Neurotoxicity Assessment in Adult Danio rerio using a Battery of Behavioral Tests in a Single Tank
Summary
Here, we present a comprehensive behavioral test battery, including the novel tank, Shoaling, and social preference tests, to effectively determine the potential neurotoxic effects of chemicals (e.g., methamphetamine and glyphosate) on adult zebrafish using a single tank. This method is relevant to neurotoxicity and environmental research.
Abstract
The presence of neuropathological effects proved to be, for many years, the main endpoint for assessing the neurotoxicity of a chemical substance. However, in the last 50 years, the effects of chemicals on the behavior of model species have been actively investigated. Progressively, behavioral endpoints were incorporated into neurotoxicological screening protocols, and these functional outcomes are now routinely used to identify and determine the potential neurotoxicity of chemicals. Behavioral assays in adult zebrafish provide a standardized and reliable means to study a wide range of behaviors, including anxiety, social interaction, learning, memory, and addiction. Behavioral assays in adult zebrafish typically involve placing the fish in an experimental arena and recording and analyzing their behavior using video tracking software. Fish can be exposed to various stimuli, and their behavior can be quantified using a variety of metrics. The novel tank test is one of the most accepted and widely used tests to study anxiety-like behavior in fish. The shoaling and social preference tests are useful in studying the social behavior of zebrafish. This assay is particularly interesting since the behavior of the entire shoal is studied. These assays have proven to be highly reproducible and sensitive to pharmacological and genetic manipulations, making them valuable tools for studying the neural circuits and molecular mechanisms underlying behavior. Additionally, these assays can be used in drug screening to identify compounds that may be potential modulators of behavior.
We will show in this work how to apply behavioral tools in fish neurotoxicology, analyzing the effect of methamphetamine, a recreational drug, and glyphosate, an environmental pollutant. The results demonstrate the significant contribution of behavioral assays in adult zebrafish to the understanding of the neurotoxicological effects of environmental pollutants and drugs, in addition to providing insights into the molecular mechanisms that may alter neuronal function.
Introduction
The zebrafish (Danio rerio) is a popular model vertebrate species for ecotoxicology, drug discovery, and safety pharmacology studies. Its low cost, well-established molecular genetic tools, and conservation of key physiological processes involved in the morphogenesis and maintenance of the nervous system make zebrafish an ideal animal model for neuroscience research, including neurobehavioral toxicology1,2. The main endpoint for evaluating the neurotoxicity of a chemical was, until recently, the presence of neuropathological effects. Lately, however, behavioral endpoints have been incorporated into neurotoxicological screening protocols, and these functional outcomes are now commonly used to identify and determine the potential neurotoxicity of chemicals3,4. Moreover, behavioral endpoints are highly relevant from an ecological point of view, as even a very mild behavioral change in fish could endanger the survival of the animal in natural conditions5.
One of the most used behavioral assays in adult zebrafish research is the novel tank test (NTT), which measures anxiety-like behavior6,7. In this assay, fish are exposed to novelty (fish are placed in an unfamiliar tank), a mild aversive stimulus and their behavioral responses are observed. NTT is used to assess basal locomotor activity, geotaxis, freezing, and erratic movements of fish, principally. Erratic8 is characterized by abrupt changes of direction (zigzagging) and repeated episodes of accelerations (darting). It is an alarm reaction and is usually observed before or after freezing episodes. Freezing behavior corresponds to a complete cessation of the fish's movements (except for opercular and ocular movements) while on the bottom of the tank, as distinguished from immobility caused by sedation, which causes hypolocomotion, akinesia, and sinking8. Freezing is usually related to a high state of stress and anxiety and is also part of submissive behavior. Complex behaviors are excellent indicators of the state of anxiety of animals. NTT has been shown to be sensitive to pharmacological and genetic manipulation9, making it a valuable tool for studying the neural basis of anxiety and related disorders.
Zebrafish are a highly social species, so we can measure a wide range of social behaviors. The shoaling test (ST) and the social preference test (SPT) are the most used assays to assess social behavior10. The ST measures the tendency of fish to group together11 by quantifying their spatial behavior and movement patterns. ST is useful for studying group dynamics, leadership, social learning, and understanding the social behavior of many fish species12. The SPT in adult zebrafish was adapted from Crawley's preference for social novelty test for mice13 and quickly became a popular behavioral assay for the study of social interaction in this model species14. These two tests have also been adapted for use in drug screening assays and have shown promise for identifying novel compounds that modulate social behavior15,16.
In general, behavioral assays in adult zebrafish are powerful tools that can provide valuable information on the behavior mechanisms or the neurophenotypes of active compounds and abused drugs17. This protocol details how to implement these behavioral tools7 with basic material resources and how to apply them in toxicity assays to characterize the effects of a wide range of neuroactive compounds. In addition, we will see that the same tests can be applied to assess the neurobehavioral effects of acute exposure to a neuroactive compound (methamphetamine) but also to characterize these effects after chronic exposure to environmental concentrations of a pesticide (glyphosate).
Protocol
Strict compliance with ethical standards guarantees the welfare and proper treatment of the zebrafish used for experimentation. All experimental procedures were carried out under the guidelines established by the Institutional Animal Care and Use Committees (CID-CSIC). The protocols and results presented below were performed under the license granted by the local government (agreement number 11336).
1. Animal housing for behavioral testing
- Perform all tests (presented in Figure 1) in an isolated behavioral room at 27-28 °C between 10:00 and 17:00.
- Wash both control and exposed fish several times in clean fish water [reverse-osmosis purified water containing 90 mg/L aquarium systems salt, 0.58 mM CaSO4·2H2O, and 0.59 mM NaHCO3] before starting the experiments to avoid any potential contamination of the experimental tank.
- Acclimate animals to the behavior room 1 h before starting the experiments.
- Ensure that the animals (≈50:50 male: female ratio) are experimentally naïve and perform all behavioral testing in a blind manner with observers unaware of the experimental group.
- To obtain meaningful results in behavioral assays, have a total number of 18 subjects per condition (n = 18), ideally obtained between two or more independent experiments. For example, in individual tests, analyze the behavior of 9 animals per condition, per replicate. In group tests, analyze the behavior of a shoal of 6 to 9 animals per condition, per replicate.
- Carry out all tests following a battery test approach (see planning proposals in Figure 2). Ethically more suitable, this method allows to reduce the number of animals needed for the study, complying with the 3R reduction principle7.
- Most of the time, behavioral assays are connected to biological assays, so sacrifice the animals following euthanasia guidelines18 before collecting and analyzing samples (OMICs or chemicals). If the endpoint does not prove to be sampling, re-stable the control group at the end of the experiment. Reuse the control animals for breeding or experimental purposes after a few days.
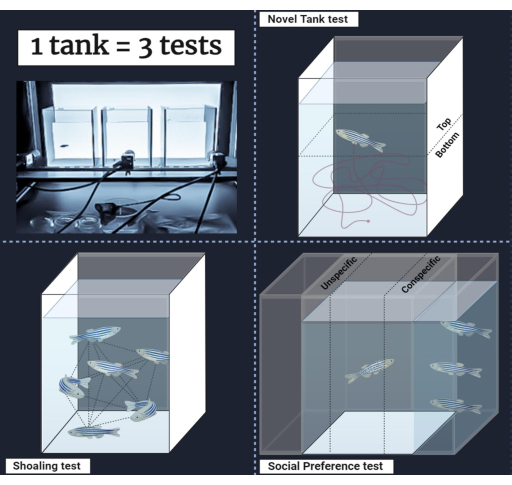
Figure 1: Experimental setups. Three configurations of the square tank to study a wide range of behaviors in adult zebrafish. Please click here to view a larger version of this figure.
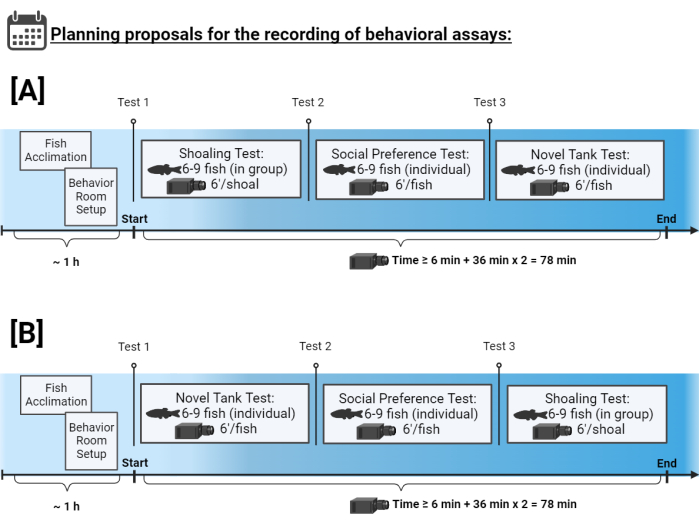
Figure 2: Experimental timeline. Two planning proposals for the recording of behavioral assays. Please click here to view a larger version of this figure.
2. Experimental configurations of the tank
- Anxiety-like behavior: The Novel Tank Test (NTT)
- Adjust the experimental setup (number of tanks, cameras, and computers) to record the maximum number of fish simultaneously. Individual behavior assays are time-consuming, so optimize time, material, and space.
- Prepare the experimental tanks for NTT: Square tank (20 cm length, 20 cm width, 25 cm height) covered with acrylic panels on lateral walls and bottom to avoid reflection and interference between subjects.
- Fill the experimental tanks with 7 L (water column height: 20 cm height) of well-oxygenated fish water at 28 °C.
- Adjust the position of the tank in front of the camera to avoid distorted image.
- Check the illumination setup. LED backlight (10000 lux) provide a homogenate illumination on all part of the tank for video recording in good conditions.
- Turn on the cameras and adjust them following section 3.
- Introduce the subjects, one by one, into the bottom of the experimental tanks before starting to record as quickly as possible.
NOTE: It is important to start recording with the animal at the bottom of the tank. - Take care not to disturb the animals during recording. Use of a curtain or panel to limit visual interaction not only between tanks but also between the support and the outside.
- At the end of the recording (standard recording time is 6 min), transfer the animals that have already passed through the test to another tank so as not to mix them with the naïve animals.
- Repeat the procedure with all available subjects. It is advisable to have a total number of 18 subjects per condition to obtain meaningful results in individual trials (from two or more independent replicates).
- Randomize the experimental group assigned to each tank between trials to avoid any potential tank effects (if you are recording several conditions at the same time).
- Social grouped behavior: The Shoaling Test (ST)
- The experimental configuration of ST is the same as that of NTT (the same tanks can be reused directly).
- Follow the steps 2.1.1-2.1.6. to set up the ST.
- Introduce the shoal (6 to 9 subjects at the same time) at the bottom of the experimental tanks before starting to record as quickly as possible.
NOTE: It is important to start recording with the animal at the bottom of the tank. - Follow the steps 2.1.8-2.1.11. to perform the ST.
- Repeat the procedure with all available subjects. To obtain meaningful results in this assay, make at least two independent replicates with the same bank size in each replicate.
- Maintain the size of the shoal consistent for all the experimental groups and replicates inside the same experiment.
- Social individual behavior: The Social Preference Test (SPT)
- Adjust the experimental setup to optimize the experimental space and time of recording.
- Prepare the experimental tanks for SPT: Square tank (20 cm length, 20 cm width, 25 cm height) transparent (glass or plastic) to offer lateral visibility. The single focal fish is free to interact with a conspecific virtual zone – a fish's shoal placed into the one-sided external housing tank, or with the unspecific virtual zone – a one-sided external empty housing tank.
- Fill the experimental tanks with 5 L (water column height: 15 cm, same height as the water column in the extern housing tanks) of clean fish water at 28 °C.
- Adjust the position of the tank in front of the camera to avoid distorted image.
- Check that the system receives homogeneous lighting.
- Introduce the subjects, one by one, into the bottom of the experimental tanks before immediately starting recording with the animal down in the center.
- Avoid visual interactions between observers and animals during recording.
- At the end of the 6 min recording, transfer the present animals to another tank so as not to mix them with the naïve animals.
- Repeat the procedure with all available subjects. Have a total number of 18 subjects per condition to obtain meaningful results in individual trials (from two or more independent replicates).
3. Video recording for behavioral tests
- Open the camera manager to check the availability of the GigE camera on each computer.
- Launch the GigE camera controlling software (such as uEye Cockpit, described here). Open the Camera option, select Monochrome mode, and adjust the image size (1:2).
- Open Camera Properties
- Under Camera, set the Pixel Clock to Maximum, set the Frame Rate to 30 frames per second (fps), and adjust the Exposure (Auto or Manual adjust if the image is too dark).
- Under Image, set the Gain to 0 (Auto) and the Black Levels (Auto or Manual adjust to obtain a good contrast).
- Under Size, adjust the size of the window to the region that needs to be engraved (Width: Width-Left, Height: Height-Top). This step allows to reduce the size of the image and, therefore, the final size of the video.
- Close Camera Properties.
- Create a general folder for the experiment session to save the camera settings and videos.
- To save the camera settings, set File > Save Parameters > To File and select the experiment folder recently created.
NOTE: The camera settings file can thus be reloaded in the application to continue working with the same image parameters at any time (e.g. when the camera is suddenly switched off or to reuse the same settings, reducing the setup time and homogenizing the experimental conditions). If, in one moment, the camera freezes between videos, stop recording, exit, and turn off the camera. Turn it back on, reload the camera parameters by going to File > Load Parameters > To File, and restart recording. Check if the current video has been completely acquired to discard or repeat the fish (before repeat, give the animals some time to re-acclimatize). - Repeat this camera setup procedure (steps 3.1-3.5) on all the cameras.
- When all the cameras are correctly configured, open Record Video Sequence.
- Select Create to save as a new video file, select the experiment folder recently created, and report in the name of the video file the information of the subject, type of experiment, and the date.
- Select Max. Frames. Type 10800 in the frame box. Standard video is recording 6 min (Video 1) at 30 fps in AVI format; therefore, 6 min x 60 s x 30 fps= 10800 frames in total.
- Select Calc. Frame Rate or indicate the frame rate manually (velocity of recording: 30 fps).
- Repeat the video file creation procedure on all the computers.
- Introduce the subjects, one by one, at the bottom of each experimental tanks. All assays will be run at once.
- Start the records quickly by clicking on Record and wait to get the maximum number of requested frames (step 3.10).
- Once the videos are recorded, a chat box appears with the message Maximal number of frames achieved!. Select Accept.
- Select Close to finish the recording and close the video file.
- Remove the fish that have just been observed. Be careful to separate them from the naive fish.
- Directly select Create and repeat the process to continue recording videos.
- Once all the recordings are done, select Exit.
- To turn off the cameras, select Close Camera and Exit the program.
4. Analysis of recorded videos
- Launch the analysis software (see Table of Materials).
- To elaborate on a new template, click on New from Template > Applied a Predefined Template > From Video File, and select a video to start setting up the template. Try to choose a representative video of the experiment with a subject exhibiting good mobility and good conditions of recording.
- In Parameters, configure the parameters in the following windows (1 to 4/7). Select the model Fish > Adult Zebrafish, the arena Open Field Square > One Arena, the number of Subject per Arena (for the ST, a multi tracking package [track various subjects in one arena] is required), the type of Detection by Center-Point and finally adjust the frame rate to 30 fps. In the following windows (5 to 7/7), do not change parameters; default configuration is OK.
- Name the experiment as a template and place it in the same folder as the rest of the stored video. The template will be created as an experiment folder with several subdivisions containing all the setup information.
- Under Experiment Settings, check the defined setup (from video file, arena, number of subjects, frame per seconds). Here, the system units can be modified.
- Under Arena Settings, right-click on the center of the screen and select Grab. From File in the display. Choose a good quality video image and Accept to capture this image for the background settings. First, Calibrate the image, generating a calibrated rule. Use the width of the tank as a scale (19 cm). Then, draw the arena. Be careful to make the square just enough to avoid the reflections of the animal when the latter approaches the surface or any eventual confusion of the fish software with the black areas of the tank. Finally, draw the shape zones with the Frame function.
- For NTT and ST, divide the front of the tank into two equal virtual zones, top, and bottom (see Figure 1). Draw two equal horizontal boxes. Boxes cover half an arena for each one. Name the Top and Bottom for the upper and lower zones, respectively. Be careful that the boxes have the same width (9-10 cm) and length (8-9 cm), do not exceed arena boundaries (orange square), and do not overlap, checking that each arrow zone indicates exactly its zones.
- For SPT, divide the experimental arena conceptually into three equal-sized zones: empty, center, and conspecific (see Figure 1). Draw three equal vertical boxes. Name the box oriented to the shoal tank as Conspecific, the box oriented to the empty tank as Empty, and the middle one as Center. Be careful that the boxes have the same width (6 cm) and length (18-19 cm), do not exceed arena limits, and do not overlap.
- Under Detection Settings, verify which video to be dealt with in the Video File. Then, check detection quality (fish in yellow, red center point). Click on Auto Detect to adjust the detection, refocusing the animal (choose an image that the animal is swimming in profile on the white background, draw the picture by taking its entire body, and validate the detection with Yes). Open Advanced to improve detection by selecting Dynamic Subtraction, Darker Subject, Background Settings, Background Learning, Subject Size, Noise Reduction, etc.
- Under Trials Settings, put one trial and delete the others (right-click and delete)
- Under Data Settings, create Results dialog windows. Parameterize Results per time and per zone. For example, create one Results window for data output by minutes and another for data output by total time (6 min). Request the data output for each zone (request it if the distance in each zone is needed). Link the different Results windows to the Start window with arrows.
- Under Analyze Settings, select the Parameters to analyze and the type of Statistics for each parameter. These parameters will be automatically calculated based on the data acquired from the tracking.
- For NTT and SPT, select options as defined below:
- Select Distance Moved (select Total) to obtain the distance traveled in the arena (cm) and the distance traveled in the respective zones (cm).
- Select In Zones (select Zones, Frequency, Cumulative, and Latency to First) to have the time spent in the zones (s) and the latency to first entrance in the zones (s).
- Select Zone Transition (select Threshold: 0 cm, Add Zone 1 > Zone 2; Zone 2 > Zone 1, in any zones, Frequency) to obtain the number of entrances in the zones.
- Select Mobility Sate (fill in High mobile above 70%, Immobile below 3%, minimum 150 frames, and select frequency, cumulative, and latency to first) to have the duration of hypermobility (s), the duration of freezing (s).
NOTE: See the Discussion section for more details about the approximation of freezing behavior using the automated analysis and the number and duration (s) of freezing episodes. - Select Acceleration and Turn Angle (select frequency and cumulative) to evaluate the occurrence of complex behaviors such as darting and erratic (fast acceleration movements).
- For the ST, in addition to the above exploratory parameters, select the option Distance Between Subjects (select all the subjects, mean, maximum, minimum) to get the average distance between fish (cm), the average distance between the nearest neighbor (cm), and the average distance between the farthest neighbor.
- For NTT and SPT, select options as defined below:
- The template is ready for its use. Save the last modifications and Close the template without acquiring any data from the video (maintain the template file; it is light and easy to manage and copy). If there are several software licenses, analyze the videos from the same template copied to each computer.
- To copy and use the template, there are two options:
- Open the template file with the behavior analysis software, go to File > Save as to create a new identical file.
- In the welcome interface, select New from Template > Applied a Custom Template > From Video File (choose template. EthXV file). Name the new experiment and select its location. The software may take a few minutes to copy the information from the template file.
- Go to Arena Settings to re-adjust the template if the video was recorded with a different camera (follow steps 4.6 and 4.7).
- Go to Detection Settings or Acquisition to check which video is selected and change the video file if necessary.
- Under Acquisition, select DDS > Ready to Start. It may take a few minutes for the software to process the video.
- When the acquisition is finished, go to Track Editor. Select acceleration x16 to read the processed video faster and check if the tracking is correct.
NOTE: Sometimes, there may be "losses" in the tracking (due to reflections or confusion of the software itself). They can be edited manually from this part if they are few; otherwise, it is preferable to reprocess the whole experiment, improving the definition of the canvas and the detection. - Under Statistics, click on Calculate > Export Data. Data exportation is directly located in the experiment folder.
- Under Track Visualization or Heatmaps, generate and export (right click, export image, select the folder Export Files of the experiment to save this data with the spreadsheet report) tracking images of the animal.
- Go to File to close the active experiment and repeat this procedure for the next video.
5. Statistical analysis
- Analyze the normality (Shapiro-Wilk test) of data in each group.
- Assess homoscedasticity with Levene's test.
- Use one-way ANOVA followed by Dunnett's and Tukey's multiple comparison tests to test differences between groups when criteria of normality and homoscedasticity cannot be rejected.
- Use the Kruskal-Wallis test followed by a pairwise comparison using the Bonferroni correction to test differences between groups when criteria of normality and homoscedasticity are rejected.
- Plot the data with graphical software.
Representative Results
In this section, we will look at some possible applications of these behavioral tools in fish neurotoxicology. The following results correspond to the characterization of the acute or binge effects of methamphetamine (METH), a recreational drug, and the sub-chronic effects of glyphosate, one of the main herbicides found in aquatic ecosystems.
Characterization of a methamphetamine binge neurotoxicity model in adult zebrafish
When evaluating the effect of 40 mg/L METH on NTT (Figure 3), the Kruskal-Wallis test confirmed that the exposed animals presented a positive geotaxis, characterized by a decrease in the exploration time in the upper zone of the experimental tank (H(2) = 35.964, P = 1.55 x 10-8), as well as in the distance traveled in this part (H(2) = 32.272, P = 9.82 x 10-8), and in the number of visits (H(2) = 36.527, P = 1.17 x 10-8). We also observed a significant increase in the latency time preceding the first visit to the upper zone (H(2) = 17.264, P = 0.00018). It is important to remark that the differences observed in the parameters measured in the NTT after METH exposure are consistent over time, as confirmed by the Bonferroni correction (P > 0.8). A significant effect of exposure time was found for freezing behavior (H(2) = 13.120, P = 0.0014).
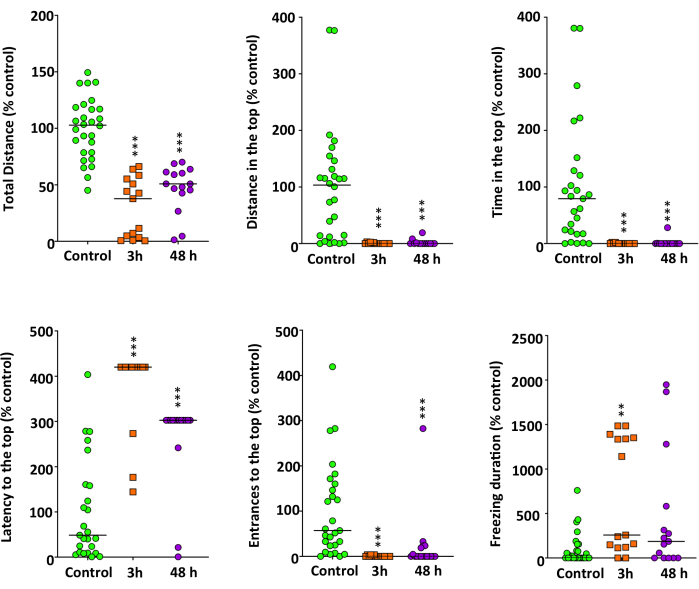
Figure 3: Anxiety-like behavior assessed in standard 6-min novel tank test (NTT) of adult zebrafish exposed to 40 mg/L methamphetamine (METH) for 3 h and 48 h. Data from each experiment were normalized to the corresponding control values. The combined data is reported as a scatter plot with the median (n = 14-15), **p < 0.01, ***p < 0.001; Kruskal Wallis test with Bonferroni correction for NTT endpoints. Data from 2 independent experiments. This figure has been reproduced with permission from Bedrossiantz et al.15. Please click here to view a larger version of this figure.
Freezing movements can be quantified by assessing the frequency, latency, duration, or location of freezing. The best way to score them is undoubtedly the eye of an experienced observer, which is quite laborious and complex, so we tried an automated alternative using EthoVision software to detect freezing behavior19. We found that the number, latency, and duration of freezing attacks calculated by the software (Table 1A) correlate with good accuracy with the episodes scored manually by the observer (Table 1B). Whereas the two methods are equivalent in terms of results (P = 0.958, Student's test), we used the automated approach to assess the freezing here. After 3 h of exposure to METH, freezing time increased significantly (P = 0.0012), whereas no difference was found with the control after 48 h of exposure (P = 0.16). METH produced no effect on erratic movements at either time.
We used two experimental paradigms to evaluate the effects on social behavior after acute exposure to METH. The ST (Figure 4) revealed that the average distance and farthest distance between individuals were significantly greater for METH-treated fish (H(2) = 53.261, P = 2.72 x 10-12; H(2)=52.504, P = 3.97 x 10-12 for average and farthest interfish distances, respectively), pointing to a behavioral phenotype of social isolation. Again, we remark that no time effect was found using the Bonferroni post hoc test (P > 0.5).
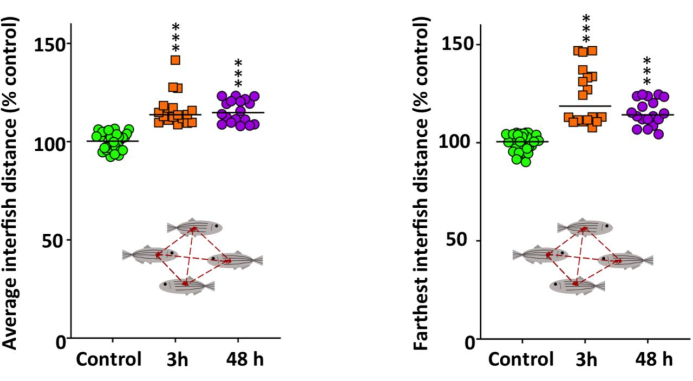
Figure 4: Social behavior of adult zebrafish waterborne exposed to 40 mg/L methamphetamine (METH) for 3 h and 48 h. Shoaling test (ST) results, including the average and the farthest interfish distances. The combined data is reported as a scatter plot with the median (n = 18), *p < 0.05, **p < 0.01, ***p < 0.001; Kruskal Wallis test with Bonferroni correction. Data from 2 independent experiments. This figure has been reproduced with permission from Bedrossiantz et al.15. Please click here to view a larger version of this figure.
In the SPT (Figure 5), treated fish show a significant decrease in time spent and distance traveled in the conspecific zone (F(2,74) = 14.497, P = 4.87 x 10-6; F(2,73) = 13.461, P = 0.00001 for time spent and distance traveled in the conspecific zone, respectively). These results reaffirm the social isolation phenotype suggested by the TS results. Tukey's Honest Significant Difference (HSD) post hoc test ruled out no possible differences between the two analysis times (P > 0.5).
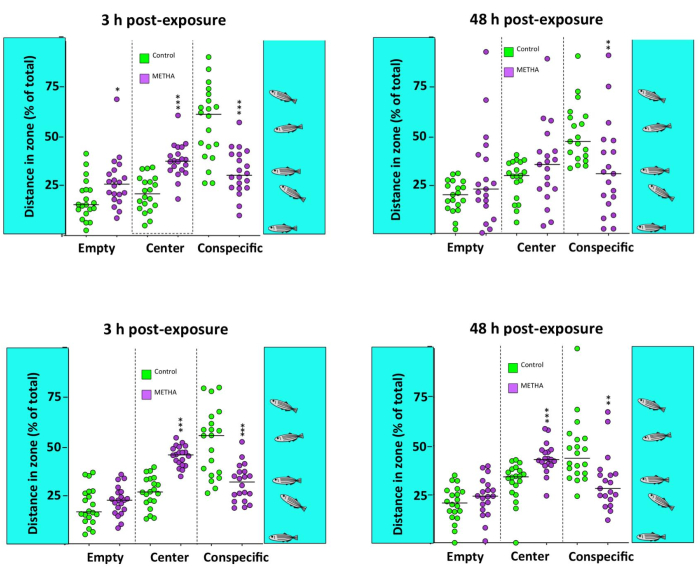
Figure 5: Social behavior of adult zebrafish waterborne exposed to 40 mg/L methamphetamine (METH) for 3 h and 48 h. The social preference test (SPT) results, including time and distance of the fish in each of the three virtual zones of the experimental tank: empty, center, and conspecific. Data from each experiment were normalized to the corresponding control values. The combined data is reported as a scatter plot with the median (n = 17-20), *p < 0.05, **p < 0.01, ***p < 0.001; one-way ANOVA with Dunnett's multiple comparison test. Data from 2 independent experiments. This figure has been reproduced with permission from Bedrossiantz et al.15. Please click here to view a larger version of this figure.
Behavioral effect of sub-chronic exposure to environmental levels of glyphosate
Behavioral analysis of the effects of sub-chronic exposure to 3 µg/L glyphosate on the NTT (Figure 6) reveals a significant decrease in time spent exploring the top (F2,77 = 8.744, P = 0.0004), distance traveled in this part (F2,77 = 9.118, P = 0.0003), and number of visits (F2,77 = 3.441, P = 0.037). These effects are characteristic of positive geotaxis behavior, as is the increased effect observed on the latency time preceding the first visit to the top of the tank (H(2) = 9.628, P = 0.008). The expression of erratic and freezing behaviors of the exposed animals was also analyzed in the NTT. The duration (H(2) = 17.261, P = 0.025) and number of erratic episodes (F2,76 = 10.073, P = 0.0001) were significantly increased by glyphosate. In contrast, no freezing differences were found with the control (Pearson Chi-Square(2) = 2.964, P = 0.253). Applied to an ecological context, the observations made at NTT suggest that glyphosate could significantly decrease the exploratory behavior of fish, jeopardizing their ability to survive in the wild.
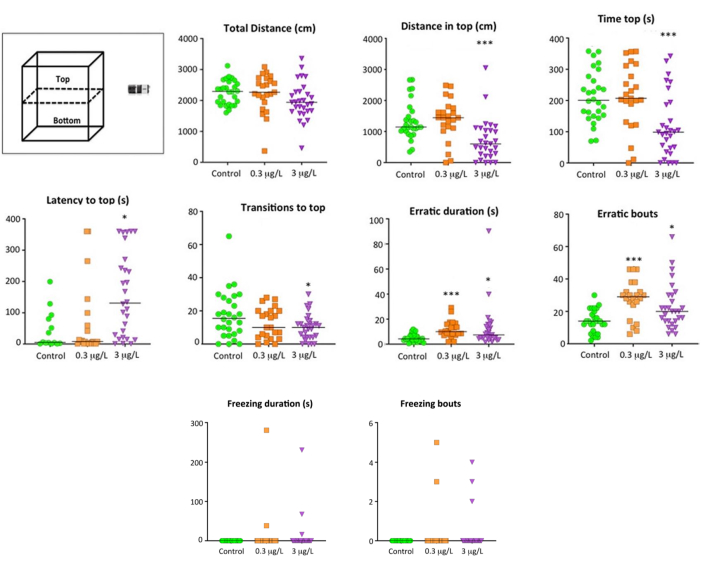
Figure 6: Anxiety-like behavior assessed in standard 6-min novel tank test (NTT) of adult zebrafish exposed to 0.3 µg/L and 3 µg/L glyphosate for 2 weeks. Behavioral parameters analyzed, as well as a cartoon of the experimental tank divided into two equal virtual zones, top and bottom. Data reported as a scatter plot with the median (n = 23-29), *p < 0.05, **p < 0.01, ***p < 0.001; one-way ANOVA with Dunnett's multiple comparison test (Total distance, Distance in top, Time in top, Transitions to top, Erratic bouts, High mobility frequency) or Kruskal Wallis test with Bonferroni correction (Latency to the top, Erratic duration). No differences (P > 0.05) were found in the freezing duration and freezing bouts. Data from 2-4 independent experiments. This figure has been reproduced with permission from Faria et al.20. Please click here to view a larger version of this figure.
Schooling, non-polarized groups of conspecifics that are held together by social pressure to protect themselves from predators, is a natural tendency of Danio rerio. The school can "tighten" or "expand" depending on the animals' level of anxiety or fear, a particular visual effect that is very easy to identify experimentally (Figure 7). In the glyphosate experiment, the shoaling test revealed an increase in anxiety in fish exposed to 3 µg/L, reflected by a grouping of the shoal and thus a significant decrease in the average distance and farthest distance between individuals (F2,56 = 5.664, P = 0.006 and F2,56 = 7.413, P = 0.001, for the average and farthest interfish distances, respectively) compared to the control.
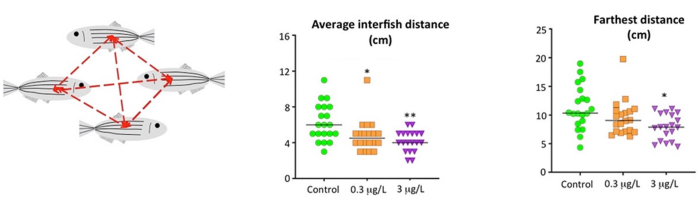
Figure 7: Social behavior of adult zebrafish waterborne exposed to 0.3 µg/L and 3 µg/L glyphosate for 2 weeks. Data reported as scatter plot with the median (n = 19-20), *p < 0.05, **p < 0.01, ***p < 0.001; one-way ANOVA with Dunnett's multiple comparison test (Average interfish distance and Farthest distance) Data from 2 to 4 independent experiments. This figure has been reproduced with permission from Faria et al.20. Please click here to view a larger version of this figure.
Table 1: An approximation of freezing behavior using an automated analysis. Data reported in this table come from the same recording (Video 1) analyzed with two different methods. (A) Approximation of freezing behavior by automated calculation with EthoVision V13 software. The variable mobility is calculated from the change of the subject area between two samples, so it depends on the acquisition frequency of this area. We set a very low threshold of immobility (less than 3% mobility) as well as the sample rate to a minimum continuous time of 5 s (more than 150 frames). (B) Analysis of freezing behavior with Behavioral Observation Research Interactive Software (BORIS, free and open-source software). BORIS is an event-logging software for video coding and live observations. With BORIS, the observer can code the freeze episode as a state event, defining the start and end points. Please click here to download this Table.
Video 1: Control fish in the novel tank test. Please click here to download this Video.
Discussion
Characteristic anxiety behaviors observed in NTT have been positively correlated with serotonin levels analyzed in brains21. For example, after exposure to para-chlorophenylalanine (PCPA), an inhibitor of 5-HT biosynthesis, fish exhibited positive geotaxis as well as decreased brain 5-HT levels22, results very similar to those obtained with METH. Therefore, the decrease in brain serotonin levels and the display of positive geotaxis in METH-exposed zebrafish suggests that the anxiety behavior produced by the drug is mediated by the serotonergic pathway. Interestingly, a similar behavioral phenotype, i.e., an anxiogenic effect on geotaxis, can be seen in adult zebrafish exposed for 2 weeks to 0.3 3 µg/L and 3 µg/L, two environmentally relevant concentrations of glyphosate. An increase in geotaxis was also previously reported for adult zebrafish with the neurotoxicant acrylamide6,23. In all these cases, this behavioral phenotype (an increase of geotaxis in the NTT, characteristic of anxiogenic substance) was associated with the diminution of monoaminergic neurotransmitter levels. Therefore, the NTT paradigm combined with neurochemical analysis of the brain provides ecologically relevant information, exploratory behavior, and foraging efficiency and connects behavior neurophenotypes with neurotransmitter modulations.
On the other hand, an impairment of social behaviors in both assays, the ST and SPT, was also observed in METH-treated fish. The result obtained in this study is consistent with several studies with rats and monkeys, where the acute and chronic exposure of the study animals to METH results in social withdrawal24. Social behavior changes associated with METH abuse have been explained in humans by impairments in social-cognitive function24. An anxiogenic effect on the shoal size was found in zebrafish exposed for 2 weeks to 3 µg/L glyphosate. We observed a phenocopy of this effect in zebrafish exposed to 53 mg/L (0.75 mM) acrylamide for 3 days6,23.
The NTT, ST, and SPT assays allow to effectively determine the potential neurotoxic effects25 of a wide range of chemicals as illustrated by the study of acute methamphetamine and sub-chronic glyphosate toxicity models in adult zebrafish. Behavior is, in toxicology, a relevant apical endpoint, characterizing the effects at organismal levels of a chemical for neurotoxicity and environmental research. Besides being a sublethal endpoint in laboratory conditions, changes in behaviors, such as exploratory or social behavior, can be deleterious in nature. Moreover, the proposed behavioral analysis battery is an easy-to-implement, semi-automated method11 and, therefore, very efficient if the assays are consciously planned (reduction principle)26. Performing these assays as a test battery using a single tank reduces the number of animals and the experimental time and waste generation.
The order of the assays in the battery is an important consideration if we want to study the response profile of an individual in each trial. For this purpose, conducting the individual assays followed (see Figure 2) allows for keeping the animal identified and to relate its exploratory behavior to its social preference. In addition, the animal’s behavioral responses can be related to other biological data, such as its neurotransmitter profile or gene expression, if the fish are kept identified until the end point of sampling (Figure 2A).
Usually, behavioral analysis allows for the observation of differences between groups. First, individual responses are calculated on the basis of animal tracking27 before pooling the data by group. Then, the means and the difference in variance with respect to the control group are compared for each behavioral parameter calculated. With shoaling analysis12, it is critical to be very clear that the unit of variance is the group of test fish, not individual fish because the behavior of each individual fish is influenced by the other fish in the shoal. This is the way used in most papers to process behavioral data28. However, it might be useful to rethink the analysis of behavioral parameters not on a parameter-by-parameter basis but as an overall response per trial. For example, one could calculate the covariance of each measurement made in a trial and report it as a different way of measuring the same thing: anxious, exploratory, or gregarious behavior. There are many ways to calculate and interpret behavioral data28,29. Depending on the number of conditions, type of tests, and image acquisition (2D or 3D)30,31 the analysis can be completely rethought in order to get the best out of the data.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by "Agencia Estatal de Investigación" from the Spanish Ministry of Science and Innovation (project PID2020-113371RB-C21), IDAEA-CSIC, Severo Ochoa Centre of Excellence (CEX2018-000794-S). Juliette Bedrossiantz was supported by a PhD grant (PRE2018-083513) co-financed by the Spanish Government and the European Social Fund (ESF).
Materials
| Aquarium Cube shape | Blau Aquaristic | 7782025 | Cubic Panoramic 10 (10 L, 20 cm x 20 cm x 25 cm, 5 mm) |
| Ethovision software | Noldus | Ethovision XT | Version 12.0 or newer |
| GigE camera | Imaging Development Systems | UI-5240CP-NIR-GL | |
| GraphPad Prism 9.02 | GraphPad software Inc | GraphPad Prism 9.02 | For Windows |
| IDS camera manager | Imaging Development Systems | ||
| LED backlight illumination | Quirumed | GP-G2 | |
| SPSS Software | IBM | IBM SPSS v26 | |
| uEye Cockpit software | Imaging Development Systems | version 4.90 |
References
- Raldúa, D., Piña, B. In vivo zebrafish assays for analyzing drug toxicity. Expert Opinion on Drug Metabolism & Toxicology. 10 (5), 685-697 (2014).
- Faria, M., Prats, E., Bellot, M., Gomez-Canela, C., Raldúa, D. Pharmacological modulation of serotonin levels in zebrafish larvae: Lessons for identifying environmental neurotoxicants targeting the serotonergic system. Toxics. 9 (6), 118 (2021).
- Faria, M., et al. Zebrafish models for human acute organophosphorus poisoning. Scientific Reports. 5, 15591 (2015).
- Faria, M., et al. Glyphosate targets fish monoaminergic systems leading to oxidative stress and anxiety. Environment International. 146, 106253 (2021).
- Faria, M., et al. Screening anti-predator behaviour in fish larvae exposed to environmental pollutants. Science of the Total Environment. 714, 136759 (2020).
- Faria, M., et al. Acrylamide acute neurotoxicity in adult zebrafish. Scientific Reports. 8 (1), 7918 (2018).
- Kalueff, A. V., Stewart, A. M. Zebrafish Protocols for Neurobehavioral Research. Neuromethods. , (2012).
- Kalueff, A. V., et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish. 10 (1), 70-86 (2013).
- Egan, R. J., et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behavioural Brain Research. 205, 38-44 (2009).
- . Social behavior in Zebrafish Available from: https://www.noldus.com/applications/social-behavior-zebrafish (2012)
- Green, J., et al. Automated high-throughput neurophenotyping of zebrafish social behavior. Journal of Neuroscience Methods. 210 (2), 266-271 (2012).
- Miller, N., Gerlai, R. Quantification of shoaling behaviour in zebrafish (Danio rerio). Behavioural Brain Research. 184 (2), 157-166 (2007).
- Landin, J., et al. Oxytocin receptors regulate social preference in zebrafish. Scientific Reports. 10 (1), 5435 (2020).
- Ogi, A., et al. Social preference tests in zebrafish: A systematic review. Frontiers in Veterinary Science. 7, 590057 (2021).
- Bedrossiantz, J., et al. A zebrafish model of neurotoxicity by binge-like methamphetamine exposure. Frontiers in Pharmacology. 12, 770319 (2021).
- Hamilton, T. J., Krook, J., Szaszkiewicz, J., Burggren, W. Shoaling, boldness, anxiety-like behavior and locomotion in zebrafish (Danio rerio) are altered by acute benzo[a]pyrene exposure. Science of the Total Environment. 774, 145702 (2021).
- Kane, A. S., Salierno, J. D., Brewer, S. K. Chapter 32. Fish models in behavioral toxicology: Automated Techniques, Updates, and Perspectives Methods in Aquatic Toxicology. Volume2, (2005).
- Faria, M., et al. Glyphosate targets fish monoaminergic systems leading to oxidative stress and anxiety. Environment International. 146, 106253 (2021).
- Maximino, C., Costa, B., Lima, M. A review of monoaminergic neuropsychopharmacology in zebrafish, 6 years later: Towards paradoxes and their solution. Current Psychopharmacology. 5 (2), 96-138 (2016).
- Maximino, C., et al. Role of serotonin in zebrafish (Danio rerio) anxiety: Relationship with serotonin levels and effect of buspirone, WAY 100635, SB 224289, fluoxetine and para-chlorophenylalanine (pCPA) in two behavioral models. Neuropharmacology. 71, 83-97 (2013).
- Faria, M., et al. Therapeutic potential of N-acetylcysteine in acrylamide acute neurotoxicity in adult zebrafish. Scientific Reports. 9 (1), 16467 (2019).
- Homer, B. D., Solomon, T. M., Moeller, R. W., Mascia, A., DeRaleau, L., Halkitis, P. N. Methamphetamine abuse and impairment of social functioning: A review of the underlying neurophysiological causes and behavioral implications. Psychological Bulletin. 134 (2), 301-310 (2008).
- Linker, A., et al. Assessing the maximum predictive validity for neuropharmacological anxiety screening assays using zebrafish. Neuromethods. 51, 181-190 (2011).
- Hartung, T. From alternative methods to a new toxicology. European Journal of Pharmaceutics and Biopharmaceutics. 77 (3), 338-349 (2011).
- Cachat, J. M., Kalueff, A., Cachat, J., et al. Video-Aided Analysis of Zebrafish Locomotion and Anxiety-Related Behavioral Responses. Zebrafish Neurobehavioral Protocols. Neuromethods. 51, (2011).
- Rosemberg, D. B., et al. Differences in spatio-temporal behavior of zebrafish in the open tank paradigm after a short-period confinement into dark and bright environments. PLoS ONE. 6 (5), e19397 (2011).
- Blaser, R., Gerlai, R. Behavioral phenotyping in Zebrafish: Comparison of three behavioral quantification methods. Behavioral Research Methods. 38 (3), 456-469 (2006).
- Cachat, J., et al. Three-dimensional neurophenotyping of adult zebrafish behavior. PLoS ONE. 6 (3), e17597 (2011).
- Cachat, J. M., et al. Deconstructing adult zebrafish behavior with swim trace visualizations. Neuromethods. 51, 191-201 (2011).

