostic criteria found that among the 3,585,911 people in these trials, primarily from North America, Northern Europe, and Eastern Asia, the incidence of AKI in different inpatient settings ranged from 20%-32%3. In the inpatient setting, AKI is commonly identified in the intensive care unit and is associated with increased mortality4. Point-of-care ultrasound (POCUS) machines are readily available in settings such as the ICU, but diagnostic kidney-GU ultrasound is often underused despite being able to quickly evaluate several etiologies of AKI5.
Compared to consultative ultrasound, in which a patient’s primary provider orders a formal ultrasound to be performed by a radiology technician and read by a radiologist, diagnostic POCUS is performed and interpreted by a patient’s primary provider at the point of care6. There is growing evidence that non-radiologists can effectively and accurately use diagnostic POCUS for a variety of conditions7. For instance, the 2019 Hospitalist-Operated Compression Ultrasonography: a Point-of-Care Ultrasound Study (HOCUS-POCUS) prospective cohort study compared hospitalist-performed deep vein thrombosis (DVT) compressive POCUS to consultative technician/radiologist performed vascular DVT ultrasound. The study showed similar accuracy of hospitalist-performed POCUS to technician/radiologist-performed consultative vascular ultrasound with sensitivity of 100% and specificity of 96%8. Similarly, a 2020 study found kidney-GU POCUS performed by Emergency Department providers of varied experience had moderate accuracy (specificity of 72% and sensitivity of 77%) in detecting hydronephrosis when compared to computed tomography scan9. Importantly, primary-provider performed POCUS allows for more timely diagnosis and intervention compared to technician/radiologist imaging.
Causes of AKI can be divided into pre-renal (hemodynamically mediated injury), intra-renal (glomerular or interstitial pathologies), and post-renal (urologic etiologies, most commonly obstructive uropathy). The latter, especially, can be diagnosed with POCUS. Obstructive uropathy has an annual incidence of 1.7 per 1000 people and has been estimated to account for about 10% of acute and chronic kidney disease10. From prostatic hyperplasia to nephrolithiasis, there are many causes of urinary obstruction. The main pathologic manifestation of these conditions at the level of the kidney is hydronephrosis. This is easily visualized on POCUS, and speed in diagnosis can be critical in treating acutely ill patients with kidney failure.
Beyond AKI, POCUS remains a cost-effective and safe modality for evaluating chronic kidney disease. POCUS can be used to identify lesions indicative of renal cell carcinoma, given the ability to visualize cysts greater than or equal to 3 cm and to discriminate characteristics deemed concerning for malignancy11. POCUS allows for the rapid evaluation of autosomal dominant polycystic kidney disease (ADPKD), avoiding unnecessary scheduling of kidney biopsies and expensive lab work. Additionally, ultrasonographic measurement of kidney length was shown to prognosticate risk of progression in early ADPKD when compared to the gold standard magnetic resonance-based, height-adjusted total kidney volume measurement (htTKV)12.
While computed tomography scans have an advantage in detecting neoplasms, stones, and calcifications, there has been no proven benefit of computed tomography over ultrasonography in the diagnosis of AKI etiologies13. Furthermore, some patients may be too sick to move out of their rooms, precluding transport to the CT scanner or even the radiology suite for a technician/radiologist to perform the consultative ultrasound. In these cases, POCUS provides a safe and reliable diagnostic alternative. Despite this, diagnostic kidney-GU POCUS remains an underused tool, likely due to a lack of training among frontline clinicians14. To address this knowledge gap, this narrative review brings together the expertise of multiple specialties (hospital medicine, critical care, anesthesiology, and nephrology) to propose an evidence-based kidney-GU POCUS image acquisition protocol, including indications/contraindications, patient positioning, transducer selection, acquisition sequence, and exam limitations. Finally, we describe foundational concepts in kidney-GU ultrasound image interpretation, including key abnormal findings that every bedside clinician performing this modality should know.
Protocol
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Duke University Health System institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Images included were performed on the authors themselves for normal images and as part of routine educational ultrasound scans done for teaching purposes for positive images, with preceding consent per institutional standards. Patients were included based on the following criteria: any patient with acute kidney injury, decreased urinary output, or any other reason to suspect abnormal kidney function. Exclusion criteria included patients with an open abdomen. Pain is a relative contraindication to ultrasound exams, especially when the pain is so severe that it prohibits probe placement (e.g., intraabdominal hypertension). The reagents and the equipment used are listed in the Table of Materials.
Terminology: the three planes of the body are called coronal, sagittal, and transverse
This can be confusing because the latter term ("transverse") can also be used to refer to the short axis of a single organ alongside the use of the term "longitudinal" to refer to the long axis of the organ. To minimize confusion, this protocol will exclusively use coronal, sagittal, and transverse to refer to planes in the human body and will use long-axis and short-axis to refer to the planes of the kidney. Further, since the bladder's dynamic shape prevents it from having a permanent long- or short-axis, the two views of the bladder will only be named based on which one of two to the plane of the body that the view aligns with: sagittal or transverse.
1. Transducer selection
- Select a low-frequency transducer: preferably, this should be the curvilinear transducer (2-5 MHz). If a curvilinear transducer is not available, a sector array (aka "phased array"; 1-5 MHz) can serve as a substitute.
NOTE: The curvilinear transducer is the best choice for visualizing the kidney through a single view because its footprint creates a large field of view, and its low-frequency piezoelectric crystals permit penetration deep enough into the body. However, the curvilinear probe can be difficult to use when looking at kidneys through the ribs.
2. Machine settings
- Set the depth so that the kidney will appear in the middle third of the ultrasound screen (the typical setting is between 16 cm and 20 cm).
- Set the gain such that the renal medullary pyramids appear anechoic (black), the renal sinus complex appears hyperechoic (bright), and the kidney cortex appears intermediate between these extremes.
3. Mode and presets
- Select two-dimensional (2D) mode, also called brightness mode (B-mode). This is a 2-dimensional greyscale ultrasound mode.
- After activating the 2D mode, select the abdominal preset.
4. Patient positioning
- For most of the exam, position the patient supine.
NOTE: When imaging the right kidney, it may become necessary to reposition the patient in the left lateral recumbent position for better visualization of the right kidney. When imaging the left kidney, it may become necessary to reposition the patient in the right lateral recumbent position for better visualization of the left kidney. - Before scanning, expose the patient's lower chest and abdomen.
- Position the ultrasound machine so that the sonographer's dominant hand can hold the ultrasound probe. This allows for finer manipulation of the ultrasound probe and frees up the non-dominant hand for operating the ultrasound machine.
NOTE: Right-handed sonographers should position themselves with the patient on their right side and vice versa.
5. Imaging the right kidney
- Gel application
- Apply gel to the ultrasound probe directly to maximize scanning efficiency prior to acquiring each image.
- Coronal view
- Place the probe on the right flank, along the mid-axillary line, 5th-7th intercostal space, indicator pointing cranially (Figure 1).
- Adjust the probe position (sliding up/down, angling anterior/posterior) until the right kidney is seen in its maximal longitudinal extent (see Figure 2).
- Fan through anteriorly to posteriorly to screen for hydronephrosis and other gross abnormalities (Video 1).
- Cine clip acquisition: For machines configured for retrospective image acquisition, click on acquire prior to step 5.2.4. For machines configured for prospective image acquisition, click on acquire after step 5.2.4.
- Kidney long-axis diameter measurement
- Center the kidney in the image, click on Freeze, and measure the height of the kidney (Figure 3).
- Click on Save (or the equivalent button).
- Transverse view
- After centering on the right kidney, rotate the probe 90 degrees clockwise until the probe marker is facing anteriorly, revealing the kidney in the transverse view (Figure 4).
- Adjust the probe position (sliding up/down, angling anterior/posterior) until the right kidney is seen in its maximal size in this transverse plane (Figure 5).
- Fan through cranially to caudally to screen for hydronephrosis and other gross abnormalities (Video 2). Repeat the step.
6. Imaging the left kidney
- Repeat steps 5.1-5.3 for the left kidney.
7. Imaging the bladder
- Gel application: repeat step 5.1.1.
- Transverse view
- Probe position: position the probe just cranial to the pubic symphysis with the probe indicator pointing toward patient's right side (Figure 6).
- Angle the ultrasound beam caudally into the pelvis until the bladder is visible in its maximal size.
- Image optimization
- Adjust depth until the bladder is visible in the middle third of the screen.
- Adjust gain until the bladder lumen is grossly anechoic (black) and the tissue plane directly posterior to the bladder is slightly hyperechoic (bright).
- Cine clip acquisition
- Fan through the bladder from cranial to caudal to visualize the entire structure. Repeat step 5.2.3.
- Transverse bladder dimension measurements
- Center the view on the bladder's maximal dimension, click on Freeze and measure the anterior-posterior and lateral-to-medial diameters of the bladder (Figure 7, left panel).
- Click on Save (or the equivalent button).
- Sagittal view
- Maintaining the transverse view, center the view on the bladder and rotate the probe 90 degrees clockwise until the probe marker is facing cranially, revealing the kidney in the sagittal plane (Figure 8).
- Cine clip acquisition
- Fan through the bladder from side to side to visualize the entire structure. Repeat step 5.2.3.
- Transverse bladder dimension measurements
- Center the view on the bladder's maximal dimension, click on Freeze and measure the cranial-to-caudal diameter of the bladder (Figure 7, right panel).
- Click on Save (or the equivalent button).
Representative Results
Sonographically normal exam
Normal kidney ultrasound
The echogenicity of the kidney capsule and limited anatomic variability (except for the occasional pelvic kidney or the even more rare horseshoe kidney) allow for easy identification of the kidneys with bedside POCUS. The kidneys will have a typical bean-shaped appearance, measuring on average 9-13 cm, though size varies with patient height and weight (Figure 2, Video 1). The outer cortex is typically hypoechoic relative to the adjacent spleen and liver. Interior to the cortex is the kidney medulla, which is comprised of anechoic pyramids with cortical tissue extending into the medulla and separating the pyramids. The renal sinus is comprised of the renal pelvis, collecting system, sinus fat, vessels, and lymphatics. The sinus fat contributes to the hyperechoic appearance of this region of the kidney (Figure 9). The transverse or short axis of the kidney, as seen by rotating the probe 90 degrees, is characterized by the mid-portion of the kidney appearing as a C-shaped pattern with the localization of renal vessels and ureter (Figure 5, Figure 10, and Video 2, Video 3, and Video 4).
Normal bladder ultrasound
Whenever feasible, the bladder should be visualized in both the transverse and sagittal planes. The transverse view is obtained with the probe indicator pointing towards the patient's right side and normally shows the bladder in a circular cross-section (Figure 7, left). The sagittal bladder view is obtained with the probe indicator pointing cranially, and it normally shows the bladder as an irregular structure with a broad portion caudally and a narrow portion cranially (Figure 7, right). As the bladder distends, the sagittal view will tend to show a bladder shape that is increasingly circular/ovoid.
Key abnormal findings
Key abnormal kidney findings
Identification of kidney abnormalities with bedside ultrasound can provide critical clues to a patient's pathology and causes of kidney dysfunction. Assessment of kidney size, presence and degree of hydronephrosis, and identification of stones, cysts, and masses are the predominant objectives of bedside ultrasound.
Assessment of kidney size
Measurement of kidney length may help distinguish AKI from chronic kidney disease (CKD) or acute-on-chronic kidney injury as kidney size is expected to decrease with decreases in kidney function15. Normal kidney size is 9-13 cm in its longest dimension, 3-7 cm wide (in the coronal plane) and 3-6 cm thick (in the transverse plane)16. Also, as kidney size varies within the adult population, differences in size between the left and right kidneys can suggest differential kidney function between them. The kidney cortex is also known to decrease in size in the setting of CKD from its normal thickness of 7-10 mm as well as become more echogenic17 (Video 5). Certain kidney pathologies may also be associated with an increase in kidney size. Inflammatory states (such as AIN or glomerulonephritis) and infiltrative processes may be associated with an increase in kidney size.
Hydronephrosis
An obstructive process that leads to a dilated renal pelvis and calyces is termed hydronephrosis. In contrast, the term pelvicaliectasis is used when the renal pelvis and calyces are dilated without any evidence of obstruction. POCUS is frequently used to assess hydronephrosis as an indicator of obstruction. Obstruction may be due to a stone distal to the kidney, such as ureteropelvic junction, ureter, or ureterovesical junction. Additionally, a bladder mass obstructing a ureterovesical junction or urethral orifice (Figure 11), a prostatic mass, or hypertrophy can lead to hydronephrosis. Finally, extrinsic compression from a mass or lymphadenopathy is another potential cause. Hydronephrosis of the kidneys, independent of the cause, has a similar ultrasonographic appearance. However, one should note if hydronephrosis is unilateral or bilateral, as this will support or refute certain diagnoses.
As noted above, normal kidney parenchyma includes fat, which gives it a hyperechoic appearance. The obstructed urinary tract results in a distended collecting system, changing the ultrasonographic appearance of the kidney from hyperechoic to hypoechoic and sometimes anechoic. As the severity of hydronephrosis increases, the appearance becomes more anechoic, extending proximally to the kidney cortex. Hydronephrosis is graded as being either mild, moderate, or severe. Mild hydronephrosis is characterized by dilation of the renal pelvis and the calyces (Figure 12A, Video 6). As hydronephrosis progresses to moderate, the tips of the renal pyramids flatten and become concave due to increasing urine volume and pressure (Figure 12B,C, Video 7). In cases of severe hydronephrosis, the renal pelvis and calyces undergo further dilation, while the cortex begins to get thinner, resulting in a frequently described bear claw appearance (Figure 12D and Figure 13, Video 8). Further, an important false positive for hydronephrosis is a common normal variant termed extra-renal pelvis18. This describes the finding of a renal pelvis located outside the kidney itself and may be present in as much as 10% of the population18. The calyces in cases of an extra-renal pelvis are not sonographically visible, in contrast to the readily visible calyces in hydronephrosis due to their dilatation (Figure 14, Video 9).
Kidney cysts
Kidney cysts, both sporadic and inherited, are commonly seen on POCUS and characterized as simple or complex. Simple cysts are observed as round or oval, well-defined, thin-walled structures exhibiting acoustic enhancement on the distal wall (enhanced echogenicity attributed to increased echoes). CT imaging (Figure 15, Video 10, Video 11) uses the Bosniak criteria for the classification of cysts. While the validation of the Bosniak criteria in ultrasound imaging is not as extensive, they are commonly employed to categorize patients into two groups: those requiring follow-up imaging and those who do not. Any cyst that fails to meet the following criteria: being oval/circular, having a thin wall without septations, and lacking posterior acoustic enhancement on the far wall, should undergo further imaging evaluation19–22. If multiple cysts are observed bilaterally, one should consider the possibility of polycystic kidney disease or acquired cystic kidney disease23.
Kidney masses
As noted above, abnormalities seen on kidney ultrasound that are not consistent with a simple cyst should receive further radiographic evaluation. The prevailing malignancy is renal cell carcinoma, distinguished by a partially cystic mass with heterogeneous features (Figure 16, Video 12, and Video 13). Benign kidney masses most commonly are angiomyolipomas and oncocytomas24.
Key abnormal bladder findings
In the evaluation of oliguria/anuria and/or AKI, bladder and kidney POCUS can aid in narrowing the differential diagnosis (Figure 17). The initial step, outlined in the algorithm, is to determine if the patient has an indwelling invasive urinary (Foley) catheter. If a Foley catheter is present, the subsequent examination involves checking for the catheter balloon in the bladder. If the balloon is not visible, it suggests catheter misplacement (e.g., the catheter tip is in the urethra). If the catheter balloon is visible but the bladder is distended (Figure 18), obstruction is likely. If the bladder is small/decompressed (Figure 19), it indicates either complete ureteral obstruction, pre-renal/intrinsic kidney disease, or unilateral obstruction in patients with a solitary kidney. To differentiate, kidney ultrasound is crucial; hydronephrosis significantly raises the probability of ureteral obstruction, while its absence argues against it. Note that this algorithm serves as a heuristic rather than a definitive diagnostic tool, as some cases of low urine output and AKI can coexist, such as a patient with chronic hydronephrosis developing acute pre-renal/intrinsic kidney disease.
In reference to the algorithm in Figure 17, when the patient lacks an indwelling urinary catheter, the sonographic screening for low urine output and/or AKI commences with the qualitative or quantitative evaluation of bladder size. A grossly distended bladder (Figure 20) suggests potential causes such as bladder outlet obstruction (e.g., benign prostatic hyperplasia) or ineffective detrusor muscle action (e.g., spinal or epidural anesthesia). In cases where the bladder is small/decompressed, the differential diagnosis mirrors that of an indwelling urinary catheter with a small/decompressed bladder-either (1) complete ureteral obstruction or (2) pre-renal or intrinsic kidney disease. To distinguish between these possibilities, an assessment of the patient's kidneys for signs of hydronephrosis, as described earlier, is essential.
During the assessment of the bladder for factors contributing to low urine output, it is possible to occasionally observe echodensities within the bladder lumen. For instance, Figure 11 (see also Video 14) illustrates a bladder containing a sizable, irregular echodensity, determined to be a combination of a bladder tumor and blood clots. In practical terms, the use of ultrasound faces challenges in differentiating between masses and blood clots within the bladder. However, both findings are considered grossly abnormal and necessitate further imaging and/or consultation with Urology for guidance on long-term care.
Figure 1: Curvilinear probe to the mid axillary line on the right side to visualize the right kidney in the longitudinal view. The probe marker is pointing to the patient's head and hence is not visible in the image. Please click here to view a larger version of this figure.
Figure 2: Longitudinal view of a normally functioning right kidney. The cranial to the right kidney, the liver, and the diaphragm are seen. Medial to the kidney, the individual vertebrae are seen. Please click here to view a larger version of this figure.
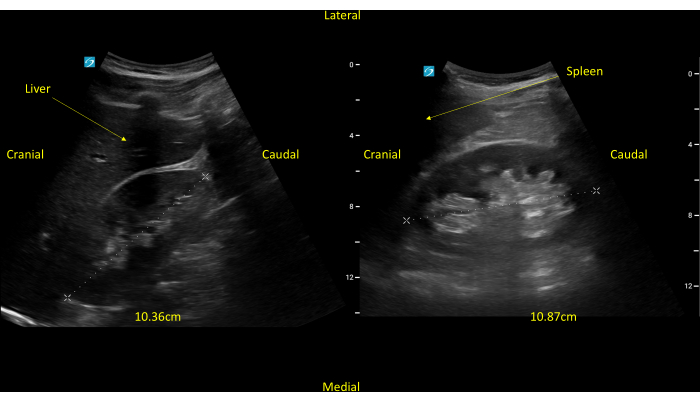
Figure 3: Longitudinal views of both the right and left kidney, respectively. The kidneys measure a little over 10 cm each. Please click here to view a larger version of this figure.
Figure 4: Curvilinear probe to the mid axillary line on the right side to visualize the right kidney in the short axis view. The probe marker is pointing posteriorly and hence, is not visible in the image. Please click here to view a larger version of this figure.
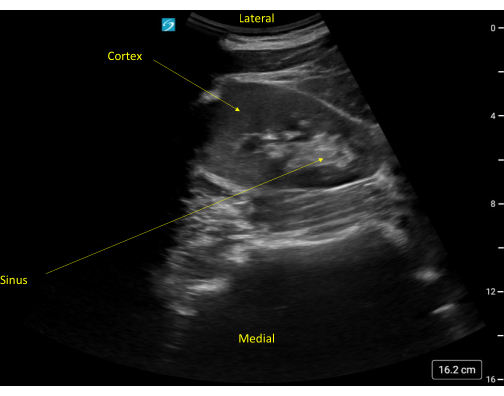
Figure 5: Short axis view of a normal appearing kidney. Note the rib shadow to the right of the kidney. Please click here to view a larger version of this figure.
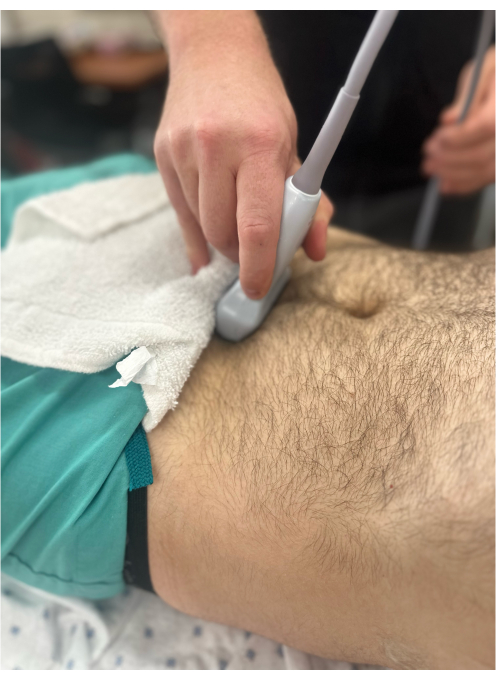
Figure 6: Curvilinear probe to the suprapubic area to visualize the bladder in the transverse orientation. The probe marker is pointing to the patient's right and, hence, is not visible in the image. Please click here to view a larger version of this figure.
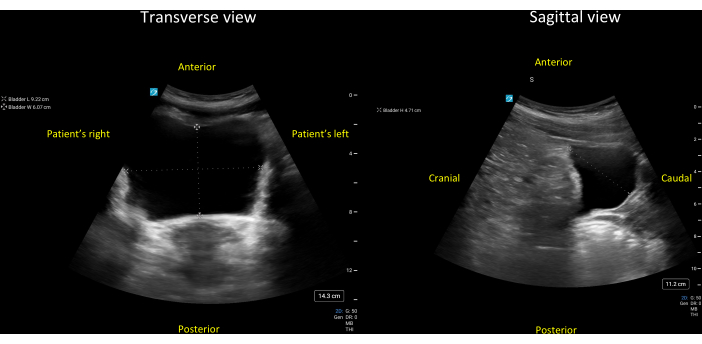
Figure 7: Transverse (left panel) and sagittal (right panel) views of a grossly normal bladder. The three diameters of this bladder have been measured as 9.2 cm (lateral-to-medial), 6.1 cm (anterior-to-posterior), and 4.7 cm (cranial-to-caudal). Entering these three diameters and a correction factor for the triangular bladder of 0.69 (Protocol step 7.3.3.1), one gets the following estimate for the bladder volume = 0.69 * 9.2cm * 6.1cm * 4.7cm = 182cm3 (mL). Please click here to view a larger version of this figure.
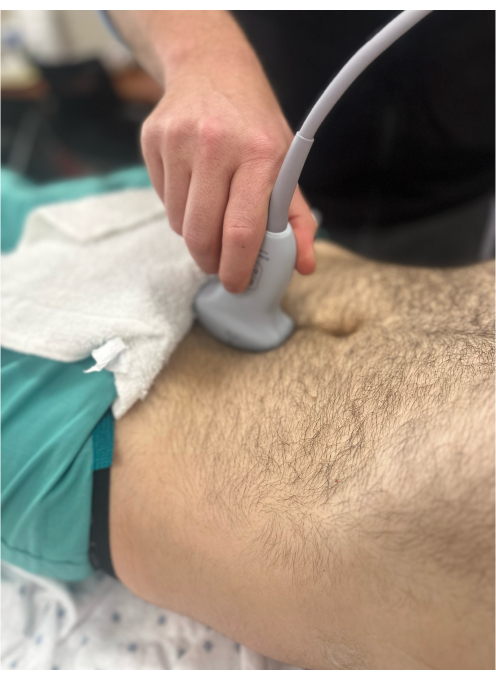
Figure 8: Curvilinear probe to the suprapubic area to visualize the bladder in the sagittal orientation. The probe marker is pointing to the patient's head and, hence, is not clearly visible in the image. Please click here to view a larger version of this figure.
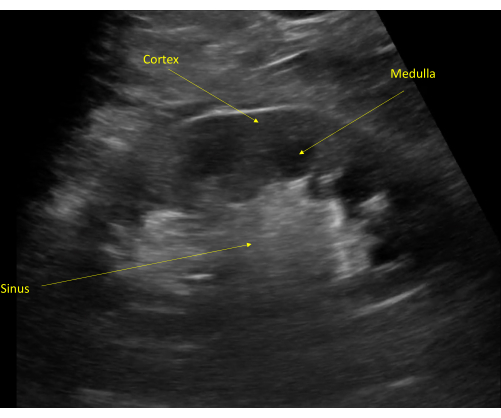
Figure 9: The kidney cortex is typically seen as hypoechoic to the liver (unlabeled, superior to the kidney). The kidney medulla is visible distal to the cortex with visible renal pyramids. The collecting system is seen as a hyperechoic structure due to the renal sinus fat. Please click here to view a larger version of this figure.
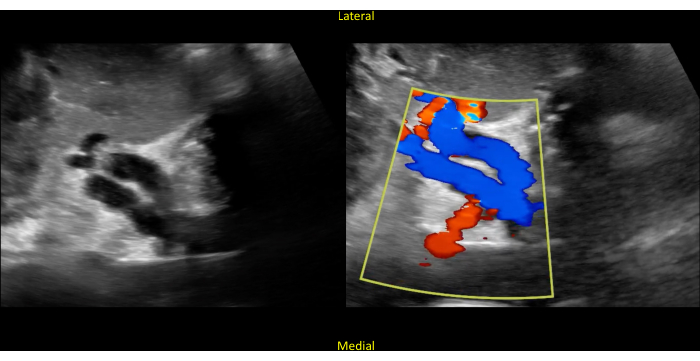
Figure 10: Short axis view of the kidney. The image shows hollow structures in the center (left panel). Color Doppler confirms that these structures represent the renal vasculature (right panel). Blue represents flow away from the probe, and red represents flow towards the probe (remembered by the mnemonic "BART"; blue away, red towards). Please click here to view a larger version of this figure.
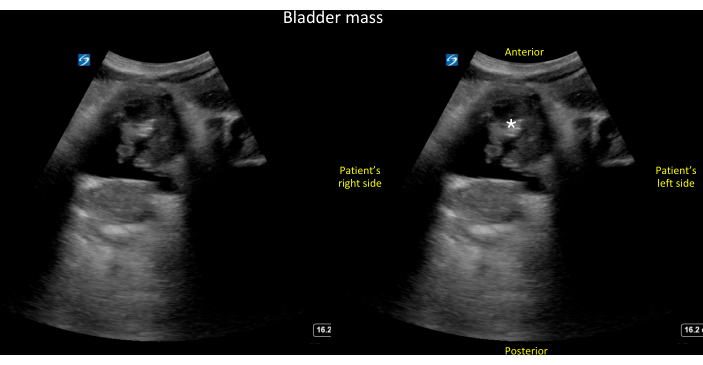
Figure 11: Transverse view of the bladder. Unlabeled (left panel) and labeled (right panel) transverse view of the bladder showing a large, irregular echo density within the bladder lumen that turned out to be a combination of tumor and blood clots (see also Video 14). Please click here to view a larger version of this figure.
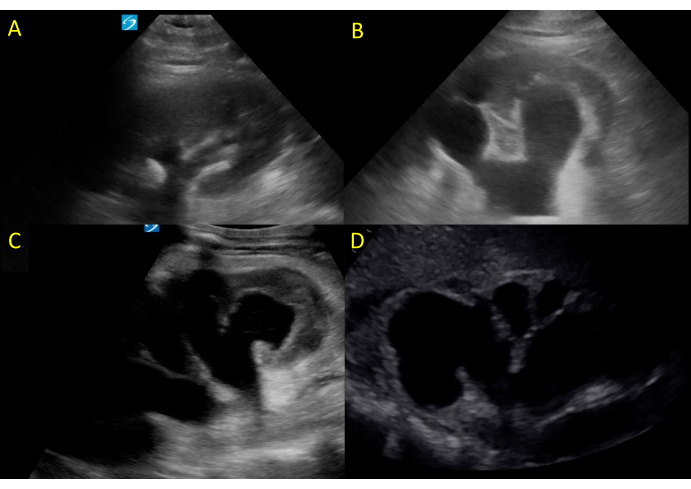
Figure 12: Hydronephrosis of varying degrees. (A) shows mild hydronephrosis, (B,C) moderate hydronephrosis, and (D) severe hydronephrosis. Please click here to view a larger version of this figure.
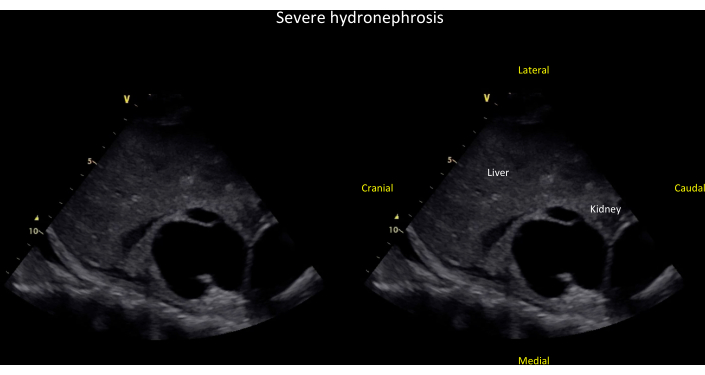
Figure 13: Coronal view of the right kidney showing severe hydronephrosis. Unlabeled (left panel) and labeled (right panel) coronal view of the right kidney showing severe hydronephrosis, defined by dilation of major and minor calyces and cortical thinning (see also Video 8). Please click here to view a larger version of this figure.
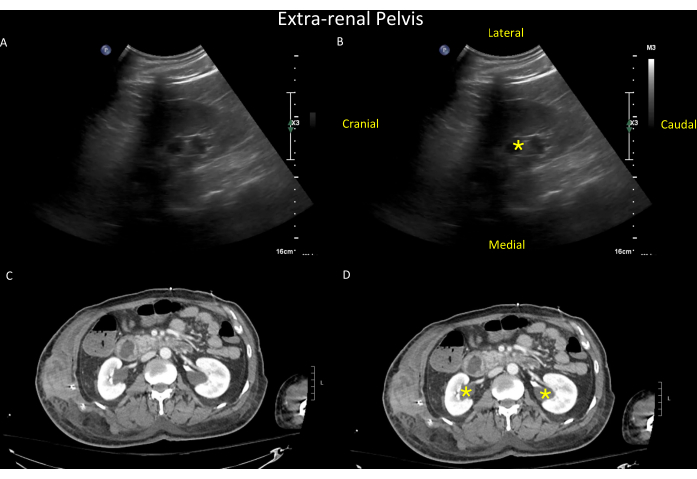
Figure 14: Sonographic findings in the right kidney. Unlabeled (top left, A) and labeled (top right, B) views of the right kidney showing the sonographic findings of an extra-renal pelvis (yellow asterisk). The extra-renal pelvis is a normal variant found in up to 10% of the population, where the renal pelvis lies outside the kidney. Extra-renal pelvis can mimic hydronephrosis except that in hydronephrosis, the calyces are visible and dilated, whereas, in extra-renal pelvis, the calyces are normal-sized and hence not visible. For illustrative purposes, a CT scan of the same patient is shown in the bottom panels (unlabeled in C and labeled in D, with extra-renal pelvis seen bilaterally (yellow asterisk in D) (see also Video 9). Please click here to view a larger version of this figure.
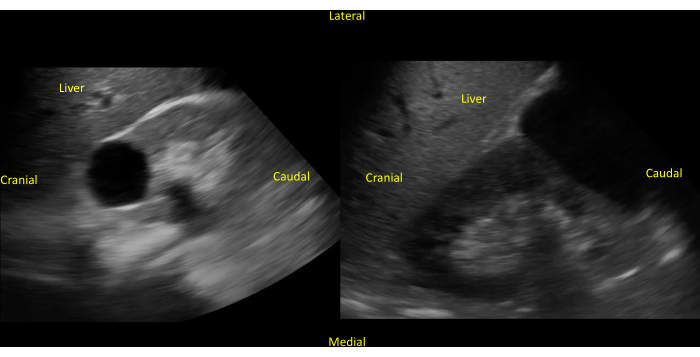
Figure 15: Presence of renal cyst in the right kidney. An unlabeled (left panel) image of a simple renal cyst in the center of the right kidney is viewed on the long axis. Unlabeled (right panel) image of a larger simple renal cyst in the inferior pole of the right kidney. Please click here to view a larger version of this figure.
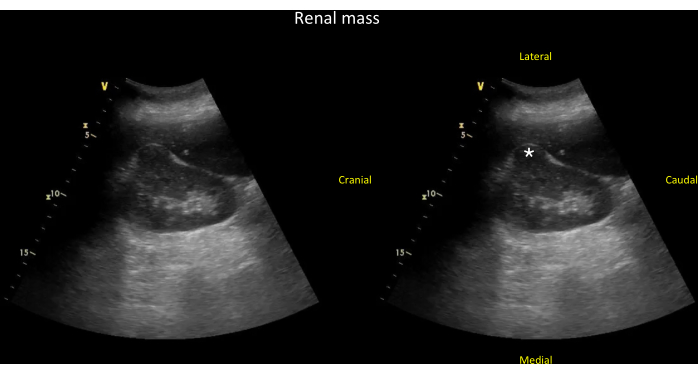
Figure 16: Coronal view of the right kidney showing a kidney mass. Unlabeled (left panel) and labeled (right panel) coronal view of the right kidney showing a kidney mass (*) that is arising from the kidney cortex and has disrupted the expected normal oval shape typically seen in this view (see Video 13). Please click here to view a larger version of this figure.
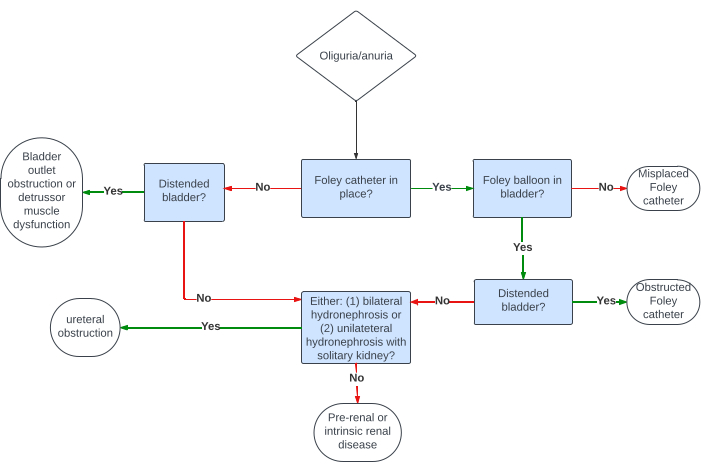
Figure 17: An algorithm showing how the combined bladder and kidney ultrasound can be used to narrow the differential diagnosis of oliguria/anuria. Please click here to view a larger version of this figure.
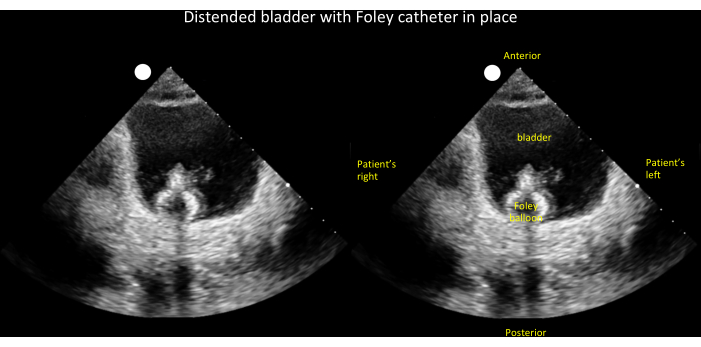
Figure 18: Unlabeled (left panel) and labeled (right panel) transverse view of a distended bladder with Foley balloon visible inside. This finding, when coupled with low urine output, narrows the differential diagnosis of oliguria/anuria to an obstructed Foley catheter (see Figure 17). Please click here to view a larger version of this figure.
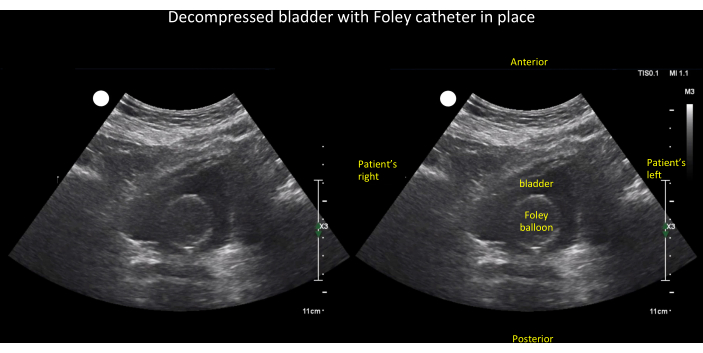
Figure 19: Unlabeled (left panel) and labeled (right panel) transverse view of an empty bladder with Foley balloon visible inside. This finding, when coupled with low urine output, narrows the differential diagnosis of oliguria/anuria to (1) ureteral obstruction, (2) intrinsic kidney, or (3) pre-renal disease (see Figure 17). Please click here to view a larger version of this figure.
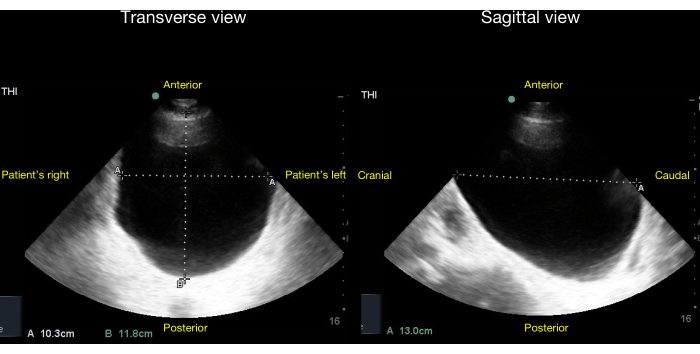
Figure 20: Transverse (left panel) and sagittal views (right panel) of a bladder with no Foley balloon inside and gross bladder distension visible. The volume of urine in the bladder can be quantified by the method described in the Discussion section of this article. After choosing a correction factor of 0.81 for an ellipsoid bladder, the estimated bladder volume in this case = 0.81 x diameter1 x diameter2 x diameter3 = 0.81 * 10cm * 12cm * 13cm = 1,263mL +/- 13%. Please click here to view a larger version of this figure.
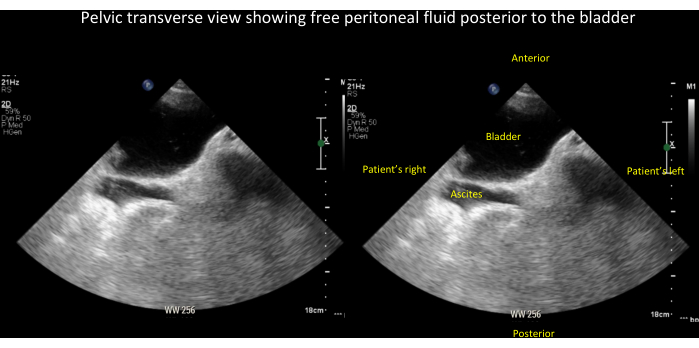
Figure 21: Unlabeled (left panel) and labeled (right panel) images of free peritoneal fluid (in this case, ascites). These are seen posterior to the bladder in the pelvic transverse view (see also Video 15). Please click here to view a larger version of this figure.
Video 1: Fanning through the right kidney in the long view. Please click here to download this Video.
Video 2: Fanning through the right kidney in the short view. Please click here to download this Video.
Video 3: Renal vasculature coming off the aorta. Please click here to download this Video.
Video 4: Renal vasculature with color Doppler. Please click here to download this Video.
Video 5: Kidney in long view with chronic medical renal disease. Note the small size of the kidney. Please click here to download this Video.
Video 6: The right kidney in the coronal plane showing mild hydronephrosis. Please click here to download this Video.
Video 7: The right kidney in the coronal plane showing moderate hydronephrosis, defined by dilation of major and minor calyces, but without cortical thinning. Please click here to download this Video.
Video 8: The right kidney in the coronal plane showing severe hydronephrosis, defined by dilation of major and minor calyces and cortical thinning. Please click here to download this Video.
Video 9: The right kidney in a patient with bilateral extra-renal pelvis. Please click here to download this Video.
Video 10: The right kidney in the longitudinal view with a simple cyst in the center. Please click here to download this Video.
Video 11: The right kidney in the longitudinal view with a larger, simple appearing cyst in the inferior pole of the kidney. Please click here to download this Video.
Video 12: The right kidney in the longitudinal view with a mass in the center. Note that it is not as hypoechoic as the cysts that were previously shown (Video 11). Please click here to download this Video.
Video 13: The right kidney in the coronal plane showing a kidney mass that is arising from the kidney cortex and has disrupted the expected normal oval shape typically seen in this view. Please click here to download this Video.
Video 14: The bladder in a transverse view showing a large, irregular echo density within the bladder lumen that turned out to be a combination of tumor and blood clots. Please click here to download this Video.
Video 15: Pelvic transverse view showing free peritoneal fluid (in this case ascites) posterior to the bladder. See also Figure 21 for a labeled still image of this video. Please click here to download this Video.
Discussion
AKI commonly manifests in critically ill hospitalized patients, amplifying the risk of mortality. To proficiently execute the steps outlined above and differentiate normal from pathologic findings, a comprehensive understanding of normal anatomy and ultrasonographic appearances is essential, along with precise adherence to the protocol's specific steps.
Critical anatomy/steps in the protocol
Kidney– The kidneys are retroperitoneal organs that lie in an oblique coronal plane in the body, requiring the transducer to be positioned obliquely for accurate imaging. To aid in visualizing the left and right kidney, the spleen and liver, respectively, can be used by clinicians as acoustic windows. Further, the left kidney tends to lie in a more cranial and posterior position, whereas the right kidney tends to be more caudal and lateral. Views of true long- and short-axes are essential for precise measurement. Additionally, fanning or tilting through long- and short axes is crucial to fully assess for hydronephrosis, cysts, atrophy, and nephrolithiasis16.
Bladder– The bladder is a fluid-filled structure with a highly variable shape depending on the volume of its contents at any given time and adjacent anatomical structures. For reference, the adult bladder has a capacity of 400-600 mL, starts to signal an urge to avoid at around 150 mL, and activates tension receptors at about 300 mL, at which point a sense of fullness is expected25. Given the bladder's complex and dynamic shape, it is prudent to visualize this organ in both transverse and sagittal planes to estimate the volume of urine present most accurately. The volume can be obtained by multiplying the diameter in all three dimensions by a correction factor for the shape. A cuboid bladder would be closest to a rectangle, so the correction factor is closest to 1.0 at 0.89, followed by an ellipsoid bladder at 0.81 and a triangular-prism-shaped bladder at 0.69. A correction factor of 0.72 is used when the bladder shape does not fit into a predefined prism26.
Limitations and troubleshooting
Kidney – For optimal imaging, the patient is placed in the supine position. If kidney visualization is suboptimal, have the patient roll to the contralateral side, adjusting the probe to the mid-axillary line (on the right) and posterior axillary line (on the left). Note that rib shadows can obscure the image, necessitating probe adjustments (e.g., rotating the probe obliquely so that the ultrasound plane lies between the ribs). A sector array probe (often colloquially referred to as a "phased array") allows imaging between the ribs but may not capture the entire kidney in one view. The curvilinear probe can easily capture the kidney's entire long axis in a single view, but its wide footprint may encounter rib shadows as a trade-off. These same limitations apply equally to handheld ultrasound devices. Ureters are typically challenging to visualize due to bowel gas obstruction, except in the case of hydroureteronephrosis, when the ureters can be visualized as distended tubules extending from the renal pelvis16.
Bladder - Free fluid in the pelvis can easily be mistaken for urine in the bladder (Figure 21 and Video 15). Although this limitation most clearly impacts automated bladder scanners, it is also relevant to greyscale ultrasound assessment of the bladder. POCUS providers should take care to differentiate fluid in the bladder from free peritoneal fluid. Free peritoneal fluid typically lacks a clear border, and fluid in the bladder is confined by a rim of hyperechoic tissue representing the detrusor muscle and bladder wall. Further, one can be misled about the dimensions of the bladder when only visualizing it in a single two-dimensional plane. Although the renal-GU protocol described in this manuscript does not include color or power Doppler, it is occasionally possible to visualize ureteral jets in the bladder using greyscale ultrasound alone. However, POCUS providers should be aware that the presence of such jets does not reliably exclude ureteral obstruction, and their absence does not rule in obstruction16.
Significance
Kidneys – Hydronephrosis is visualized as an anechoic fluid collection within the renal sinus, causing dilation of the major and/or minor calyces. Severity is categorized as mild, moderate, or severe, often correlating with the size of an obstructing kidney stone27. Classically, the terms are defined as follows: (1) mild hydronephrosis entails enlarged major calyces with preserved renal papillae; (2) moderate involves dilation of both major and minor calyces with obliterated papillae; and (3) severe features major and minor calyceal ballooning with cortical thinning28. However, criteria for precise grading of hydronephrosis vary depending on different specialty- and modality-specific guidelines, so in cases when findings appear to overlap two of these categories, it is reasonable to classify hydronephrosis as such. For example, hydronephrosis, which has both mild and moderate features, could be classified as mild-moderate29. Complications of hydronephrosis include calyceal rupture and urine extravasation, posing a heightened infection risk. Hydronephrosis can be either unilateral or (rarely bilateral). The most common etiology is nephrolithiasis ("kidney stones"), but the differential diagnosis also includes ureteral compression, retroperitoneal lymphadenopathy, or bladder outlet obstruction. The absence of hydronephrosis does not exclude nephrolithiasis, as small calculi may not cause significant obstruction16. Hypovolemia may lead to an underestimation of hydronephrosis, making it imperative to repeat imaging post-resuscitation. Conversely, aggressive hydration can create the radiographic appearance of mild hydronephrosis with no clinical consequence. Additionally, various conditions can mimic the ultrasound appearance of hydronephrosis, such as dilated renal vessels, cortical and parapelvic cysts, extra-renal pelvis, and medullary pyramids. Scrutiny of kidney architecture is crucial, and color Doppler can be employed to distinguish between renal vessels and dilated calyces16. In the process of screening for hydronephrosis, POCUS providers may routinely encounter renal cysts. Although renal cysts are usually benign, a subset of them may harbor malignancy. To determine which renal cysts encountered with POCUS require further follow-up, one can employ the Bosniak criteria. While the Bosniak criteria are primarily validated for CT imaging, a conservative application of these criteria is commonly used to categorize renal cysts into two groups: those that require follow-up imaging and those that do not. Cysts that do not need follow-up imaging have ALL of the following benign characteristics: (1) are smooth and thin-walled (wall should be less <2 mm); (2) have no septations, calcifications, internal echoes, or solid elements; (3) are round or oval shaped, well-demarcated from the adjacent parenchyma and appear homogenous in all imaging planes; and (4) generate posterior acoustic enhancement behind the cyst. Any kidney mass or kidney cyst detected incidentally that does not meet the above criteria should be worked up further19,20,21,22.
Bladder – POCUS users should be familiar with several sonographic findings one may encounter in the bladder. Bladder masses often present as irregular, echogenic projections from the bladder wall or as areas of increased thickness. The differential diagnosis for a bladder mass encompasses malignancy, bladder diverticula, congenital outpouchings, blood clots, and bladder wall thickening due to chronic or recurrent cystitis. Normally, the bladder wall thickness ranges from 3 to 6mm but varies with bladder filling16.
Assessing the kidneys and bladder with point-of-care ultrasound holds increasing value for bedside clinicians. Yet, the broader adoption of this POCUS modality is impeded by a lack of standardized training in image acquisition and interpretation7,14,30. To address this educational gap, this narrative review presents a comprehensive framework for renal-GU image acquisition and interpretation from a multi-disciplinary group encompassing acute and chronic care providers from four specialties (internal medicine, nephrology, anesthesiology, and critical care). The resulting protocol holds the potential for enhancing the teaching and learning of renal-GU POCUS across a multitude of bedside care scenarios.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
None.
Materials
| Curvilinear Transducer | Philips | C5-2 USB | 2-5 MHz, also called the abdominal probe |
| Curvilinear Transducer | SonoSite | C5-1 | 1-5 MHz, also called the abdominal probe |
| Edge 1 ultrasound machine | SonoSite | Used to obtain a subset of the Figures and Videos | |
| Phased-Array Transducer | Philips | 1-5 MHz, also called the cardiac probe | |
| Phased-Array Transducer | SonoSite | P5-1 | 1-5 MHz, also called the cardiac probe |
| Ultrasound system | Philips | Affiniti30 | Used to obtain a subset of the Figures and Videos |
Referenzen
- Rewa, O., Bagshaw, S. M. Acute kidney injury – epidemiology, outcomes and economics. Nat Rev Nephrol. 10 (4), 193-207 (2014).
- National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Renal Data System. 2023 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. , (2023).
- Susantitaphong, P., et al. World incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol. 8 (9), 1482-1493 (2013).
- Kellum, J. A., et al. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 26 (9), 2231-2238 (2015).
- Khaled Shawwa, K., et al. Heterogeneity in acute kidney injury management in critically ill patients: National survey. J Nurse Pract. 19 (10), 104776 (2023).
- Diaz-Gomez, J. L., Mayo, P. H., Koenig, S. J. Point-of-care ultrasonography. N Engl J Med. 385 (17), 1593-1602 (2021).
- Koratala, A., Bhattacharya, D., Kazory, A. Point of care renal ultrasonography for the busy nephrologist: A pictorial review. World J Nephrol. 8 (3), 44-58 (2019).
- Fischer, E. A., et al. Hospitalist-operated compression ultrasonography: A point-of-care ultrasound study (hocus-pocus). J Gen Intern Med. 34 (10), 2062-2067 (2019).
- Sibley, S., et al. Point-of-care ultrasound for the detection of hydronephrosis in emergency department patients with suspected renal colic. Ultrasound J. 12 (1), 31 (2020).
- Yaxley, J., Yaxley, W. Obstructive uropathy – acute and chronic medical management. World J Nephrol. 12 (1), 1-9 (2023).
- Rahbari-Oskoui, F., O’Neill, W. C. Diagnosis and management of acquired cystic kidney disease and renal tumors in ESRD patients. Semin Dial. 30 (4), 373-379 (2017).
- Bhutani, H., et al. A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease. Kidney Int. 88 (1), 146-151 (2015).
- Liu, C., Wang, X. Clinical utility of ultrasonographic evaluation in acute kidney injury. Transl Androl Urol. 9 (3), 1345-1355 (2020).
- Wong, J., et al. Barriers to learning and using point-of-care ultrasound: A survey of practicing internists in six north American institutions. Ultrasound J. 12 (1), 19 (2020).
- Ozmen, C. A., et al. Ultrasound as a diagnostic tool to differentiate acute from chronic renal failure. Clin Nephrol. 74 (1), 46-52 (2010).
- Soni, N. J., Arntfield, R., Kory, P. . Point-of-care ultrasound. Second edition. , 229-230 (2020).
- O’Neill, W. C. Sonographic evaluation of renal failure. Am J Kidney Dis. 35 (6), 1021-1038 (2000).
- Koratala, A., Bhattacharya, D. Extra-renal pelvis mimicking hydronephrosis: A case for caution. Clin Case Rep. 5 (10), 1720-1721 (2017).
- Muglia, V. F., Westphalen, A. C. Bosniak classification for complex renal cysts: History and critical analysis. Radiol Bras. 47 (6), 368-373 (2014).
- Silverman, S. G., et al. Bosniak classification of cystic renal masses, version 2019: An update proposal and needs assessment. Radiology. 292 (2), 475-488 (2019).
- Burgan, C. M., Sanyal, R., Lockhart, M. E. Ultrasound of renal masses. Radiol Clin North Am. 57 (3), 585-600 (2019).
- Weber, T. M. Sonography of benign renal cystic disease. Radiol Clin North Am. 44 (6), 777-786 (2006).
- Pei, Y., Watnick, T. Diagnosis and screening of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 17 (2), 140-152 (2010).
- Hines, J. J., Eacobacci, K., Goyal, R. The incidental renal mass- update on characterization and management. Radiol Clin North Am. 59 (4), 631-646 (2021).
- Baldini, G., Bagry, H., Aprikian, A., Carli, F. Postoperative urinary retention: Anesthetic and perioperative considerations. Anesthesiology. 110 (5), 1139-1157 (2009).
- Bih, L. I., Ho, C. C., Tsai, S. J., Lai, Y. C., Chow, W. Bladder shape impact on the accuracy of ultrasonic estimation of bladder volume. Arch Phys Med Rehabil. 79 (12), 1553-1556 (1998).
- Goertz, J. K., Lotterman, S. Can the degree of hydronephrosis on ultrasound predict kidney stone size. Am J Emerg Med. 28 (7), 813-816 (2010).
- Noble, V. E., Brown, D. F. Renal ultrasound. Emerg Med Clin North Am. 22 (3), 641-659 (2004).
- Onen, A. Grading of hydronephrosis: An ongoing challenge. Frontiers in Pediatrics. 8, 458 (2020).
- Koratala, A., Reisinger, N. Point-of-care ultrasound training in nephrology: A leap forward, not merely a check mark. Kidney Med. 6 (1), 100752 (2024).

