Anterior High-Resolution Optical Coherence Tomography in the Diagnosis and Therapeutic Monitoring of Ocular Surface Squamous Neoplasia
Summary
Anterior segment high-resolution optical coherence tomography (HR-OCT) is a promising non-invasive modality for the diagnosis and therapeutic evaluation of ocular surface squamous neoplasia (OSSN). Here, the system setup, scanning technique, and representative diagnostic results are presented.
Abstract
Ocular surface squamous neoplasia (OSSN) is the most common tumor of the ocular surface, ranging from mild dysplasia to invasive squamous carcinoma. Traditionally, the diagnosis of OSSN relies on histopathological confirmation followed by a full-thickness biopsy. However, in the past two decades, the therapeutic approach to OSSN has shifted from surgical intervention to topical chemotherapy regimens in clinical settings. This shift emphasizes the need for less invasive or non-invasive methods to diagnose ocular surface pathologies. Among various imaging devices, commercially available high-resolution optical coherence tomography (HR-OCT) has emerged as a powerful tool for the characterization of OSSN. HR-OCT provides an in vivo, cross-sectional view of ocular surface lesions, offering an “optical biopsy” for OSSN with high sensitivity and specificity. It provides valuable information in differentiating intraepithelial or invasive OSSN from other benign lesions. Additionally, HR-OCT can be used to monitor the response to topical chemotherapy and to detect subclinical OSSN during follow-up visits. In this article, the scanning protocol for image acquisition is presented, and image interpretation for OSSN is outlined. This standardized, practical, and reproducible approach is recommended in clinical workflows and is expected to assist clinicians in the management of OSSN.
Introduction
Ocular surface squamous neoplasia (OSSN) is the most common non-pigmented tumor of the conjunctiva and cornea. The term OSSN encompasses a broad spectrum of squamous neoplastic changes, including dysplasia (graded I-III), intraepithelial neoplasia (i.e., carcinoma in situ, CIS), and invasive squamous cell carcinoma (SCC)1. The diagnosis of OSSN can be made clinically by slit lamp examination by detecting the typical appearance of elevated, leukoplakic or papilliform mass, often presented at limbus straddling conjunctiva to the cornea with feeder vessels2. Sometimes, they may present less distinctively. The gold standard for the diagnosis of OSSN remains histopathologic confirmation followed by incisional or excisional biopsy1,3.
Recently, the therapeutic pattern of OSSN has shifted from surgical management to the use of topical chemotherapy. This has greatly promoted the adoption of biopsy to less or non-invasive modalities4. Various imaging devices have been studied for the characterization of OSSN, namely HR-OCT, in vivo confocal microscopy (IVCM)5, impression cytology (IC)6, ultrasound biomicroscopy (UBM)7, and methylene blue stain4. Currently, several studies have provided insights into the characterization of OSSN using anterior segment HR-OCT. Commercially available HR-OCT devices using spectral-domain technology can attain an axial resolution of approximately 5 µm. These images provide in vivo, cross-sectional views of ocular surface lesions and display distinct features of OSSN that parallel the changes seen in histopathology. Hence, HR-OCT enables clinicians to obtain an “optical biopsy” to expediently diagnose and monitor OSSN in clinics.
To promote the application of HR-OCT in the management of OSSN, a standardized, practical, and reproducible guidance for image acquisition is presented in detail to ensure good quality for clinical use. Also, characteristics of OSSN and other common benign lesions on HR-OCT are elucidated for better image interpretation.
Protocol
All the protocols described below follow the guidelines of the Human Research Ethics Committee of the First Affiliated Hospital of Harbin Medical University and adhere to the tenets of the Declaration of Helsinki. The approval number is 2023IIT008. Written, informed consent was obtained from all study participants. The study included participants who presented with conjunctival masses. Exclusion criteria for enrollment included females who were pregnant or breastfeeding and conditions that prevented performing study investigations. Participants who met the inclusion criteria underwent a complete ophthalmological examination by an ocular surface specialist (CLK). AS-OCT was performed at the initial and follow-up visits, with impression cytology and/or biopsy for histology at the initial visit or the time of surgery.
1. Slit lamp examination and imaging procedure
- Ask the subject to sit behind the slit lamp. Explain the testing procedures to the subject.
- Edit the subject's information.
- Disinfect the setup: Wipe down the slit lamp head and chinrest with an alcohol swab.
- Position the subject for examination.
- Ask the subject to place their chin on the chinrest. Instruct the subject to rotate their eyeball to fully expose the lesion. Observe the lesion under the slit lamp.
- Note details of the lesion during the clinical examination, such as laterality, appearance, quadratic location, extent of ocular surface involvement, dimensions of the lesion, presence of keratin, pigmentation, intrinsic vascularity, feeder vessels, cystoid spaces, and scleral involvement.
- Flip the eyelids to observe whether fornical or palpebral conjunctiva are affected.
- Perform anterior segment photography with the slit lamp to document clinical features.
2. Imaging in the HR-OCT device
NOTE: The following is a general procedure for acquiring anterior segment OCT images scanned by the Fourier-domain optical coherence tomography system (see Table of Materials), which has an axial resolution of 5 µm, transverse resolution of 8 µm, and a scanning speed of 26,000 A-scans/s. To ensure image quality, spectral-domain OCT with shorter wavelengths of light, such as HR-OCT (in-depth resolution of 5-7 µm) or ultra-high-resolution OCT (in-depth resolution of about 2-3 µm), is recommended to better visualize the features of ocular lesions.
- Ask the subject to sit behind the imaging device. Explain the testing procedures to the subject.
- Double-click on the RTVue icon to start the.
- Edit the subject's information.
- Click on the New Patient button to create a new patient. Fill out the information fields, such as last name, first name, gender, birth date, and ethnicity. Click on the Save button.
- Disinfect the setup: Wipe down the OCT head and chinrest with an alcohol swab.
- Select the desired scan pattern by clicking on the Cornea < Cross Line button.
- Attach the anterior segment lens to the system scanner.
- Select the eye(s) to be scanned according to the anterior segment slit lamp photography.
- Position the subject for imaging.
- Ask the subject to place their chin on the chinrest. Instruct the subject to roll the eyeball to fully expose the ocular surface lesion.
- Align the lesion of interest with the center of the scan. Click on the Scan tab.
- Press Auto P tab to enhance signal strength. Adjust the scanner head to get the best ocular image. Capture the image using the 8×8 mm automated segmentation OCT scan.
- Review the OCT slices. Click the Save tab to save the scan.
3. Determining the region of interest (ROI)
- Observe the dynamic sectional view during alignment. Align the cross line with the thickest point of the lesion and capture the scan.
- Pay attention to the adjacent parts of normal and abnormal tissue. Capture the border of the lesion.
4. OCT image analysis
NOTE: Evaluate the AS-OCT parameters, such as lesion location (epithelium, subepithelium, or sclera), epithelium reflectivity and thickness, abrupt transition zone from normal to abnormal tissue, visibility of the separation zone between epithelial and subepithelial layers, lesion uniformity, cystoid spaces presence, back-shadowing presence, tumor's posterior extent delineation, and underlying sclera visibility.
- Open the system software and navigate to the Measuring Tools section. Click on the View B-Scans < measuring tool.
- Within the Measuring Tools, select the Distance Tool < measuring tool. Measure the maximal epithelial and sub-epithelial thickness values of the lesion.
- Begin by choosing an initial point on the anterior epithelium boundary, then move vertically to the visible separation between the epithelial and subepithelial layers. Save the maximal area measurement data as epithelial thickness.
- Next, select an anchor point on the visible separation between the epithelial and subepithelial layers, then proceed vertically to the posterior border of the lesion (typically the anterior boundary of the sclera). Save the maximal area measurement data as subepithelial thickness.
- Click on Snapshot Tool < measuring tool to export a .jpg image of the measurement report screen.
5. Follow up procedures
NOTE: Perform OCT examinations monthly during topical chemotherapy and at each follow-up visit following surgical resection and medical or para-surgical treatments.
- Slit lamp examination (repeat step 1): Observe and document the changes in the lesion.
- Perform AS-OCT examination.
- Open the system software and locate the existing patient by typing their Name or Birth Date in the "Search By" space. Input the patient's relevant information and click on the Search button.
- Once the patient is located, click on their name to list all the visits. Choose the "latest visit" and review the OCT images.
- Click on the SCAN button to obtain the current AS-OCT image and assess the parameters on AS-OCT (repeat step 2 to Step 4).
Representative Results
Figure 1A,B illustrate HR-OCT images of OSSN, revealing three key characteristics: (1) Significantly thickened hyper-reflective epithelium; (2) An abrupt shift from normal to abnormal epithelium, marked by a sudden rise in both brightness and thickness of the epithelium; (3) Occasionally, a division plane is visible between the epithelium and underlying tissue. However, in very thick lesions, the lower edge of the lesion may be somewhat obscured by shadowing.
The sub-epithelial tissue is usually of normal thickness in simple OSSN lesions. However, unlike OSSN, the HR-OCT findings of other ocular lesions like pterygia, pseudopterygium, and dermoid often reveal normal to slightly thickened epithelium accompanied by dense, hyperreflective thickened subepithelial mass (Figure 1C–H). The corneal epithelium is usually dark and thin, while that of the conjunctiva is thin but mildly hyper-reflective and sometimes intense as well, which may be caused by actinic changes. These unique characteristics were always parallel to the changes seen on histopathologic examination, which makes HR-OCT an important modality for differentiating OSSN from various ocular surface pathologies8.
HR-OCT can also be used to monitor the chemotherapeutic response of OSSN. During topical chemotherapeutic treatment, a decrease in epithelial thickness and hyperreflectivity can be seen after 2 cycles of topical chemotherapy, which indicates the time point of clinical regression of OSSN, which can help clinicians determine the therapy duration (Figure 2). In addition, the restoration of normal anatomy can also be detected during follow-up visits.
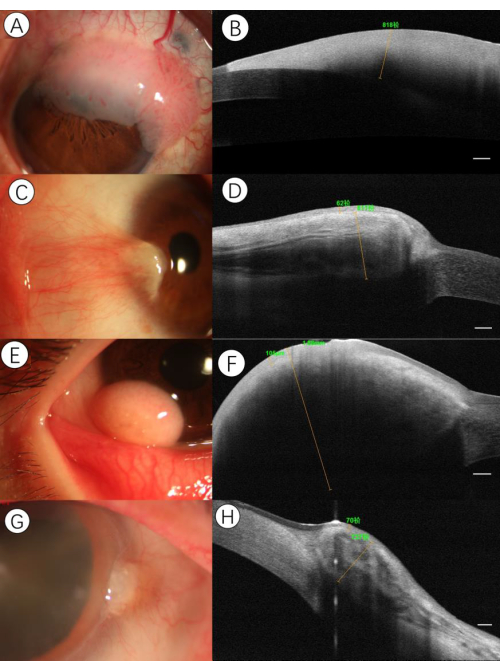
Figure 1: Sample imaging of various ocular surface lesions. (A) Slit-lamp photograph demonstrating gelatinous OSSN. A 58-year-old male presented with a limbal lesion in the upper quadrant of the left eye. (B) HR-OCT disclosed a well-circumscribed, severely hyper-reflective epithelium with a shadowing effect. An abrupt transition between normal and thickened hyperreflective epithelium could be seen, as well as a plane of cleavage between the lesion and the underlying tissue. The maximal epithelial thickness value of the lesion was over 818 µm. (C) Slit-lamp photograph demonstrating pterygia. A 36-year-old female presented with a conjunctival and corneal lesion consistent with pterygium. (D) HR-OCT disclosed a thickened sub-epithelial lesion with a striped reflection, which likely corresponded to the vasculature and the overlying thin epithelium separated by a plane of cleavage. The maximal epithelial and sub-epithelial thickness values of the lesion were 62 µm and 811 µm, respectively. (E) Slit-lamp photograph demonstrating lipodermoid. A 13-year-old male presented with an adipose-like lesion in the nasal quadrant of the left eye. (F) HR-OCT disclosed a thin epithelium with an underlying severely thickened uniformed tissue with shadowing. The maximal epithelial and sub-epithelial thickness values of the lesion were 105 µm and 1.88 mm, respectively. (G) Slit-lamp photograph demonstrating pseudopterygium. A 70-year-old female presented with a raised conjunctival lesion at the limbus in the nasal portion of the right eye. (H) HR-OCT disclosed a moderately hyperreflective epithelium with a thickened lesion in the substantia propria intermixed with a hyporeflective cluster area. The maximal epithelial and sub-epithelial thickness values of the lesion were 70 µm and 737 µm, respectively. Scale bars: 250µm. Please click here to view a larger version of this figure.
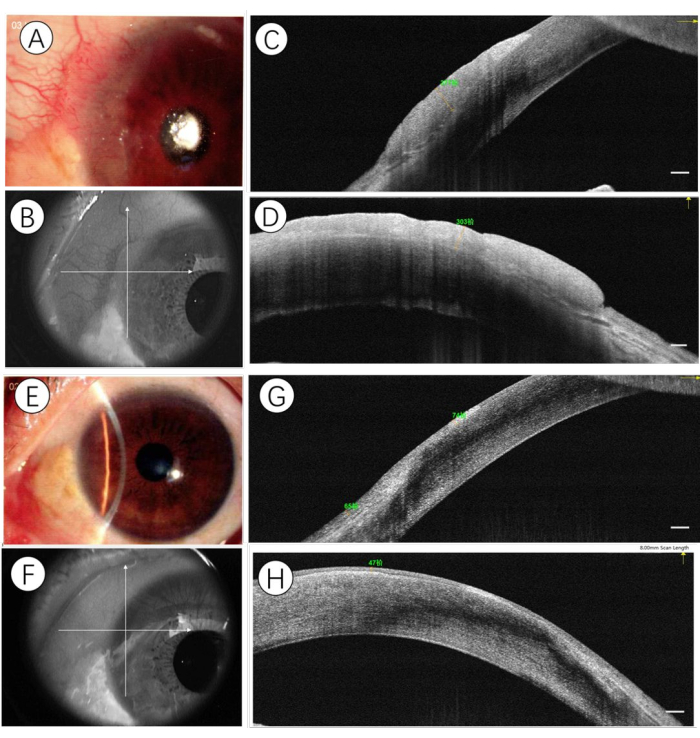
Figure 2: Sample imaging of OSSN before and after topical chemotherapy. (A) At presentation, a slit-lamp photograph demonstrating a gelatinous OSSN. The cross line is aligned to the center of the lesion (B), and HR-OCT identified the horizontal (C) and vertical (D) expanse of the lesion of a hyper-reflective thickened epithelium. After a 2-month basis of topical chemotherapy (5-fluorouracil 1%, one week-on, 3 weeks-off, given four times daily; Interferon-α2b 1MIU/mL, given four times daily), the slit-lamp photograph demonstrated a complete resolution of the lesion (E). The cross line is aligned to the original spot (F), and HR-OCT identified the horizontal (G) and vertical (H) expanse of a hyper-reflective epithelium in normal thickness. Scale bars: 250µm. Please click here to view a larger version of this figure.
Discussion
Anterior segment OCT (AS-OCT) is a promising diagnostic tool for surveying the ocular surface. It obtains an optical section of the ocular surface following the principle of Michelson's interferometry9. The systematic interpretation of AS-OCT begins with the outmost tissue of the ocular surface, namely the epithelium of the cornea, limbal, and conjunctival complex.A recent study by Vempuluru et al. showed that AS-OCT is very useful in confirming epithelial involvement where most ocular surface tumors arise, especially OSSN10. Even though many non-contact technologies can be used in the management of OSSN in clinics, HR-OCT still stands out with several strengths. It is rapid scanning, accessible for the whole ocular surface, easily interpreted, and requires minimal operator training. Yim et al. found that a favorable learning curve was observed in novice clinicians after a short learning module of the AS-OCT features11. Therefore, we focus on presenting a standardized, practical, and reproducible method for OSSN imaging to promote the application of AS-OCT in clinical workflow.
HR-OCT can expediently assist in guiding the differentiation of OSSN from various ocular lesions. It provides typical cross-sectional images with morphological patterns, namely epithelial thickening, hyper-reflectivity, and abrupt transition zone. Most non-epithelial-derived diseases usually present as normal epithelium with thickened subepithelial lesions. Many studies differentiated OSSN quantitatively by measuring the value of the epithelial thickness. Cut-off values of 120-142 µm were verified to obtain good sensitivity and specificity in differentiating OSSN from pterygia12,13. AS-OCT can also be used to identify coexistent ocular pathologies2. It is helpful in potentially reducing biopsy or directing the biopsy location for better diagnosis and treatment.
Even though histopathological examination remains the gold standard, HR-OCT can be a useful assistant in monitoring chemotherapeutic response and avoiding premature termination of therapy. During topical chemotherapy, progression toward epithelial normalization, namely reduced thickness and hyper-reflectivity, less distinct transition zone can be seen on HR-OCT images, which is defined as tumor resolving while the appearance of normalized epithelium is defined as clinical tumor resolved14. Generally, to prevent progression and recurrence of OSSN, 1-2 additional cycles of topical chemotherapy are recommended to achieve complete tumor resolution after the HR-OCT-defined tumor is resolved15. Besides, HR-OCT can be used to monitor sub-clinical disease during follow-up visits to help clinicians adjust the therapy in order to prevent tumor recurrence16.
It is important, however, to realize the limitations of HR-OCT in clinical settings. HR-OCT cannot reliably differentiate subtypes of squamous neoplasia, such as pseudoepitheliomaotus hyperplasia, papilloma, CIN, and SCC17. Besides, shadowing effects are usually seen in keratinized, pigmented tumors or lesions exceeding 500 µm in depth, which may hinder the visualization of the posterior epithelial border10. In addition, a few lesions may have overlapping characteristics, which decrease the accuracy of interpretation18. Even with the above-mentioned shortcomings, HR-OCT is still a promising modality for diagnosing tumors when they are epithelial or subepithelial in nature. This imaging adjunct can assist clinicians in better managing OSSN in clinics. What's more, those imaging biomarkers in AS-OCT can be extended further in the field of deep-learning applications to help ophthalmologists increase their diagnostic, prognostic, and monitoring accuracy, as well as decrease workloads and costs.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
None.
Materials
| 70% Ethanol | Any make | ||
| Fourier-domain optical coherence tomography (OCT) system | Optuvue Inc., Fremont, CA, USA | RTVue XR | |
| RTVue software | Optuvue Inc., Fremont, CA, USA |
Referenzen
- Cicinelli, M. V., Marchese, A., Bandello, F., Modorati, G. Clinical management of ocular surface squamous neoplasia: A review of the current evidence. Ophthalmol Ther. 7 (2), 247-262 (2018).
- Atallah, M., et al. Role of high-resolution optical coherence tomography in diagnosing ocular surface squamous neoplasia with coexisting ocular surface diseases. Ocul Surf. 15 (4), 688-695 (2017).
- Venkateswaran, N., Sripawadkul, W., Karp, C. L. The role of imaging technologies for ocular surface tumors. Curr Opin Ophthalmol. 32 (4), 369-378 (2021).
- Hӧllhumer, R., Michelow, P., Williams, S. Comparison of non-invasive diagnostic modalities for ocular surface squamous neoplasia at a tertiary hospital, south Africa. Eye. 38, 1118-1124 (2023).
- Nguena, M. B., et al. Diagnosing ocular surface squamous neoplasia in East Africa: Case-control study of clinical and in vivo confocal microscopy assessment. Ophthalmology. 121 (2), 484-491 (2014).
- Tole, D. M., McKelvie, P. A., Daniell, M. Reliability of impression cytology for the diagnosis of ocular surface squamous neoplasia employing the biopore membrane. Br J Ophthalmol. 85 (2), 154-158 (2001).
- Meel, R., et al. Ocular surface squamous neoplasia with intraocular extension: Clinical and ultrasound biomicroscopic findings. Ocul Oncol Pathol. 5 (2), 122-127 (2019).
- Singh, S., Mittal, R., Ghosh, A., Tripathy, D., Rath, S. High-resolution anterior segment optical coherence tomography in intraepithelial versus invasive ocular surface squamous neoplasia. Cornea. 37 (10), 1292-1298 (2018).
- Huang, D., et al. Optical coherence tomography. Science. 254 (5035), 1178-1181 (1991).
- Vempuluru, V. S., et al. Spectrum of as-oct features of ocular surface tumors and correlation of clinico-tomographic features with histopathology: A study of 70 lesions. Int Ophthalmol. 41 (11), 3571-3586 (2021).
- Carol, L. K. Evolving technologies for lid and ocular surface neoplasias: Is optical biopsy a reality. JAMA Ophthalmol. 135 (8), 852-853 (2017).
- Kieval, J. Z., et al. Ultra-high resolution optical coherence tomography for differentiation of ocular surface squamous neoplasia and pterygia. Ophthalmology. 119 (3), 481-486 (2012).
- Nanji, A. A., Sayyad, F. E., Galor, A., Dubovy, S., Karp, C. L. High-resolution optical coherence tomography as an adjunctive tool in the diagnosis of corneal and conjunctival pathology. Ocul Surf. 13 (3), 226-235 (2015).
- Thomas, B. J., et al. Ultra high-resolution anterior segment optical coherence tomography in the diagnosis and management of ocular surface squamous neoplasia. Ocul Surf. 12 (1), 46-58 (2014).
- Venkateswaran, N., Mercado, C., Galor, A., Karp, C. L. Comparison of topical 5-fluorouracil and interferon alfa-2b as primary treatment modalities for ocular surface squamous neoplasia. Am J Ophthalmol. 199, 216-222 (2019).
- Tran, A. Q., Venkateswaran, N., Galor, A., Karp, C. L. Utility of high-resolution anterior segment optical coherence tomography in the diagnosis and management of sub-clinical ocular surface squamous neoplasia. Eye Vis (Lond). 6, 27 (2019).
- Stevens, S., et al. Clinical and optical coherence tomography comparison between ocular surface squamous neoplasia and squamous metaplasia. Cornea. 42 (4), 429-434 (2022).
- Theotoka, D., et al. The use of high-resolution optical coherence tomography (HR-OCT) in the diagnosis of ocular surface masqueraders. Ocul Surf. 24, 74-82 (2022).

.
