Methods for Detecting Cough and Airway Inflammation in Mice
Summary
Here, we describe the measurement of cough using a noninvasive and real-time whole-body plethysmography (WBP) system and the normative procedures for harvesting tissue samples of mice and introduce some methods to assess airway inflammation.
Abstract
Chronic cough, which lasts for more than 8 weeks, is one of the most common complaints requiring medical attention, and patients suffer from a huge socioeconomic burden and a marked decrement in quality of life. Animal models can mimic the complex pathophysiology of the cough and are important tools for cough research. The detection of cough sensitivity and airway inflammation is of great significance for studying the complex pathological mechanism of cough. This article describes the measurement of cough using a noninvasive and real-time whole-body plethysmography (WBP) system and the normative procedures for harvesting tissue samples (including blood, lung, spleen, and trachea) of mice. It introduces some methods to assess airway inflammation, including pathological changes in hematoxylin and eosin (HE)-stained lung and trachea sections, the total protein concentration, the uric acid concentration, and the lactate dehydrogenase (LDH) activity in the supernatant of bronchoalveolar lavage fluid (BALF), and the leukocytes and differential cell counts of BALF. These methods are reproducible and serve as valuable tools to study the complex pathophysiology of cough.
Introduction
Cough is an important defense behavior to maintain airway patency and protect the lungs from potentially harmful substances. However, when dysregulated, cough becomes a pathological condition1. Chronic cough, usually defined as lasting eight or more weeks, is one of the most frequent symptoms requiring medical attention2. Because chronic cough frequently persists for years, patients suffer from a huge socioeconomic burden and a marked decrement in quality of life3,4,5. Chronic cough is widely considered a cough hypersensitivity syndrome and is characterized by troublesome coughing often triggered by low levels of thermal, mechanical, or chemical exposure6. The occurrence of cough hypersensitivity is closely related to airway inflammation7. However, the pathophysiological mechanisms underlying the modulation of cough sensitivity need to be further elucidated.
Animal models can mimic the complex pathophysiology of the cough and are important tools for cough research8,9. Previous studies have found that viral infection, intrapulmonary Interferon-γ (IFN-γ) instillation, esophageal perfusion of hydrochloric acid, pollutant exposure, cigarette smoke, and citric acid can induce cough in animals10,11,12,13,14,15,16,17. In order to better evaluate cough and airway inflammation, a mouse model of cough was established using a nonlethal dose of the H1N1 virus in this study. For the detection of cough, some cough measurement tools have been established clinically to measure cough, including subjective and objective methods18. The subjective evaluation tools to assess the cough severity primarily include a visual analog scale, the cough score, and quality of life questionnaires, etc19,20. However, they are unlikely to be used to assess cough in animals. In addition, cough can be objectively assessed through a cough challenge test and cough frequency monitoring. The cough challenge test using a whole-body plethysmography (WBP) system is an objective method widely used in animal studies to measure cough sensitivity and reveal the underlying mechanisms of cough13,16. Based on the neuroanatomical characteristics of the cough reflex, citric acid, capsaicin, adenosine 5'-triphosphate (ATP), allyl isothiocyanate (AITC), and inflammatory mediator bradykinin are commonly used as the tussive agents to induce cough21,22. Citric acid is one of the earliest and the most widely used tussive agents triggering cough reflexes, which has been validated for measuring cough sensitivity. Besides, the citric acid challenge has good safety, feasibility, and tolerability and is suggested to assess cough reflex sensitivity in response to cough therapies23. Therefore, this article will describe the method for measuring cough sensitivity in response to citric acid in mice using a noninvasive and real-time WBP system.
The studies of the pathophysiology of cough require test samples, including samples from the blood, bronchoalveolar lavage fluid (BALF), and lung and trachea tissues to verify changes in the levels of key factors24. Currently, there is a lack of normative procedures for harvesting tissue samples of mice, and related studies employ different approaches that complicate the assessment of airway inflammation. Bronchoalveolar lavage is an important method to evaluate airway inflammation in respiratory diseases25. Different methods of bronchoalveolar lavage will lead to a lack of comparability between related studies. Moreover, different bronchoalveolar lavage methods have an impact on inflammatory cells and inflammatory cytokines in BALF. Therefore, this article will describe the establishment of a mouse model of cough with a nonlethal dose of the H1N1 virus, the measurement of cough using a WBP system, and a reliable, safe, and highly successful bronchoalveolar lavage method of mice.
Protocol
All the procedures were approved by the Animal Care and Use Committee of Guangzhou Medical University (20240248) and were performed in strict accordance with approved guidelines. Male specific pathogen-free C57BL/6 mice weighing 20-25 g were used in this study. All mice were housed under controlled temperature (22 ± 2 °C), humidity (50% ± 20%), and lighting (6:30 AM to 6:30 PM) in solid bottom cages with food and water available ad libitum. The time line of the protocol is shown in Figure 1.
1. Establishment of a mouse model of cough
- Use influenza A/California/7/2009 (H1N1) virus. Determine the lethal dose of the H1N1 virus in mice.
- Briefly, anesthetize mice (n = 10 per group) with pentobarbital sodium (80 mg·kg-1) and then intranasally infect with a 10-fold serially diluted virus.
- Monitor the death over a period of 15 days. Calculate the median lethal dose (LD50) by the Reed-Muench method26.
- Infect the mouse with the H1N1 virus.
- Dissolve 0.8 x LD50 of H1N1 virus in 50 µL of phosphate-buffered saline (PBS).
- Anesthetize the mice with pentobarbital sodium (80 mg·kg-1).
- When the mice were deeply anesthetized, place the mouse in a supine position with the nostrils facing upward.
- Instill 5-10 µL of H1N1 virus solution or PBS solution (as a control) intranasally into one nostril of mice using a pipette (Figure 2A).
- Then, hold the mouse's mouth closed with the thumb to make its nose inhale hard so that the H1N1 virus solution in the nasal cavity is completely inhaled into the lungs (Figure 2B).
- Repeat steps 1.2.4 and 1.2.5 in the other nasal cavity of the mice.
- When all H1N1 virus prepared in step 1.2.1 is intranasally instilled into the mouse's lungs, place it in the supine position for rest (Figure 2C).
- Measure the cough sensitivity of the mouse after model establishment using the Buxco small animal whole-body plethysmography system. On day 21, intraperitoneally anesthetize the mouse with pentobarbital sodium (150 mg·kg-1). Collect and process blood, spleen, BALF, lung, and trachea tissues (Figure 1).
2. The cough sensitivity measurement
- Prepare citric acid (0.4 M): Place 0.1537 mg of citric acid into a 5 mL centrifuge tube and add normal saline to a volume of 2 mL.
- Instrument inspection
- Check the channels: Connect the whole-body plethysmograph chambers according to the manufacturer's instructions and click the calibration button-the calibration button changes from orange to green, indicating that calibration is successful (Figure 3A).
- Calibrate the nebulizer:
- After connecting the nebulizer, add 500 µL of normal saline into the nebulizer and click the nebulization button.
- When the liquid in the nebulizer is completely aerosolized, click the nebulization button again to see the power of the nebulizer, which is generally about 0.3 mL/min. Click the nebulization button again to accept the current nebulized power.
- After checking the channels, ensure the error value is less than 0.5%.
- Setting parameters
- Click Create New Study, select the Cough option, select Mouse in Species, and click Next.
- Select the CCnt parameters: Set the duration of the acclimation period to 1 min, the response time to 10 min, the aerosol volume to 1 mL, and the delivery duration to 10 min.
- Place conscious unrestrained mouse in individual plastic transparent whole-body plethysmograph chambers. Input the Weight and Subject ID of the mouse and click Next.
- Add 1 mL of citric acid (0.4 M) solution to the nebulizer and observe real-time changes in CCnt (Figure 3B).
- After citric acid is completely used, click File and End Session to complete the experiment.
3. Harvesting of blood, spleen, BALF, lung, and trachea tissues of mouse (Figure 4)
- Collect blood.
- After cough detection, anesthetize the mouse with pentobarbital sodium (150 mg·kg-1) by intraperitoneal injection. Collect blood from the orbits of the deeply anesthetized mouse. Mix 1 mL of blood in 0.1 mL of anticoagulant buffer (9.9 mg/mL heparin sodium dissolved in PBS) at 4 °C (Figure 4A).
- Shake the blood collection tube to fully mix the blood and anticoagulant to prevent blood coagulation.
- Centrifuge the collected blood at 800 x g for 5 min at 4 °C. Collect the supernatant, store it at -80 °C (used for cytokine measurement), and resuspend the pellet in 1 mL of D-Hank's solution. Spread 10 µL of the blood cell suspension on the glass slide to determine cell profiles.
- Harvest the spleen.
- Open the mouse's chest, collect the blood, and exsanguinate via the artery (Figure 4B).
- Remove the left auricle, and then perfuse the pulmonary and systemic circulation with 5 mL of normal saline (Figure 4C, D). Remove the whole spleen from the mouse using surgical forceps (Figure 4E).
- After measuring the weight of the spleen, cut it into two halves. Fix the first half with 4% paraformaldehyde at room temperature (RT) for histopathological analysis. Store the second half at -80 °C for cytokine measurement.
- Bronchoalveolar lavage
- Make the bronchoalveolar lavage tube using a Pasteur pipet. Heat the Pasteur pipette using an alcohol lamp. When it becomes soft, lengthen it to form a thin tube. The length of the bronchoalveolar lavage tube is 5 cm. The upper diameter is 5 mm, and the lower diameter is 1 mm (Figure 5).
- For BALF collection, separate the right lung by ligating at the right mainstem bronchus. Collect the BALF from the left lungs by lavaging three times with 0.5 mL of PBS pre-cooled on ice. The recovery ratio of BALF is more than 80% (Figure 4F).
- Centrifuge the collected BALF at 800 x g for 5 min at 4 °C. Collect the supernatant and measure the total protein concentration, uric acid concentration, LDH activity, and cytokine concentrations.
NOTE: The inflammatory markers, including total protein concentration, the uric acid concentration, and the LDH activity in the BALF supernatant, are detected using the assay kit according to the manufacturer's guidelines24. - Resuspend the pellet in 200 µL of PBS, and count the leukocytes in BALF with the help of a counting slide.
- To determine cell profiles by differential counts, smear 50 µL of the cell suspension onto the glass slides and allow it to dry.
- Fix the slide with 4% paraformaldehyde overnight, and then stain with the hematoxylin-eosin (HE). Classify at least 400 cells into neutrophils, macrophages, lymphocytes, or eosinophils per slide.
- Harvesting the lung and trachea tissues for histopathological analysis
- After BALF collection, remove and fix half of the right lung (Figure 4G) and trachea (Figure 4H) with 4% paraformaldehyde at RT for histopathological analysis. Store the other half at -80 °C for western blotting, Real-time quantitative polymerase chain reaction (qPCR), and Enzyme-linked immunosorbent assay (ELISA).
Representative Results
Figure 6 shows representative images of pathological changes in HE-stained lung (Figure 6A,B), trachea (Figure 6C,D), and spleen (Figure 6E,F). H1N1 virus infection resulted in inflammatory changes in mouse lungs, including edema and many lymphocytes and neutrophil infiltration. H1N1 virus infection also induced inflammatory changes in mouse tracheae, including cilia shedding and inflammatory cell infiltrations (many lymphocytes and small numbers of neutrophils). In addition, the infection also significantly increased the ratio of the white pulp area to the whole spleen area of mice. Lymphocytes accumulate in the white pulp of the spleen. This standardized procedure for harvesting tissue samples can better evaluate airway inflammation.
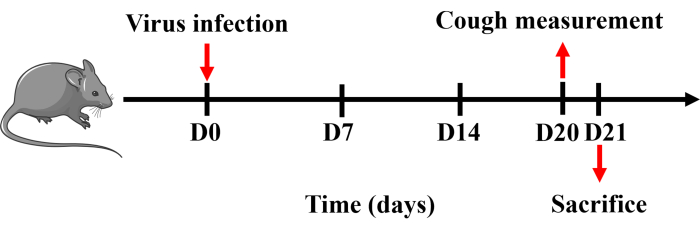
Figure 1: Protocol for establishing a mouse model of cough. Mice were anesthetized with pentobarbital sodium and intranasally instilled with 0.8 x LD50 of H1N1 virus dissolved in 50 µL of PBS once on day 0. Cough measurement was performed on day 20, and mice were sacrificed the following day. Please click here to view a larger version of this figure.
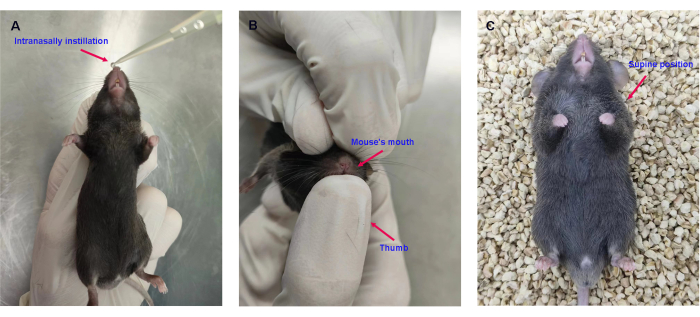
Figure 2: Intranasal instillation of the H1N1 virus. (A) Intranasal instillation of 0.8 x LD50 of H1N1 virus in 50 µL of PBS. (B) Holding the mouse's mouth closed with the thumb. (C) Placing the mice in the supine position. Please click here to view a larger version of this figure.
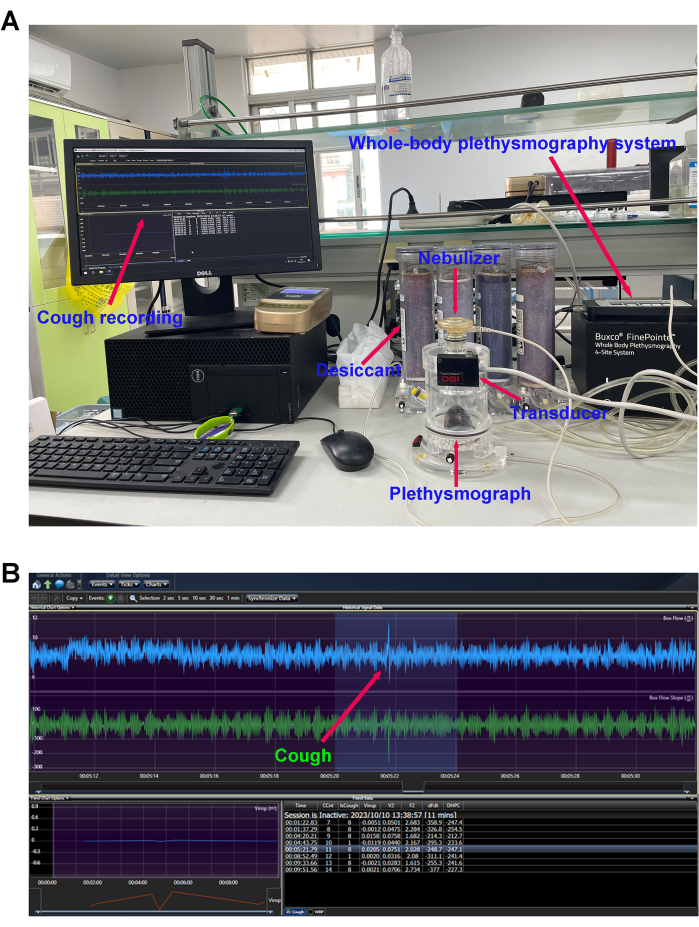
Figure 3: Cough sensitivity measurement. (A) Mouse cough detection equipment and (B) cough reflex curve. The number of cough events in response to the nebulized citric acid solution (0.4 M) was detected using the whole-body plethysmography (WBP) system after modeling. Please click here to view a larger version of this figure.
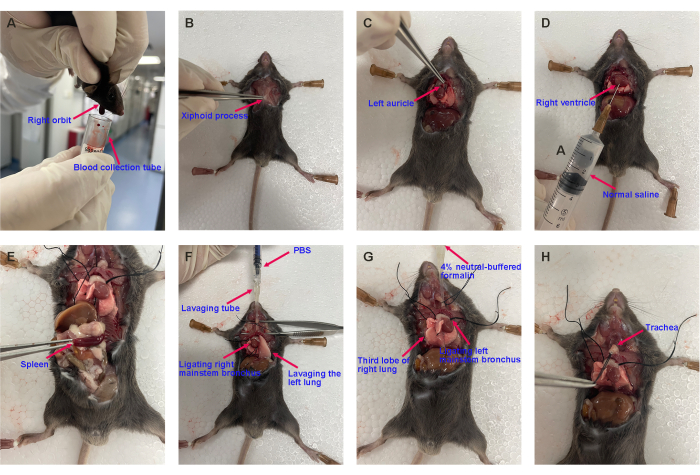
Figure 4: Representative images of harvesting the mouse's blood, spleen, BALF, lung, and trachea. (A) Blood collection, (B) opening the chest, (C) cutting off the left auricle, (D) pulmonary circulation perfusion, (E) harvesting the spleen, (F) bronchoalveolar lavage, and (G) harvesting the lung lobe and (H) the trachea for histopathological analysis. Please click here to view a larger version of this figure.
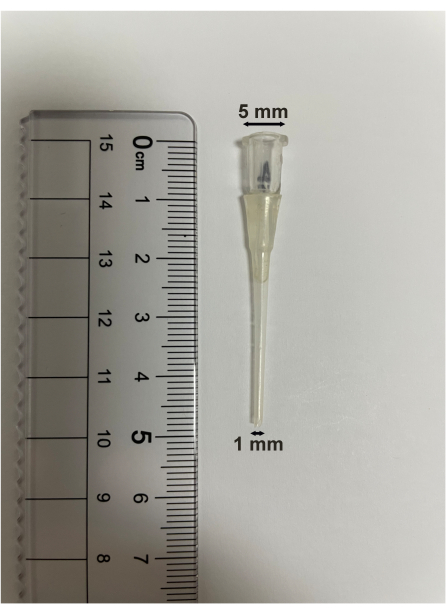
Figure 5: Bronchoalveolar lavage tube specification. The length of the bronchoalveolar lavage tube is 5 cm. The upper diameter is 5 mm, and the lower diameter is 1 mm. Please click here to view a larger version of this figure.
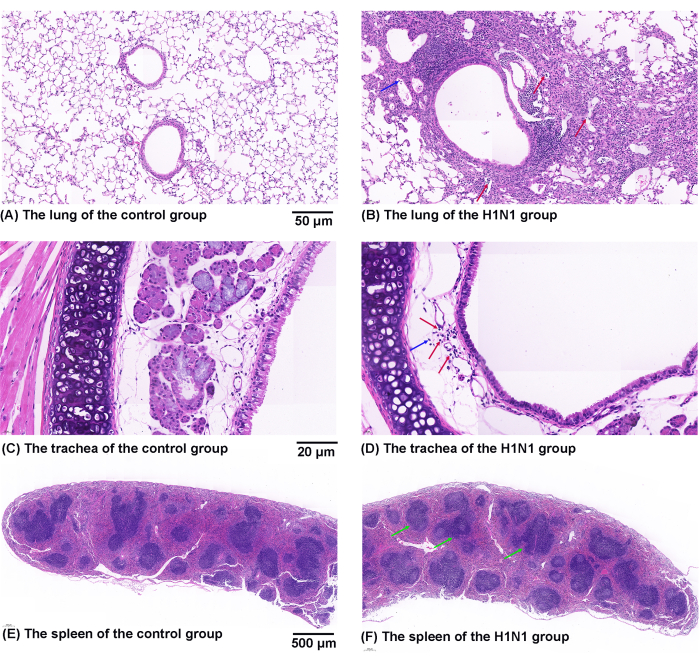
Figure 6: Effects of H1N1 virus on pathological changes in the mouse's lung, trachea, and spleen. (A,B) Representative figures of pathological changes in HE-stained lung sections from the (A) control and (B) H1N1 groups. The symbol of "↑" marks the infiltration of lymphocytes (red) and neutrophils (blue). Scale bars: 50 µm. (C,D) Representative figures of pathological changes in HE-stained trachea sections from the (C) control and (D) H1N1 groups. The symbol of "↑" marks the infiltration of lymphocytes (red) and neutrophils (blue). Scale bars: 20 µm. (E,F) Representative figures of pathological changes in HE-stained spleen sections from the (E) control and (F) H1N1 groups. The symbol of "↑" marks the white pulp (green). Scale bars: 500 µm. Please click here to view a larger version of this figure.
Discussion
Some chronic refractory and postinfectious coughs are common conditions associated with respiratory virus infection27. In order to better evaluate cough sensitivity and airway inflammation, a mouse model of cough was established using the H1N1 virus in this study. Appropriate mouse cough models should be selected for other studies according to the purpose of the study. Most previous studies used the guinea pig as an animal model in mechanistic studies or new drug trials for cough28,29,30. Recent studies have suggested that mice might be used to assess cough pathophysiology owing to their shorter reproductive cycle, more reagents, and readiness for genetic manipulations despite their cough behavior still being debated16,31. The citric acid was used as a tussive agent to induce cough in this study. The mechanisms of cough reflex induced by citric acid may be related to the activation of jugular C-fibers and nodose Aδ-fibers32. Besides, the WBP system measures the changes in cough reflex in mice in a noninvasive manner and minimizes the effects of psychological stress. The effect of the external environment on mice should be minimized when cough sensitivity is detected. The mouse should be placed in the testing room and then covered with a plastic bag to reduce the irritation caused by the external environment.
This study details the normative procedures for harvesting blood, spleen, BALF, lung, and trachea tissues of mice and introduces some measurements to assess airway inflammation. To detect airway inflammation, the lung and trachea sections are stained with hematoxylin and eosin to assess general histopathology24. The increased concentration of total protein and uric acid in the supernatant of BALF are associated with inflammation and cell injury in the airway33. LDH activity in the supernatant of BALF reflects cell damage and necrosis34. The leukocytes in BALF and blood can reflect the degree of inflammation of the disease35. Differential cell counts of BALF are widely used in assessing airway inflammation in chronic airway diseases and provide important information in studying pathogenesis, making diagnoses, and management strategies for chronic respiratory diseases36.
The limitation of this experiment is that collecting a large number of tissue samples makes mice be operated for a long time, which may affect the activity of the tissue samples. Therefore, mouse tissue samples need to be placed on ice immediately after collection. In addition, the bronchoalveolar lavage tube used in this study is suitable for mice but not for bigger animals.
In summary, we provide a detailed description of the detection methods of cough and airway inflammation in mice. These methods provide researchers with tools to study the complex pathophysiology of cough.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Guangzhou Science and Technology Planning Project (202002030151), the Major Project of Guangzhou National Laboratory (GZNL2024A02001), and the grant of State Key Laboratory of Respiratory Disease (SKLRD-Z-202202).
Materials
| 4% paraformaldehyde | Biosharp | BL539A | |
| Buxco Small Animal Whole Body Plethysmography System | DSI | — | |
| Calcium-free and magnesium-free Hank’s Balanced Salt Solution | Beyotime | C0219 | |
| Citric acid | Sigma-Aldrich | C2404 | |
| Hematoxylin-Eosin | BASO Biotechnology | BA-4098 | |
| Heparin sodium | Alfa Aesar | A16198 | |
| Influenza A/California/7/2009 (H1N1) virus | ATCC | VR-1894 | |
| Isoflurane | RWD | R510-22 | |
| Lactate dehydrogenase assay kit | Nanjing Jiancheng Bioengineering Institute | A020-2-2 | |
| Normal saline | Guangzhou Zhongbo Biotechnology | 1234-1 | |
| Pasteur pipet | NEST | 318415 | |
| Pentobarbital sodium | Merck | P3761 | |
| Phosphate buffered saline | Meilunbio | MA0015 | |
| Total protein assay kit | Nanjing Jiancheng Bioengineering Institute | A045-3 | |
| Uric acid assay kit | Thermo Fisher Scientific | A22181 |
Referenzen
- Brooks, S. M. Perspective on the human cough reflex. Cough. 7, 10 (2011).
- Lai, K., Long, L. Current status and future directions of chronic cough in china. Lung. 198 (1), 23-29 (2020).
- Zeiger, R. S., et al. Patient-reported burden of chronic cough in a managed care organization. J Allergy Clin Immunol Pract. 9 (4), 1624-1637.e10 (2021).
- Marchant, J. M., et al. What is the burden of chronic cough for families. Chest. 134 (2), 303-309 (2008).
- Chamberlain, S. A., et al. The impact of chronic cough: A cross-sectional european survey. Lung. 193 (3), 401-408 (2015).
- Morice, A. H., et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J. 44 (5), 1132-1148 (2014).
- Chung, K. F., et al. Cough hypersensitivity and chronic cough. Nat Rev Dis Primers. 8 (1), 45 (2022).
- Deng, Z., et al. Pulmonary IFN-γ causes lymphocytic inflammation and cough hypersensitivity by increasing the number of IFN-γ-secreting t lymphocytes. Allergy Asthma Immunol Res. 14 (6), 653-673 (2022).
- Hiramatsu, Y., et al. The mechanism of pertussis cough revealed by the mouse-coughing model. mBio. 13 (2), e0319721 (2022).
- Lin, L., et al. The duration of cough in patients with H1N1 influenza. Clin Respir J. 11 (6), 733-738 (2017).
- Deng, Z., et al. IFN-γ enhances the cough reflex sensitivity via calcium influx in vagal sensory neurons. Am J Respir Crit Care Med. 198 (7), 868-879 (2018).
- Chen, Z., et al. Dorsal vagal complex modulates neurogenic airway inflammation in a guinea pig model with esophageal perfusion of HCl. Front Physiol. 5, 536 (2018).
- Zhi, H., et al. Gabapentin alleviated the cough hypersensitivity and neurogenic inflammation in a guinea pig model with repeated intra-esophageal acid perfusion. Eur J Pharmacol. 959, 176078 (2023).
- Fang, Z., et al. Traffic-related air pollution induces non-allergic eosinophilic airway inflammation and cough hypersensitivity in guinea-pigs. Clin Exp Allergy. 49 (3), 366-377 (2019).
- Xiang, J., et al. Fructus mume protects against cigarette smoke induced chronic cough guinea pig. J Med Food. 23 (2), 191-197 (2020).
- Chen, Z., et al. A descending pathway emanating from the periaqueductal gray mediates the development of cough-like hypersensitivity. iScience. 25 (1), 103641 (2022).
- Chen, Z., et al. Glial activation and inflammation in the nts in a rat model after exposure to diesel exhaust particles. Environ Toxicol Pharmacol. 83, 103584 (2021).
- Mai, Y., et al. Methods for assessing cough sensitivity. J Thorac Dis. 12 (9), 5224-5237 (2020).
- Lee, K. K., et al. A longitudinal assessment of acute cough. Am J Respir Crit Care Med. 187 (9), 991-997 (2013).
- Birring, S. S., et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester cough questionnaire (LCQ). Thorax. 58 (4), 339-343 (2003).
- Mazzone, S. B., Farrell, M. J. Heterogeneity of cough neurobiology: Clinical implications. Pulm Pharmacol Ther. 55, 62-66 (2019).
- Morice, A. H., Kastelik, J. A., Thompson, R. Cough challenge in the assessment of cough reflex. Br J Clin Pharmacol. 52 (4), 365-375 (2001).
- Nurmi, H. M., Lätti, A. M., Brannan, J. D., Koskela, H. O. Comparison of mannitol and citric acid cough provocation tests. Respir Med. 158, 14-20 (2019).
- Ding, W., et al. Amg487 alleviates influenza a (H1N1) virus-induced pulmonary inflammation through decreasing IFN-γ-producing lymphocytes and IFN-γ concentrations. Br J Pharmacol. 181 (13), 2053-2069 (2024).
- Connett, G. J. Bronchoalveolar lavage. Paediatr Respir Rev. 1 (1), 52-56 (2000).
- Wu, X., et al. Correlation of adhesion molecules and non-typeable haemophilus influenzae growth in a mice coinfected model of acute inflammation. Microbes Infect. 23 (8), 104839 (2021).
- Capristo, C., Rossi, G. A. Post-infectious persistent cough: Pathogenesis and therapeutic options. Minerva Pediatr. 69 (5), 444-452 (2017).
- Kollarik, M., Brozmanova, M. Cough and gastroesophageal reflux: Insights from animal models. Pulm Pharmacol Ther. 22 (2), 130-134 (2009).
- Driessen, A. K., et al. A role for neurokinin 1 receptor expressing neurons in the paratrigeminal nucleus in bradykinin-evoked cough in guinea-pigs. J Physiol. 598 (11), 2257-2275 (2020).
- Ruhl, C. R., et al. Mycobacterium tuberculosis sulfolipid-1 activates nociceptive neurons and induces cough. Cell. 181 (2), 293-305.e211 (2020).
- Chen, L., Lai, K., Lomask, J. M., Jiang, B., Zhong, N. Detection of mouse cough based on sound monitoring and respiratory airflow waveforms. PLoS One. 8 (3), e59263 (2013).
- Wallace, E., Guiu Hernandez, E., Ang, A., Hiew, S., Macrae, P. A systematic review of methods of citric acid cough reflex testing. Pulm Pharmacol Ther. 58, 101827 (2019).
- Ding, W., et al. Intrapulmonary ifn-γ instillation causes chronic lymphocytic inflammation in the spleen and lung through the CXCR3 pathway. Int Immunopharmacol. 122, 110675 (2023).
- Liu, B., et al. Anti-IFN-γ therapy alleviates acute lung injury induced by severe influenza a (H1N1) pdm09 infection in mice. J Microbiol Immunol Infect. 54 (3), 396-403 (2021).
- Domagała-Kulawik, J. Bal in the diagnosis of smoking-related interstitial lung diseases: Review of literature and analysis of our experience. Diagn Cytopathol. 36 (12), 909-915 (2008).
- Miyata, Y., et al. The effect of bronchoconstriction by methacholine inhalation in a murine model of asthma. Int Arch Allergy Immunol. 181 (12), 897-907 (2020).

.
