Preparation of Food Samples Using Homogenization and Microwave-Assisted Wet Acid Digestion for Multi-Element Determination with ICP-MS
Summary
The presented protocol describes sample homogenization with a laboratory mixer, acid digestion of food samples using a mixture of 68 wt% HNO3 and 30 wt% H2O2 via microwave-assisted wet acid digestion, and multi-element determination performed with inductively coupled plasma mass spectrometry.
Abstract
Sample preparation is crucial for elemental determination, and various techniques are available, one of which involves homogenization followed by acid digestion. Special care is required during sample handling in the preparation stage to eliminate or minimize potential contamination and analyte loss. Homogenization is a process that simultaneously reduces particle size and uniformly distributes sample components. Following homogenization, the sample undergoes acid digestion, wherein it is digested with acids and auxiliary chemicals at elevated temperatures, transforming solid samples into a liquid state. In this process, metals in the original sample react with acids to form water-soluble salts. Samples prepared through acid digestion are suitable for elemental analysis using techniques such as inductively coupled plasma mass spectrometry, inductively coupled plasma optical emission spectroscopy, atomic absorption spectroscopy, electrochemical methods, and other analytical techniques. This work details the preparation of food samples for multi-element determination using inductively coupled plasma mass spectrometry. The step-by-step procedure involves the homogenization process using a laboratory-sized mixer with ceramic blades, followed by acid digestion in closed vessels using microwave-assisted wet acid digestion. A mixture of 5.0 mL of 68 wt% HNO3 and 1.0 mL of 30 wt% H2O2 serves as an auxiliary reagent. This guide provides an explanation of the processes involved in both stages.
Introduction
Elemental analysis is an analytical process for determining the elemental composition of various samples. It can be used to control the intake of metals into human bodies (especially heavy metals1) since their high concentrations may cause unwanted health problems. Heavy metals are also one of the main environmental contaminants, therefore, control of their presence in the environment is necessary2. Moreover, elemental analysis can be employed to determine the geographical origin of food products3 and to control the quality of food and water resources4. In addition, it is used for the determination of micro- and macronutrients in soils5 and to gain insights into geological processes throughout history by examining the chemical composition of minerals and sediments6. Studies have also been made to determine the presence of rare metals in electrical and electronic waste for further metal regeneration7, to evaluate the success of drug treatments8, and to verify the elemental composition of metal implants9.
Inductively coupled plasma mass spectrometry (ICP-MS) and inductively coupled plasma optical emission spectroscopy (ICP-OES) are commonly used techniques for the elemental analysis of various samples10. They allow simultaneous determination of multiple elements with limits of detection (LOD) and limits of quantification (LOQ) as low as ng/L. In general, ICP-MS has lower LOD values11 and a wider linear concentration range compared to ICP-OES12. Other techniques to determine elemental composition are microwave-induced plasma optical emission spectrometry13 and several variants of atomic absorption spectroscopy (AAS), including flame atomic absorption spectroscopy, electrothermal atomic absorption spectroscopy2, cold vapor atomic absorption spectroscopy, and hydride generation atomic absorption spectroscopy14. Furthermore, elemental determination with low LOD and LOQ is possible with different electroanalytical methods, especially with anodic stripping voltammetry15,16. Of course, there are other methods to determine the elemental composition of samples, but they are not as frequently employed as the above-mentioned methods.
Direct elemental determination of solid samples is feasible using laser-induced breakdown spectroscopy and X-ray fluorescence17. However, for elemental determination with ICP-MS, ICP-OES, and AAS it is necessary to convert solid samples into a liquid state. For this purpose, acid digestion is performed using acids and auxiliary reagents (in most cases H2O2). Acid digestion is carried out at elevated temperature and pressure, converting the organic part of the sample into gaseous products and converting the metal elements into water-soluble salts, thus dissolving them in the solution18.
There are two main types of acid digestion, open vessel digestion and closed vessel digestion. Open vessel digestion is cost-effective14 but has limitations, such as the maximum digestion temperature, which coincides with the boiling temperature of acids at atmospheric pressure. The sample can be heated on hot plates, heating blocks, water baths, sands baths2, and by microwaves19. By heating the sample in that manner, much of the generated heat is lost to the surroundings20, which extends the digestion time14. Other disadvantages of open vessel digestion include greater chemical consumption, the greater possibility of contamination from the surrounding environment, and possible loss of analytes due to the formation of volatile components and their evaporation from the reaction mixture21.
Closed vessel systems are more convenient for the digestion of organic and inorganic samples compared to open vessel systems. Closed vessel systems utilize a variety of energy sources to heat the samples, such as conduction heating and microwaves22. Digestion methods which use microwaves include microwave-induced combustion23, single reaction chamber systems24, and commonly used microwave-assisted wet acid digestion (MAWD)25,26. MAWD allows digestion at higher operating temperatures, ranging between 220 °C and 260 °C and maximum pressures up to 200 bar, depending on the instrument's working conditions27.
The efficiency and rate of MAWD depend on several factors, including the chemical composition of the samples, the maximum temperature, the temperature gradient, the pressure in the reaction vessel, the amount of acids added, and the concentration of acids used28. In MAWD, complete acid digestion can be achieved in a few minutes due to the elevated reaction conditions compared to longer-lasting digestions in open vessel systems. Lower volumes and concentrations of acids are required in MAWD, which is in line with current green chemistry guidelines29. In MAWD, a smaller amount of sample compared to open vessel digestion is needed to perform acid digestion, usually up to 500 mg of sample is sufficient30,31,32. Larger sample quantities may be digested, but they require a larger amount of chemicals.
Since the instrument for MAWD automatically controls the reaction conditions and the person does not come in direct contact with the chemicals during heating, MAWD is safer to operate than open vessel digestions. However, the person should always proceed with caution when adding chemicals to the reaction vessels to prevent them from coming into contact with the body and causing harm. Reaction vessels also need to be opened slowly as the pressure is built up inside them during acid digestion.
Although acid digestion is a useful technique for preparing samples for elemental determination, the person performing it should be aware of its possible limitations. Acid digestion may not be suitable for all samples, especially those with complex matrices and those that are highly reactive or could react explosively. Therefore, sample composition should always be evaluated to select the appropriate chemicals and reaction conditions for complete digestion that dissolves all desired elements in the solution. Other concerns that the user must consider, and address are impurities and loss of analytes at every step of sample preparation. Acid digestion must always be performed according to specific rules or using protocols.
The protocol described below provides instructions for the homogenization of food samples in a laboratory-sized mixer, a procedure for cleaning the mixer's components, properly weighing the sample, adding chemicals, performing acid digestion by MAWD, cleaning the reaction vessels after the digestion is complete, preparing the samples for elemental determination, and performing a quantitative multi-element determination with ICP-MS. By following the instructions given below, one should be able to prepare a sample suitable for elemental determination and perform the measurements of digested samples.
Protocol
1. Sample homogenization
- Using a clean ceramic knife, manually cut the food samples (broccoli, mushrooms, sausages, and noodles) into smaller pieces to speed up the drying process. Prepare enough samples for a minimum of 6 replicates of the acid digestion (ensure that the minimum mass of the dried samples is 1500 mg).
NOTE: Increasing the surface area of the sample exposes a larger portion of the sample to the heated surrounding air, increasing the rate of evaporation of the water. - Place the sample in a 250 mL glass beaker and dry it at 105 °C to a constant weight using a dryer.
- Remove the glass beaker with the sample from the dryer and insert it in the desiccator.
- Allow the sample to cool down to room temperature.
NOTE: Samples must be weighed at a constant temperature to ensure that the weight accurately reflects the mass. Temperature variations can affect the volume and density of the samples measured. - Open the desiccator and transfer the glass beaker with the sample on the analytical balance. Measure the weight of the glass beaker with the sample.
- After the weighing is completed, put the sample back into the dryer.
NOTE: If the sample shrank significantly during drying, one could transfer it to a smaller glass beaker using a plastic spatula for more convenient weighing. - Repeat the process as described in steps 1.3-1.6 until a constant weight of the sample is achieved.
- Place the dried heterogeneous sample into the mixer beaker (see Table of Materials), ensuring it does not exceed the maximum volume of the mixer beaker.
- Insert the mixer beaker into the mixer and close the guard door (Figure 1).
- Press the start button to activate the blades for grinding and mixing the sample.
- Perform the grinding until the sample turns into a fine powder or a homogenous paste. To achieve such a product, repeat the grinding process several times.
- When the sample is homogenized, switch off the mixer, open the guard door, and remove the mixer beaker.
- Remove the homogenized sample from the mixer beaker and transfer it to a clean 50 mL glass beaker using a clean plastic spatula (Figure 2).
NOTE: If the sample is too hard and could potentially damage the mixer's components, such as the blades and mixer beaker, it can be homogenized by other means, such as crushing it in mortars. Mixers are usually unsuitable for homogenizing hard materials, frozen samples, or easily flammable samples, which could damage the mixer's components. The use of organic solvents in the mixer is discouraged.
CAUTION: Use safety gear and ensure that the mixer's door is adequately closed as the mixer's blades rotate at high speeds.
2. Mixer cleaning
- Add ultrapure water (see Table of Materials) to the mark of the empty mixer beaker.
- Insert the mixer beaker into the mixer and perform the standard mixing procedure.
- Take the beaker out of the mixer and pour out the wastewater. If necessary, repeat the process with ultrapure water several times until the water remains clean even after mixing.
- Remove the contaminated blades and diaphragm seal from the mixer and clean them thoroughly with ultrapure water.
NOTE: Use neutral detergents to improve cleaning efficiency, especially when dealing with samples with a high fat content, as fat easily adheres to the surface of the laboratory inventory.
CAUTION: Wear appropriate protective equipment, such as gloves, when removing and cleaning the blades to reduce the risk of potential injuries from their sharp edges. - Dry the cleaned components in the dryer at 105 °C and reinsert them into the mixer.
NOTE: Ensure that the mixer's components are completely dry before reinstalling them in the mixer, to prevent carry-over of the water to the following sample.
3. Sample weighing
- Remove the cover lid from the 100 mL trifluoromethoxyl-polytetrafluoroethylene TFM-PTFE reaction vessel33.
- Place the open reaction vessel on the analytical balance and ensure that the balance is leveled and zeroed before each measurement (Figure 3).
NOTE: Weighing must be performed at room temperature. Avoid areas where severe temperature fluctuations and air flow could affect the measured weight. Ensure that the weighing area is clean and free of any contaminants. - Transfer the homogenized sample into the reaction vessel using a plastic spatula and weigh 250 mg of the sample. Do not weigh the sample below the minimum weight limit of the analytical balance.
- Once the weighing is complete, place the cover lid on the reaction vessel to protect the sample from contamination.
NOTE: Exceeding the weight limit of the digestion procedure can result in incomplete digestion. Handle the sample and reaction vessels with care to avoid any external contamination.
4. Acid addition
- Pour approximately 40.0 mL of 68 wt% HNO3 and 5.0 mL of 30 wt% H2O2 into separate 50 mL glass beakers, respectively.
NOTE: Chemicals must be of high purity with trace metal impurities of less than 1.0 µg/L (ppb), ideally in the ng/L (ppt) range. Trace metal impurities affect the accuracy and repeatability of elemental determination. - Place the reaction vessels in a fume hood, open the cover lids, and add the below mentioned volumes of 68 wt% HNO3 and 30 wt% H2O2 with 1 mL or 5 mL automatic pipettes, according to the following specifications:
- Broccoli, mushrooms, sausages, and noodles; for 250 mg of sample add 5.0 mL 68 wt% HNO3 and 1.0 mL 30 wt% H2O2. Prepare three replicates for every sample.
- To determine the accuracy of the method (in terms of recovery, Rec), use the procedure described in step 4.2.1 and add 37.5 µL of 100 mg/L ICP multi-element standard solution (see Table of Materials) into the reaction vessels using a 200 µL automatic pipette. For every sample, prepare three replicates.
NOTE: The volume of 37.5 µL was selected as it corresponds to an increase of 15.0 µg/L for the spiked solutions of samples compared with the concentration in the non-spiked solutions of samples. Moreover, the increase in concentration for the spiked solution of samples corresponds to the final concentration that is still in the linear concentration range for every analyte measured. - Prepare a sample blank using the same volume of 68 wt% HNO3 and 30 wt% H2O2 as used for the digestion of food samples in step 4.2.1. For a sample blank, do not add the sample to the reaction vessels.
CAUTION: HNO3 used for digestion is corrosive and produces fumes. For this reason, acid addition must be performed in a fume hood. Standard laboratory protective equipment must be employed (gloves, safety goggles, and laboratory coat). If there is contact with acid, the affected area should be immediately rinsed under the stream of cold water, and medical help should be sought.
- Place the cover lid on reaction vessels and allow samples to react with the added 68 wt% HNO3 and 30 wt% H2O2 for 2-3 min.
- Screw the thread cover on the vessel and tighten the cover lids.
- Shake the reaction vessel using light hand movements to fully incorporate the samples into chemicals.
NOTE: Do not leave the specimens on the walls or lids of the reaction vessels, as there is a possibility that they will not be completely digested.
5. Microwave-assisted wet acid digestion
- Turn on the microwave system (see Table of Materials) for acid digestion by pressing the start button (Figure 4).
- Open the microwave oven door and remove the rack.
- Distribute the closed reaction vessels symmetrically in the rack to ensure even irradiation of the samples by microwaves.
- Insert the rack into the microwave chamber and mount it on a holder (Figure 5).
- Close the microwave oven door.
- Set a suitable digestion program on the microwave oven touch screen using a pen shaped tool. Choose an appropriate temperature gradient, the final temperature, and the number of samples to be digested. The recommended digestion program for different food samples is listed below:
- Broccoli, mushrooms, sausages, and noodles: 10 min increase to 160 °C, 10 min increase to 200 °C, 15 min at 200 °C, maximum power 900 W.
- Start the digestion program and monitor the change in reaction conditions on the screen. Stop the digestion process if the temperature does not increase according to the prescribed program.
NOTE: During digestion, sudden temperature spikes may be seen on the microwave oven screen. They occur when the samples react exothermically with the chemicals. The microwave system will automatically regulate the temperature by adjusting the output power. - Wait until microwave-assisted digestion is completed and the temperature of the sample decreases.
- Open the microwave oven door and remove the rack from the microwave oven chamber. Close the door and switch off the instrument.
- Remove the reaction vessels from the rack and wait for them to cool down to room temperature.
- Slowly open the lid covers manually to release the gases formed during acid digestion. Turn the reaction vessels in the direction of the fume hood (Figure 6).
- Completely remove the cover and rinse the cover and the walls of the reaction vessel with a small amount of ultrapure water.
- Quantitatively transfer the contents of the reaction vessel into a clean 25 mL glass volumetric flask through a glass funnel by repeated rinsing of the cover and reaction vessel with ultrapure water.
- Dilute the sample with ultrapure water to the mark of the volumetric flask. Close the volumetric flask with a stopper and mix the content of the volumetric flask.
NOTE: Further dilution of the digested samples with ultrapure water should be performed as they should contain less than 5% (V/V) of residual acid34 and less than 2 g/L of dissolved elements, also referred to as total dissolved solids35. - Take a 20 mL plastic syringe and connect it with a polyamide syringe filter (25 mm diameter, 0.20 µm pore size). Fill the plastic syringe with the diluted sample and filter its content into a 50 mL plastic centrifuge tube by applying pressure. Use a new plastic syringe and syringe filter for every sample to avoid any cross-contamination.
NOTE: Samples need to be filtered to remove any insoluble materials or solid particles that may remain undigested after MAWD. These particles may interfere with elemental determination measurements by clogging the instrument components. When filtering the samples, ensure to discard the first couple of drops. Use hydrophilic filters (made of polyamide) for aqueous solutions. Hydrophobic filters (PTFE) are not suitable for the filtration of aqueous solutions as they require a higher applied pressure, which could result in membrane rupture36. - Close the 50 mL plastic centrifuge tube with a screw cap and put the sample in the refrigerator until the measurements.
NOTE: Digested samples are stored in the refrigerator at lower temperatures to preserve them and extend their storage time.
6. Reaction vessel cleaning
- After the digested samples were transferred to 50 mL volumetric flasks, add 5.0 mL of 68 wt% HNO3 and 5.0 mL of ultrapure water with 5 mL automatic pipettes into the reaction vessels.
- Close the reaction vessels with the cover lids and insert them into the rack. Transfer the rack to the microwave oven chamber.
- Apply the following microwave program: 15 min increase to 160 °C, 10 min increase to 180 °C, maximum power 900 W.
- Monitor reaction conditions during heating. After the heating is completed, let the reaction vessels cool down.
- Open the microwave oven, remove the reaction vessels from the rack, and slowly open them in the fume hood.
- Discard the content of the reaction vessels into plastic waste containers.
- Rinse the reaction vessels with ultrapure water to remove any excess material or chemicals.
- Dry the reaction vessels in the dryer at 105 °C before the next use.
NOTE: The same microwave procedure (time, power, temperature gradient, and volume of chemicals) used for the acid digestion of samples may be used for cleaning the reaction vessels. Alternatively, the reaction vessels can be cleaned without the microwave system by submerging them in concentrated HNO3 or HCl for several hours and rinsing them with ultrapure water.
7. Multi-element determination with ICP-MS
- Take the 50 mL plastic centrifuge tubes containing the digested samples from the refrigerator and allow them to warm to room temperature.
- Dilute the samples by a factor of 10 to decrease the concentration of acid in the digested sample and to decrease the concentration of the component of the sample matrix. Using an automatic pipette, transfer 2.50 mL of the sample into a 25 mL glass volumetric flask and then fill it up to the mark with ultrapure water.
- Transfer the diluted samples into the 15 mL plastic tubes and place them into the appropriate positions in the autosampler.
- Prepare the ICP-MS instrument (see Table of Materials) for the measurements:
- Switch on the ventilation and the chiller that supplies the ICP-MS with cooling water and cools its components.
- Use the compatible software to ensure that the rinsing solution (1 wt% HNO3) flows continuously from the autosampler to the ICP-MS without pulsating.
- Open Ar (99.999% purity) and He (99.999% purity) gas cylinders to supply the ICP-MS with both gasses. Check the gas flow in the software and adjust it if necessary.
NOTE: Use collision cell with He gas when spectral interferences are expected due to the formation of polyatomic ions (e.g., 40Ar16O+ interfering with 56Fe+)37. - Start up the plasma and calibrate the instrument using the tuning solution (see Table of Materials).
- Once the instrument is calibrated (torch position, gain voltage, lens voltage, mass/resolution, pulse/analog (P/A) calibration, database (DB) calibration, and validation), select the desired measurement method and perform the measurements.
- When working with unknown samples, perform a semi-quantitative determination to obtain information on what elements are present in the sample and their approximate concentration.
NOTE: It is advisable to additionally dilute the samples for the semi-quantitative determination as the detectors have a limit of the concentration of elements they can detect at once. Lower sample concentrations can extend the lifetime of the instrument components. - After obtaining the data on the approximate concentrations of the elements in the samples, create a method for the quantitative elemental determination in the software. Select the operating conditions of the ICP-MS (Table 1) and select the desired elements (in the present case Cu, Fe, Mn, and Zn). Determine the number and concentrations of solutions of the standard required to create a calibration curve (sometimes referred to as an analytical curve or working curve) (Table 1).
NOTE: Prepare at least six different concentrations as calibration points for the calibration curve. - Prepare solutions of standard for the calibration curve. Using automatic pipettes, pipette the required volume of 100 mg/L multi-element standard solutions into 25 mL glass volumetric flasks, to prepare solutions of standards with the following concentrations: 1.0 µg/L, 2.5 µg/L, 5.0 µg/L, 10.0 µg/L, 20.0 µg/L, 30.0 µg/L, 40.0 µg/L, and 50.0 µg/L. Fill the flasks to the mark with 1 wt% HNO3. Additionally, prepare a calibration blank using the 1 wt% HNO3 solution.
- Transfer the prepared solutions of standard and samples into the 15 mL plastic tubes, place them into the autosampler, and start the instrument following the procedure described in step 7.4.
- Perform the quantitative measurement of the selected elements using the calibration curve methodology.
- Once the measurements are completed, turn off the plasma, close the Ar and He gas supplies, switch off the ICP-MS chiller, and turn-off the ventilation system.
Representative Results
Homogenization
All samples were dried to a constant mass with the laboratory dryer to eliminate any moisture. Transferring the sample to a desiccator allowed it to cool to room temperature without binding moisture from the surrounding environment. The food samples were then homogenized using the laboratory mixer to obtain a fine powder. The resulting homogenized particles were uniform in size and evenly distributed, ensuring that subsamples (samples drawn from a larger sample) used for acid digestion were representative. The samples were easily removable from the mixer beaker with the help of a plastic spatula, except for the dried meat sample, which was more challenging to remove due to its higher fat content. Higher fat content made the sample partially adhere to the glass walls of the mixer beaker. The comparison of fresh, dried, and homogenized samples is given in Figure 2.
The instrument's components had to be cleaned multiple times with ultrapure water to eliminate all food particles that remained in the mixer.
It is essential to ensure that the weighed mass of the sample does not exceed the maximum value allowed in the reaction vessels. Weighing was carried out using an analytical balance at a constant temperature, and a plastic spatula was used to avoid contamination with metals that may arise from metal spatulas.
Acid digestion
All samples used in the protocol were food samples containing various amounts of carbohydrates, proteins, and fats. HNO3, in combination with H2O2, is suitable for the digestion of these molecules, and other chemicals are not required. The chemicals were treated in a fume hood since HNO3 forms fumes. After adding the chemicals into the TFM-PTFE reaction vessels, cover lids were mounted on the top of the reaction vessels and were sealed well to avoid possible contamination and loss of analyte. The reaction vessels were symmetrically distributed in the rack to ensure uniform microwave irradiation inside the microwave system.
During acid digestion, the door of the microwave system was closed, and the door could not be opened until the end of the protocol. The entire process of acid digestion can be monitored on the screen of the device, showing the temperature change with time (Figure 7).
After acid digestion was completed and the solutions of the digested samples had cooled to room temperature, the reaction vessels were opened in the fume hood. They were opened as slowly as possible. If the pressure is released too quickly, even small droplets of the reaction mixture may escape, resulting in loss of analyte. When the reaction vessels were opened, a yellow or yellow-orange gas was released (Figure 8). The coloration of the fumes can be attributed to NO2, which forms orange fumes at higher temperatures. The pressure increase in the reaction vessels was due to the oxidation of food samples with HNO3, resulting in the formation of gases such as CO2, H2O, NO, etc. After the reaction vessels were degassed, a light-yellow or colorless solution of the digested sample remained in the reaction vessel, indicating that total acid digestion by MAWD had been achieved. This was further confirmed by the absence of visible particles left in the solution.
The final step of sample preparation involved diluting the digested samples with ultrapure water to reduce the residual acidity (RA). High RA values interfere with the measurements by increasing the background signal. Dilution also decreases the concentration of metal ions in the liquid sample26. When transferring the solution of digested samples into volumetric flasks, the components of the reaction vessel were thoroughly rinsed with ultrapure water to completely transfer the analyte. One problem which occurs is that small drops of ultrapure water, which may contain the analyte of interest, adhere to the walls of the reaction vessels. After dilution with ultrapure water to the 25 mL mark, all samples became colorless. The final solutions of digested samples contained water-soluble salts, as the metal elements present in the sample reacted with HNO3 to form highly soluble nitrates. Elemental analysis techniques can determine the metal ions that form water-soluble salts. When filtering the diluted solutions, it is important to discard the first few drops to ensure that any particles or contaminants are removed. After filtration, the solutions were tightly sealed to prevent any leakage and then stored in the refrigerator.
The main limitation of the procedure of acid digestion is sample throughput. The MAWD system can digest only a limited number of samples at a time. Additionally, each digestion and subsequent sample preparation step can take several hours to complete. Furthermore, reaction vessel cleaning is also time-consuming, but it is crucial to minimize the risk of cross-contamination between samples.
Multi-element determination with ICP-MS
For each element, a calibration curve was constructed. They were obtained by plotting the intensity as a function of analyte concentrations (Figure 9). The linear concentration ranges for all measured elements were in the range from 1.0 µg/L to 50.0 µg/L.
The LOD and LOQ for every element were calculated using Equation 1 and Equation 2, respectively. In both equations, sblank represents the standard deviation of the several measurements of calibration blank (10 replicates)38,39, while b1 represents the slope of the calibration curve.
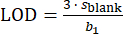 (1)
(1)
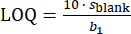 (2)
(2)
The obtained LODs were 0.5 ng/L, 2.8 ng/L, 2.8 ng/L, and 3.2 ng/L for Mn, Cu, Fe, and Zn, respectively. The obtained LOQs were 1.6 ng/L, 9.2 ng/L, 9.5 ng/L, and 10.8 ng/L for Mn, Cu, Fe, and Zn, respectively.
Six replicate digestions of every sample were performed. Three replicate digestions of every sample were performed without spiking the sample with solutions of standard, and three replicate digestions were performed with the addition of a solution of a known amount of analyte standard to test the accuracy (spike recovery test40) and precision of the entire methodology. For accuracy determination before the digestion procedure, 37.5 µL of 100 mg/L ICP multi-element standard solution was pipetted into the reaction vessels containing the sample, which resulted in an increase of concentration of 15.0 µg/L in spiked samples that were diluted by a factor of 10. This also corresponded to an increase of 15.0 µg per gram of sample for every measured metal ion. The accuracy and precision were determined using Rec and relative standard deviation (RSD), respectively.
The accuracy of an analytical method can be assessed by the spike recovery test. For this purpose, a solution of a known amount of analyte standard is added to the sample, which is then digested under the same reaction conditions as samples that are not spiked41. The Rec is calculated using Equation 3, where γi is the measured concentration of the spiked samples after digestion, while γt represents the determined concentration of the unspiked sample by considering the increase of the added solution of analyte standard. The γi and γt are averages of the three replicates. The analytical method is deemed to be accurate when Rec is in the range of 80.00%-120.00%42.
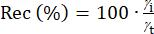 (3)
(3)
The precision of an analytical method is evaluated with RSD. It describes the closeness of agreement between independent results, which were obtained through several replicate measurements. RSD is calculated using Equation 4, where sm represents the standard deviation of the replicate measurements for the concentration determination, while 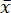 represents the average value of the determined concentrations. The analytical method is deemed precise if the RSD value is lower than 20.00%43.
represents the average value of the determined concentrations. The analytical method is deemed precise if the RSD value is lower than 20.00%43.
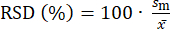 (4)
(4)
All samples were diluted with ultrapure water by a factor of 10 before the ICP-MS measurements (for the first set of measurements). The dilution decreased the concentration of the matrix components introduced into the analyzer. Moreover, by diluting the sample, the RA decreases. A high RA might compromise plasma ionization efficiency or result in matrix interference issues. If the concentration of the analytes after the first set of measurements is lower than the LOQ, the dilution factor should be lower than 10. The quantification of the metal ions was carried out using a calibration curve. The values of the calculated results should have the same precision (the same number of significant figures) as the solution of the standard employed for the calibration. The content of metal ions in the sample was expressed as µg per gram of weight (µg/g). This was achieved by multiplying the measured mass concentration of the analyzed sample with the dilution factor to obtain the concentration in the original digested sample. This mass concentration was then multiplied by the volume of the digested sample (25 mL) and then divided by the initial weighed mass of the homogenized sample (the initial weighted mass is the mass of the sample that was weighted into the reaction vessel for the MAWD). All values are reported as an average of three replicates.
The reported content of the elements below is given as 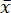 ± sm. The content of Cu, Mn, and Zn in the broccoli sample was 5.9 ± 0.5 µg/g, 32.5 ± 2.7 µg/g, and 42.8 ± 0.2 µg/g, respectively. The determined mass concentration of Fe in broccoli samples exceeded the upper limit of the linear concentration range of the calibration curve (i.e., 50.0 µg/L). Thus, the solution of the sample was diluted with ultrapure water by a factor of 2, and the ICP-MS measurement of this solution was performed. The results showed that the broccoli contained 63.0 ± 1.9 µg/g of Fe.
± sm. The content of Cu, Mn, and Zn in the broccoli sample was 5.9 ± 0.5 µg/g, 32.5 ± 2.7 µg/g, and 42.8 ± 0.2 µg/g, respectively. The determined mass concentration of Fe in broccoli samples exceeded the upper limit of the linear concentration range of the calibration curve (i.e., 50.0 µg/L). Thus, the solution of the sample was diluted with ultrapure water by a factor of 2, and the ICP-MS measurement of this solution was performed. The results showed that the broccoli contained 63.0 ± 1.9 µg/g of Fe.
For the mushroom, the content of Zn, Fe, Cu, and Mn was 35.6 ± 1.4 µg/g, 30.4 ± 1.3 µg/g, 18.5 ± 1.0 µg/g, and 5.4 ± 0.3 µg/g, respectively. Sausages contained 42.2 ± 0.9 µg/g of Fe, 25.1 ± 2.6 µg/g of Zn, and 1.0 ± 0.1 µg/g of Cu. The multi-element determination with ICP-MS of the digested solution, which was diluted 10 times, showed that the concentration of Mn was lower than the lower limit of the linear concentration range (i.e., 1.0 µg/L). Thus, the original solution of the sausage sample was diluted only by a factor of 5, and the multi-element determination with ICP-MS was repeated. The content of Mn in sausage samples was determined to be 0.9 ± 0.3 µg/g. Noodles contained 5.4 ± 2.8 µg/g of Zn, 10.3 ± 1.2 µg/g of Fe, 1.6 ± 0.3 µg/g of Cu, and 7.5 ± 0.2 µg/g of Mn.
The Rec for all analytes measured in all four samples was in the range of 80.00%-120.00%, indicating the accuracy of the analytical method. The calculations showed that the analytical method was precise, as the RSD values were below 20.00%, apart from RSD for Zn in noodle samples. The results are reported in Table 2.

Figure 1: Laboratory mixer used for the homogenization of food samples. Please click here to view a larger version of this figure.
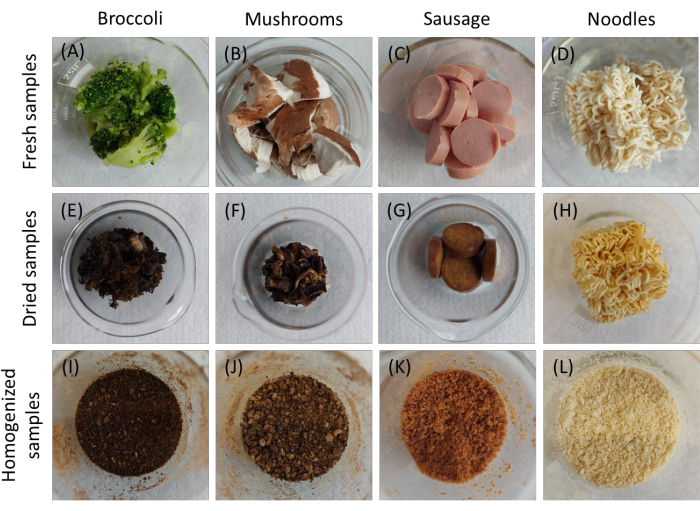
Figure 2: Comparison of fresh, dried, and homogenized samples. (A–D) Fresh samples of broccoli, mushrooms, sausage, and noodles. (E–H) dried samples of broccoli, mushrooms, sausage, and noodles. (I–L) homogenized samples of broccoli, mushrooms, sausage, and noodles. Please click here to view a larger version of this figure.

Figure 3: Weighing the sample on an analytical balance. This is performed from above by opening the upper flap. Please click here to view a larger version of this figure.

Figure 4: Microwave system. The microwave system for acid digestion with side touch screen for selecting reaction conditions and monitoring the process of acid digestion. Please click here to view a larger version of this figure.
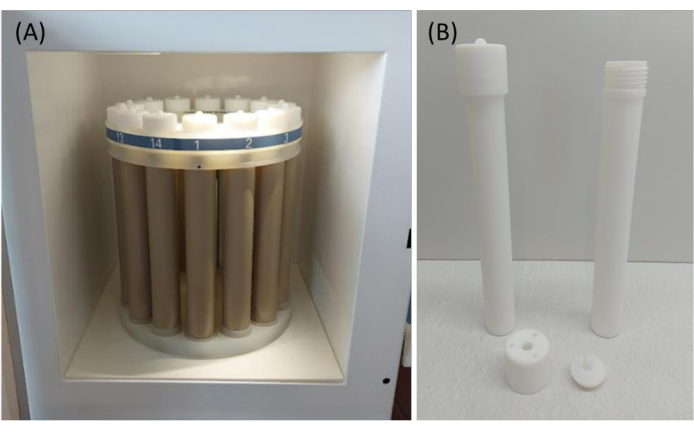
Figure 5: Components used for microwave-assisted acid digestion. (A) Rack with 14 reaction vessels for acid digestion inside the microwave oven chamber. (B) TFM-PTFE reaction vessels consist of 3 parts. Once the vessels are closed with lids, neither the sample nor gases can escape from or enter the reaction vessels. Please click here to view a larger version of this figure.
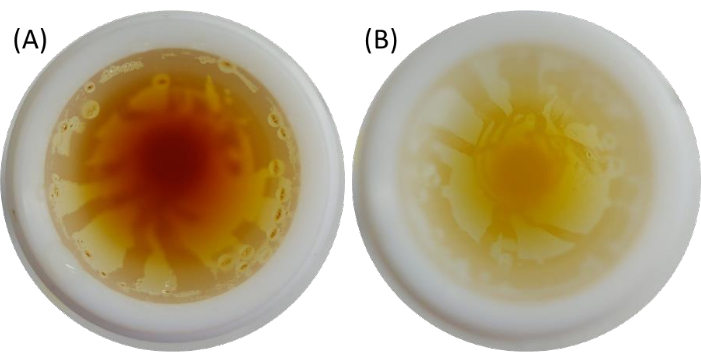
Figure 6: The interior of the reaction vessels when opened in the fume hood. (A) The yellow-orange coloration of the fumes is due to NO2 produced during acid digestion. (B) The yellow coloration of the solution of the digested sample after most of the gases have escaped from the reaction vessel. Please click here to view a larger version of this figure.
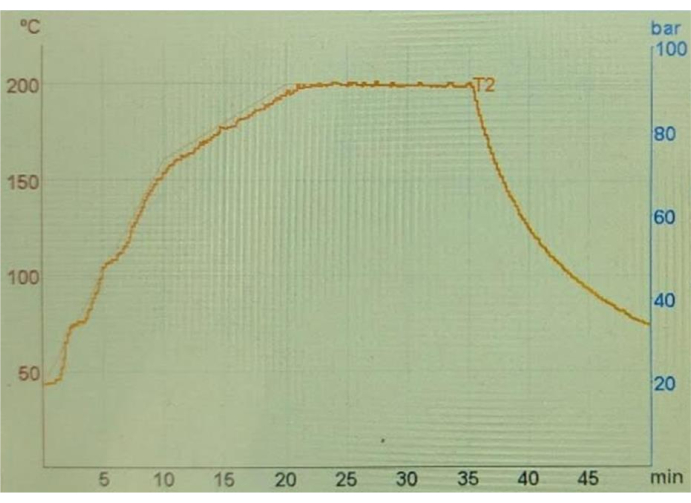
Figure 7: Change of temperature with time. A plot showing the temperature change as a function of time during acid digestion with MAWD. T2 stands for the temperature of the reaction mixture inside the reaction vessels. Please click here to view a larger version of this figure.

Figure 8: Opening the reaction vessels under the fume hood, where yellow-orange gases are released. Please click here to view a larger version of this figure.
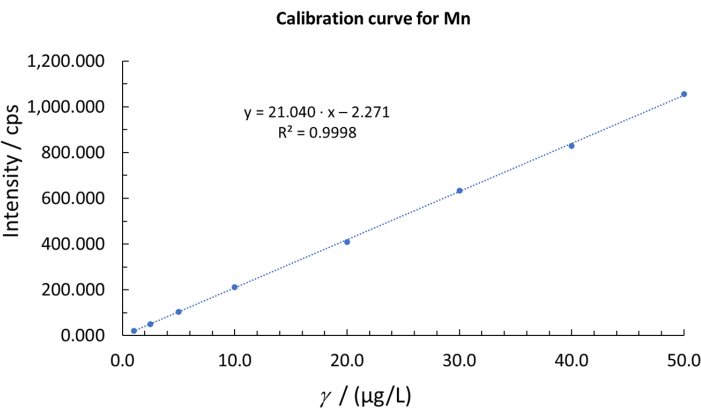
Figure 9: Example of a calibration curve for Mn. Please click here to view a larger version of this figure.
Figure 10: ICP-MS instrument used for multi-element determination. Please click here to view a larger version of this figure.
Table 1: Operating conditions of the ICP-MS instrument. Please click here to download this Table.
Table 2: Rec and RSD values of broccoli, mushrooms, sausage, and noodles. Please click here to download this Table.
Discussion
Homogenization
To ensure reproducible results in elemental determination, it is necessary to homogenize samples before acid digestion due to their complex and inhomogeneous structure and composition. Homogenization aims to eliminate constitutional and distributional heterogeneity. Mixing the sample minimizes distributional heterogeneity by evenly redistributing components throughout the sample. Similarly, by bringing the particle size down to a uniform size, constitutional heterogeneity is reduced44. The subsamples obtained from the homogenate must contain the same ratio of components as the original sample to be representative45.
Homogenization is achieved by applying a force to break down the sample into smaller particles46. Samples may be homogenized by cutting, chopping, shearing, crushing, grinding, or mixing. However, the appropriate method must consider the sample's degree of hardness, brittleness, abrasiveness, elasticity, shape, and capacity to adhere to the homogenizer's components method47.
The sample can be crushed manually with a pestle and mortar or ground in a variety of mills (knife mill, cutting mill, ball mill, mixer mill, etc.), and other forms of homogenizers48. Small laboratory-sized mixers with ceramic or metal blades are commonly used for homogenization as they rapidly reduce the particle size by disintegrating and simultaneously mixing the sample. Grinding samples into smaller homogeneous particles increases the specific surface area, which accelerates acid digestion by MAWD.
However, care must be taken to avoid any contamination due to abrasion during grinding. Samples should not be homogenized with blades containing the same metals as the elements to be determined after acid digestion. Consequently, ceramic blades are more frequently used compared to metal blades. Homogenization is often a major contributing factor to cross-contamination, usually from improperly cleaned inventory and instrument components, resulting in systematic errors. After use, each component of the mixer should be thoroughly cleaned and washed with ultrapure water.
Cryogenic grinding is used when homogenizing samples that are more difficult to break up. The sample is frozen using liquid nitrogen (which has -196 °C), making it brittle and easier to homogenize49,50.
Acid digestion
One of the most frequently used oxidizing acids in acid digestion procedures is HNO3. It is usually used in combination with a low amount of H2O2, which increases the oxidizing power of the acid, thus improving digestion efficiency20. A mixture of these two chemicals is frequently used for the digestion of organic samples, including food samples51. Digestion of organic samples in MAWD is carried out at elevated pressure and temperatures that exceeds the boiling point (121 °C) of HNO3 azeotrope at atmospheric pressure27. In MAWD the boiling point of HNO3 rises to 176 °C as the pressure is increased to 5 atm27. The temperature at which acid digestion is performed in MAWD cannot be achieved in open systems because the HNO3 would evaporate, reducing the efficiency of acid digestion.
During digestion in closed vessels by MAWD, HNO3 reacts with the organic sample under harsh reaction conditions, forming gaseous products such as CO2, H2O, and NO (Equation 5)52,53. The benefit of using closed reaction vessels is the lower volume and concentration of the acid required for digestion, as HNO3 is constantly being regenerated. This regeneration process occurs for as long as O2 is present in the reaction vessel. The primary source of O2 is H2O2, which is thermally unstable and breaks down into H2O and O2 (Equation 6). In the reaction vessel, NO reacts with O2 to form NO2 (Equation 7). Formed NO2 then dissolves in H2O, resulting in the formation of HNO3 and HNO2 (Equation 8). The produced HNO2 subsequently degrades into H2O, NO2, and NO (Equation 9), completing the regeneration mechanism53,54. The newly formed NO and NO2 then react by the above-mentioned processes.
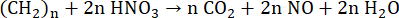 (5)
(5)
Where n stands for the number of carbon atoms.
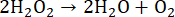 (6)
(6)
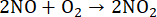 (7)
(7)
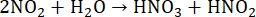 (8)
(8)
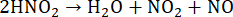 (9)
(9)
When organic samples react with HNO3, the metals present in their chemical structure will form water-soluble nitrates55. Since MAWD aims to convert solids into liquids, the formation of water-soluble salts is desired.
For different samples, different combinations of acids can be employed due to the complexity of the sample composition. Since less easily degradable organic and especially inorganic samples cannot be dissolved solely with HNO3, other acids such as HCl, HF, HClO4, and H2SO4 are also used21,56.
Non-oxidizing HCl at elevated temperatures is used for the digestion of salts such as carbonates, phosphates, oxides, borates, sulfides, and fluorides28,55. When HCl is combined with HNO3 in a molar ratio of 3:1, aqua regia is formed, which improves the oxidizing abilities compared to HCl and HNO3 alone due to the formation of nitrosyl chloride (NOCl), Cl2, and H2O (Equation 10)57. Aqua regia is able to dissolve noble metals such as Au, Pt, Pd28.
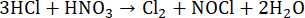 (10)
(10)
For the digestion of silicates, HF is frequently used, since it breaks strong bonds between Si and O. When HF interacts with silicate samples (minerals, soil), hexafluorosilicic acid (H2SiF6) is formed (Equation 11)19,58. However, despite HF's ability to digest silicates, it exhibits several drawbacks, including the formation of insoluble fluoride salts6, the formation of volatile products with heavy metals19, and volatile SiF427. Moreover, HF cannot be used with glassware and quartz reaction vessels as it dissolves them18.
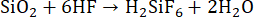 (11)
(11)
Reaction vessels for acid digestion
The reaction vessels used in MAWD are designed to withstand high temperatures and elevated pressures during acid digestion. These reaction vessels also exhibit good microwave permeability, allowing microwaves to pass through without being absorbed20. Microwaves passing through the reaction vessels will reach water molecules, which will effectively absorb them since they are polar, consequently heating up the solution containing the sample59. Only the liquid phase in the reaction vessels absorbs the microwave radiation while the gaseous phase does not, resulting in a high increase in temperature with a slight increase in pressure18.
To minimize contamination and analyte loss, reaction vessels are hermetically sealed, preventing any materials from escaping or entering the vessels.
The most commonly used materials for reaction vessels are synthetic polytetrafluoroethylene (PTFE), copolymerized PTFE known as TFM, perfluoroalkoxy alkane (PFA), and quartz52,60. These materials are chemically inert to most chemicals used in acid digestion, except for quartz vessels, in which HF dissolves. Using only one type of reaction vessel in every experiment is crucial, as using different types of vessels may result in different reaction conditions when subjected to microwave heating. At lower reaction temperatures, PTFE, PFA, and TFM-PTFE reaction vessels are used, while at temperatures above 300 °C, quartz vessels are recommended52. This is because polymers deteriorate and decompose at higher temperatures.
Evaluating the efficiency of acid digestion
There are several ways to evaluate the efficiency of acid digestion. The color of the solution can be used to evaluate whether complete or partial digestion of the sample has taken place. Usually, a colorless or slightly yellow coloration of the solution is an indicator of successful digestion, while the darker yellow, orange, green, or brown color of the solution suggests that the digestion process was unsuccessful, meaning that partial digestion took place61. In some cases, undigested organic or inorganic particulates may be present in the reaction mixture after digestion, requiring filtration before the sample can be introduced into the instrument for elemental determination. Removal of undigested particulates prevents system clogging and plasma instability in the case of ICP-OES and ICP-MS31.
The efficiency of acid digestion can also be experimentally evaluated through measurements of residual carbon content (RCC) and RA. RCC represents the amount of organic carbon remaining in the solution that was not converted to CO2 during digestion62. A lower value of RCC is preferred to reduce non-spectral and spectral interferences (e.g., 40Ar12C+) in the elemental determination63,64. RCC measurements are performed using ICP-OES. The carbon content is determined at an emission wavelength of 193.091 nm65,66,67. The efficiency of acid digestion is related to the consumption of chemicals. The more acid is consumed, the lower RCC values will be determined25.
The acid is continually consumed during digestion as it reacts with the sample. In most cases, a small amount of acid remains unreacted. The amount of RA can be determined by titration with NaOH10 or KOH25,54. Lower values of RA are preferred, as the higher acid concentration in the final digested solution can increase the background signal in analytical techniques such as ICP-MS25 and ICP-OES68. Higher RA values may also indicate the use of lower initial acid concentration for digestion69.
Multi-element determination with ICP-MS
ICP-MS consists of several components. The peristaltic pump pumps the sample solution from the autosampler to the nebulizer. The liquid sample is then converted into an aerosol by the nebulizer by mixing it with Ar gas. Subsequently, the spray chamber filters aerosol droplets, thus allowing the introduction of the finest aerosol droplet fraction into the plasma70. Ar plasma is generated and maintained within the torch by the radiofrequency coil, resulting in temperatures of approximately 10,000 K70. The aerosol is atomized and ionized in the Ar plasma. Ions then continue through the interface into the high vacuum region. Ion optics guide the ions through the collision cell, where the stream of He gas collides with the monoatomic ions of the analytes and polyatomic ions. Since polyatomic ions are larger than analytes of the same nominal mass, they collide more frequently with He, lose more kinetic energy, and are thus efficiently removed71. In the next step, ions reach the mass analyzer (in the present case, quadrupole). In the mass analyzer, the ions are separated based on their mass-to-charge ratio (m/z)72. Following the separation by m/z, the ions reach the detector (in the present case, an electron multiplier) (Figure 10).
Critical steps and limitations
There are several critical steps and some limitations within the protocol. Ensuring the samples are entirely dry before continuing the process and avoiding contamination are crucial steps in homogenization. To prevent contamination, effort must be taken to keep all glassware clean during the whole process73, as it may affect the accuracy of the analysis. In the event of contamination, the sample must be discarded, and the preparation process repeated, which can be time-consuming. When applying this protocol to other samples not described in this protocol, complete digestion might not be achieved, as some samples may require higher temperatures and different chemicals to completely dissolve all metals present in the sample. For acid digestion, high purity chemicals are needed, which can be expensive. Using high purity chemicals helps minimize interferences, ensuring improved reliability, accuracy, and precision of measurements performed by ICP-MS. The sample preparation process is time-consuming and has a low sample throughput as the preparation can last several days (drying, homogenization, acid digestion), limiting the number of samples that can be prepared in one day.
When performing multi-element determination with ICP-MS, spectral interferences (polyatomic and isobaric) can be encountered. Polyatomic interferences, which usually occur in plasma, combine at least two isotopes, while isobaric interferences represent isotopes of other elements with the same m/z as measured analytes74. Eliminating these interferences (e.g., with a collision cell) is important. In addition to spectral interferences, the results are also affected by non-spectroscopic interferences consisting of sample introduction into the ICP-MS instrument, aerosol droplet size distribution, plasma stability, ion transport through the interface etc.75.
The protocol described herein has the potential for other applications apart from that for the food samples. With slight modifications in the homogenization and acid digestion steps, it could be adapted for the preparation of inorganic samples, soil76, electronic waste28, etc. The adjustments can involve using different chemicals, varying their volumes, and altering the digestion temperature to suit different sample types. Moreover, as technology and methodologies evolve, further improvements and automation could be incorporated into the protocol, increasing its efficiency, and reducing the overall sample preparation time.
In summary, this protocol demonstrates the homogenization of food samples in a laboratory mixer, microwave-assisted wet acid digestion using a mixture of 68 wt% HNO3 and 30 wt% H2O2 at an elevated temperature and pressure, and elemental determination with ICP-MS. The protocol can be used to train personnel in preparing samples for elemental determination as the protocol provides step-by-step instructions and explains the theory behind homogenization, acid digestion, and elemental determination.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge the financial support of the Slovenian Research Agency (Grant Nos. P2-0414, P2-0118, J1-2470, NK-0001, and J1-4416).
Materials
| Ar gas | Messer | 7440-37-1 | Ar 5.0 gas (purity 99.999%). |
| AS-10 Autosampler system | Shimadzu | Autosampler connected to the ICP-MS, containing 68 ports for samples. | |
| Automatic pipettes | Sartorius | 200 µL, 1 mL, and 5 mL automatic pipettes. | |
| Balance XSE104 | Mettler Toledo, Columbus, Ohio, USA | Analytical balance scale with a maximum weighing mass of 120 g. | |
| Ceramic knife | Ceramic knife used for cutting fresh food samples. | ||
| Dessicator | Glass desiccator with lumps of silica gel. | ||
| ETHOS LEAN | Milestone, Sorisole, Italy | Microwave system for wet acid digestion in closed 100 mL vessels made of TFM-PTFE. | |
| Fume hood | Laboratory fume hood with adjustable air flow. | ||
| Glass beakers RASOTHERM | CarlRoth GmbH + Co.KG | 50 mL, 250 mL glass beakers | |
| Glass funnels | Small glass funnels fitting into the neck of volumetric flasks. | ||
| He gas | Messer | 7440-59-7 | He 5.0 gas (purity 99.999%). |
| Hydrogen peroxide | ThermoFisher Scientific | 7722-84-1 | Hxdrogen peroxide 100 volumes 30 wt.% solution. Laboratory reagent grade. |
| ICP multi-element standard solution VIII | Supelco | 109492 | 100 mg/L ICP multi-element standard solution containing 24 elements (Al, B, Ba, Be, Bi, Ca, Cd, Co, Cr, Cu, Fe, Ga, K, Li, Mg, Mn, Na, Ni, Pb, Se, Sr, Te, Tl, Zn) in 2 % dilute nitric acid. |
| ICPMS 2030 | Shimadzu | Inductively coupled plasma mass spectrometry system for multi-element analysis of digested samples. | |
| ICP-MS Tuning Solution A | CarlRoth GmbH + Co.KG | 250 mL tuning solution containing 6 elements (Be, Bi, Ce, Co, In, Mn) in 1 % nitric acid. | |
| KIMTECH Purple Nitrile gloves | Kimberly-Clark GmbH | Disposable Purple Nitrile gloves (S, M or L). | |
| Laboratory coat | Any available supplier | / | |
| Mixer B-400 | BÜCHI Labortechnik AG, Flawil, Switzerland | Laboratory mixer with ceramic blades. | |
| Nitric acid | ThermoFisher Scientific | 7697-37-2 | Nitric acid, trace analysis grade, 68 wt%, density 1.42, Primar Plus, For Trace Metal Analysis. |
| Plastic centrifuge tubes | Isolab | 50 mL plastic centrifuge tubes with screw caps, single use. | |
| Plastic syringes Injekt | B. Braun | 2 pice, single use 20 mL syringes. | |
| Plastic tubes for autosampler | Shimadzu | 046-00147-04 | Plastic tubes for autosampler, 15 mL capacity, 16 mm diameter, 100 mm length. |
| Plastic waste containers | Plastic containers for the removal of chemicals after the cleaning procedure of reaction vessels. | ||
| Protective googles | / | ||
| Samples (broccoli, sausage, noodles, zucchini, mushrooms) | Fresh samples, which were dried to a constant weight and homogenized during the procedure. The samples were purchased from a local shop. | ||
| Spatula | Plastic spatula. | ||
| Sterilizator Instrumentaria ST 01/02 | Instrumentaria | Dryer with adjustable temperature. | |
| Syringe filters | CHROMAFIL Xtra | 729212 | Syringe filters with polypropylene housing and polyamide hydrophilic membrane. Membrane diameter 25 mm, membrane pore size 0.2 µm. |
| Ultrapure water | ELGA Labwater, Veolia Water Technologies. | Ultrapure water with a resistivity of 18.2 MΩcm, obtained with laboratory water purification system. | |
| Volumetric flasks | 25 mL glass volumetric flasks. |
References
- Catenza, K. F., Donkor, K. K. Determination of heavy metals in cannabinoid-based food products using microwave-assisted digestion and ICP-MS. Food Analytical Methods. 15, 2537-2546 (2022).
- Güven, D. E., Akinci, G. Comparison of acid digestion techniques to determine heavy metals in sediment and soil samples. Gazi University Journal of Science. 24, 29-34 (2011).
- Soós, &. #. 1. 9. 3. ;., Bódi, &. #. 2. 0. 1. ;., Várallyay, S., Molnár, S., Kovács, B. Microwave-assisted sample preparation of hungarian raw propolis in quartz vessels and element analysis by ICP-OES and ICP-MS for geographical identification. Talanta. 233, 122613 (2021).
- De Oliveira, A. F., Da Silva, C. S., Bianchi, S. R., Nogueira, A. R. A. The use of diluted formic acid in sample preparation for macro- and microelements determination in foodstuff samples using ICP-OES. Journal of Food Composition and Analysis. 66, 7-12 (2018).
- Moor, C., Lymberopoulou, T., Dietrich, V. J. Determination of heavy metals in soils, sediments and geological materials by ICP-AES and ICP-MS. Microchimica Acta. 136 (3), 123-128 (2001).
- Kuznetsova, O. V., Burmii, Z. P., Orlova, T. V., Sevastyanov, V. S., Timerbaev, A. R. Quantification of the diagenesis-designating metals in sediments by ICP-MS: Comparison of different sample preparation methods. Talanta. 200, 468-471 (2019).
- Buechler, D. T., et al. Comprehensive elemental analysis of consumer electronic devices: Rare earth, precious, and critical elements. Waste Management. 103, 67-75 (2020).
- Riisom, M., Gammelgaard, B., Lambert, I. H., Stürup, S. Development and validation of an ICP-MS method for quantification of total carbon and platinum in cell samples and comparison of open-vessel and microwave-assisted acid digestion methods. Journal of Pharmaceutical and Biomedical Analysis. 158, 144-150 (2018).
- Stricker, A., et al. Impurities in commercial titanium dental implants – a mass and optical emission spectrometry elemental analysis. Dental Materials. 38 (8), 1395-1403 (2022).
- Bressy, F. C., Brito, G. B., Barbosa, I. S., Teixeira, L. S. G., Korn, M. G. A. Determination of trace element concentrations in tomato samples at different stages of maturation by ICP-OES and ICP-MS following microwave-assisted digestion. Microchemical Journal. 109, 145-149 (2013).
- Lachas, H., et al. Determination of 17 trace elements in coal and ash reference materials by ICP-MS applied to milligram sample sizes. Analyst. 124 (2), 177-184 (1999).
- Meermann, B., Nischwitz, V. ICP-MS for the analysis at the nanoscale-a tutorial review. Journal of Analytical Atomic Spectrometry. 33 (9), 1432-1468 (2018).
- Lemos, M. S., Dantas, K. G. F. Evaluation of the use of diluted formic acid in sample preparation for elemental determination in crustacean samples by mip oes. Biological Trace Element Research. 201 (7), 3513-3519 (2022).
- Mohammed, E., Mohammed, T., Mohammed, A. Optimization of acid digestion for the determination of hg, as, se, sb, pb and cd in fish muscle tissue. MethodsX. 4, 513-523 (2017).
- Sobhanardakani, S., Tayebi, L., Farmany, A., Cheraghi, M. Analysis of trace elements (cu, cd, and zn) in the muscle, gill, and liver tissues of some fish species using anodic stripping voltammetry. Environmental Monitoring and Assessment. 184 (11), 6607-6611 (2012).
- Ostapczuk, P., Valenta, P., Rützel, H., Nürnberg, H. Application of differential pulse anodic stripping voltammetry to the determination of heavy metals in environmental samples. Science of The Total Environment. 60, 1-16 (1987).
- Gamela, R. R., Costa, V. C., Sperança, M. A., Pereira-Filho, E. R. Laser-induced breakdown spectroscopy (libs) and wavelength dispersive x-ray fluorescence (wdxrf) data fusion to predict the concentration of k, mg and p in bean seed samples. Food Research International. 132, 109037 (2020).
- Hu, Z., Qi, L., Holland, H. D., Turekian, K. K. . Treatise on geochemistry (second edition). , 87-109 (2014).
- Ojeda, C. B., Rojas, F. S., Worsfold, P., Poole, C., Townshend, A., Miró, M. . Encyclopedia of analytical science (third edition). , 85-97 (2019).
- Bizzi, C. A., Nóbrega, J. A., Barin, J. S., Flores, &. #. 2. 0. 1. ;. M. d. M. . Microwave-assisted sample preparation for trace element analysis. , 179-204 (2014).
- Twyman, R. M., Worsfold, P., Townshend, A., Poole, C. . Encyclopedia of analytical science (second edition). , 146-153 (2005).
- Traversa, L. C., et al. Closed-vessel conductively heated digestion system for the elemental analysis of agricultural materials by high-resolution continuum source flame atomic absorption spectrometry (hr-cs faas). Analytical Letters. 56 (15), 2443-2456 (2023).
- Rondan, F. S. Determination of se and te in coal at ultra-trace levels by ICP-MS after microwave-induced combustion. Journal of Analytical Atomic Spectrometry. 34 (5), 998-1004 (2019).
- Muller, E. I., et al. Microwave-assisted wet digestion with H2O2 at high temperature and pressure using single reaction chamber for elemental determination in milk powder by ICP-OES and ICP-MS. Talanta. 156 – 157, 232-238 (2016).
- Pardinho, R. B., et al. Determination of toxic elements in yerba mate by ICP-MS after diluted acid digestion under O2 pressure. Food Chemistry. 263, 37-41 (2018).
- Barela, P. S., et al. Microwave-assisted digestion using diluted nitric acid for further trace elements determination in biodiesel by sf-ICP-MS. Fuel. 204, 85-90 (2017).
- Müller, E. I., Mesko, M. F., Moraes, D. P., Korn, M. D. G. A., Flores, &. #. 2. 0. 1. ;. M. M., Flores, &. #. 2. 0. 1. ;. M. d. M. . Microwave-assisted sample preparation for trace element analysis. , 99-142 (2014).
- Das, S., Ting, Y. -. P. Evaluation of wet digestion methods for quantification of metal content in electronic scrap material. Resources. 6 (4), 64 (2017).
- Nóbrega, J. A., et al. Microwave-assisted digestion of organic samples: How simple can it become. Talanta. 98, 272-276 (2012).
- Bizzi, C. A., et al. Evaluation of oxygen pressurized microwave-assisted digestion of botanical materials using diluted nitric acid. Talanta. 83 (5), 1324-1328 (2011).
- Da Silva, I. J. S., Lavorante, A. F., Paim, A. P. S., Da Silva, M. J. Microwave-assisted digestion employing diluted nitric acid for mineral determination in rice by ICP-OES. Food Chemistry. 319, 126435 (2020).
- Bizzi, C. A., Flores, E. M. M., Barin, J. S., Garcia, E. E., Nóbrega, J. A. Understanding the process of microwave-assisted digestion combining diluted nitric acid and oxygen as auxiliary reagent. Microchemical Journal. 99 (2), 193-196 (2011).
- Le Gresley, A., Ampem, G., De Mars, S., Grootveld, M., Naughton, D. P. 34;Real-world" evaluation of lipid oxidation products and trace metals in french fries from two chain fast-food restaurants. Frontiers in Nutrition. 8, 620952 (2021).
- Kutscher, D., Cui, J., Cojocariu, C. Key steps to create a sample preparation strategy for inductively coupled plasma (ICP) or ICP-mass spectrometry (ICP-MS) analysis. Spectroscopy. 37 (1), 38-42 (2022).
- Mccurdy, E., Proper, W. Improving ICP-MS analysis of samples containing high levels of total dissolved solids. Spectroscopy. 29 (11), 14 (2014).
- . Membrane filtration: How to choose the appropriate filter material for every sample Available from: https://www.cytivalifesciences.com/en/us/solutions/lab-filtration/knowledge-center/membrane-filtration-choosing-the-correct-type-of-filter (2023)
- May, T. W., Wiedmeyer, R. H. A table of polyatomic interferences in ICP-MS. Atomic Spectroscopy-Norwalk Connecticut. 19, 150-155 (1998).
- Taleuzzaman, M. Limit of blank (lob), limit of detection (lod), and limit of quantification (loq). Organic & Medicinal Chemistry International Journal. 7 (5), 127-131 (2018).
- Willner, J., et al. A versatile approach for the preparation of matrix-matched standards for la-ICP-MS analysis – standard addition by the spraying of liquid standards. Talanta. 256, 124305 (2023).
- Green, J. M. Peer reviewed: A practical guide to analytical method validation. Analytical Chemistry. 68 (9), 305A-309A (1996).
- Xu, J., et al. A critical view on spike recovery for accuracy evaluation of analytical method for medicinal herbs. Journal of Pharmaceutical and Biomedical Analysis. 62, 210-215 (2012).
- Massart, D. L., et al. . Handbook of chemometrics and qualimetrics: Part a. , (1998).
- UNOO. . Guidance for the validation of analytical methodology and calibration of equipment used for testing of illicit drugs in seized materials and biological specimens: A commitment to quality and continuous improvement. , (2009).
- Berben, G., et al. Guidelines for sample preparation procedures in GMO analysis. Publications Office of the European Union. EUR27021, JRC94042 (2014).
- Lacorte, S., Bono-Blay, F., Cortina-Puig, M., Pawliszyn, J. . Comprehensive sampling and sample preparation. , 65-84 (2012).
- Kaur, G. J., Orsat, V., Singh, A. An overview of different homogenizers, their working mechanisms and impact on processing of fruits and vegetables. Critical Reviews in Food Science and Nutrition. 63 (14), 2004-2017 (2021).
- Baudelaire, E. D., Bhandari, B., Bansal, N., Zhang, M., Schuck, P. . Handbook of food powders. , 132-149 (2013).
- Jung, H., Lee, Y. J., Yoon, W. B. Effect of moisture content on the grinding process and powder properties in food: A review. Processes. 6 (6), 69 (2018).
- Krejčová, A., Pouzar, M., Černohorský, T., Pešková, K. The cryogenic grinding as the important homogenization step in analysis of inconsistent food samples. Food Chemistry. 109 (4), 848-854 (2008).
- Balasubramanian, S., Gupta, M. K., Singh, K. Cryogenics and its application with reference to spice grinding: A review. Critical Reviews in Food Science and Nutrition. 52, 781-794 (2012).
- Potočnik, D., Jagodic Hudobivnik, M., Mazej, D., Ogrinc, N. Optimization of the sample preparation method for determination of multi-elemental composition in fruit samples by ICP-MS analysis. Measurement: Sensors. 18, 100292 (2021).
- DINEN ISO. . Theory of sample preparation using acid digestion, pressure digestion and microwave digestion (microwave decomposition). , (1972).
- Bizzi, C. A., Barin, J. S., Oliveira, J. S., Cravotto, G., Flores, E. M. Microwave-assisted oxidation of organic matter using diluted hno 3 under o 2 pressure: Rationalization of the temperature gradient effect for acid regeneration. Journal of the Brazilian Chemical Society. 28, 1673-1681 (2017).
- Castro, J. T., et al. A critical evaluation of digestion procedures for coffee samples using diluted nitric acid in closed vessels for inductively coupled plasma optical emission spectrometry. Talanta. 78 (4), 1378-1382 (2009).
- Ju, T., Han, S., Meng, Y., Song, M., Jiang, J. Occurrences and patterns of major elements in coal fly ash under multi-acid system during microwave digestion processes. Journal of Cleaner Production. 359, 131950 (2022).
- Matusiewicz, H. . Comprehensive analytical chemistry. 41, 193-233 (2003).
- Sheng, P. P., Etsell, T. H. Recovery of gold from computer circuit board scrap using aqua regia. Waste Management & Research. 25 (4), 380-383 (2007).
- Sucharova, J., Suchara, I. Determination of 36 elements in plant reference materials with different si contents by inductively coupled plasma mass spectrometry: Comparison of microwave digestions assisted by three types of digestion mixtures. Analytica Chimica Acta. 576, 163-176 (2006).
- Santos, H. M., et al. Microwave-assisted digestion using diluted HNO3 and H2O2 for macro and microelements determination in guarana samples by ICP-OES. Food Chemistry. 273, 159-165 (2019).
- Usepa, E. Method 3052: Microwave assisted acid digestion of siliceous and organically based matrices. United States Environmental Protection Agency, Washington, DC USA. , (1996).
- Elemental analysis manual, 4.7 inductively coupled plasma-mass spectrometric determination of arsenic, cadmium, chromium, lead, mercury, and other elements in food using microwave assisted digestion. Available from: https://s27415.pcdn.co/wp-content/uploads/2020/01/64ER20-7/Heavy_Metals/1-FDA-EAM-4.7-Inductively-Coupled-Plasma-MS-Determination-of-Arsenic-Cadmium-Chromium-Lead-Mercury-etc.pdf (2015)
- Leme, A. B. P., Bianchi, S. R., Carneiro, R. L., Nogueira, A. R. A. Optimization of sample preparation in the determination of minerals and trace elements in honey by ICP-MS. Food Analytical Methods. 7 (5), 1009-1015 (2014).
- Vanhoe, H., Goossens, J., Moens, L., Dams, R. Spectral interferences encountered in the analysis of biological materials by inductively coupled plasma mass spectrometry. Journal of Analytical Atomic Spectrometry. 9, 177-185 (1994).
- Loula, M., Kaňa, A., Mestek, O. Non-spectral interferences in single-particle ICP-MS analysis: An underestimated phenomenon. Talanta. 202, 565-571 (2019).
- Muller, C. C. Feasibility of nut digestion using single reaction chamber for further trace element determination by ICP-OES. Microchemical Journal. 116, 255-260 (2014).
- Muller, A. L. H., Oliveira, J. S. S., Mello, P. A., Muller, E. I., Flores, E. M. M. Study and determination of elemental impurities by ICP-MS in active pharmaceutical ingredients using single reaction chamber digestion in compliance with usp requirements. Talanta. 136, 161-169 (2015).
- Duarte, F. A., et al. Microwave-induced combustion in disposable vessels: A novel perspective for sample digestion. Analytical Chemistry. 92 (12), 8058-8063 (2020).
- Novaes, C. G., et al. A review of multivariate designs applied to the optimization of methods based on inductively coupled plasma optical emission spectrometry (ICP-OES). Microchemical Journal. 128, 331-346 (2016).
- Damak, F., Asano, M., Baba, K., Ksibi, M., Tamura, K. Comparison of sample preparation methods for multielements analysis of olive oil by ICP-MS. Methods and Protocols. 2 (3), 72 (2019).
- Thomas, R. . Practical guide to ICP-MS: A tutorial for beginners. , (2013).
- Yamada, N. Kinetic energy discrimination in collision/reaction cell ICP-MS: Theoretical review of principles and limitations. Spectrochimica Acta Part B: Atomic Spectroscopy. 110, 31-44 (2015).
- The 30-minute guide to ICP-MS. Perkin Elmer, Shelton CT Available from: https://resources.perkinelmer.com/corporate/cmsresources/images/44-74849tch_icpmsthirtyminuteguide.pdf (2001)
- Gonzálvez, A., Armenta, S., Pastor, A., De La Guardia, M. Searching the most appropriate sample pretreatment for the elemental analysis of wines by inductively coupled plasma-based techniques. Journal of Agricultural and Food Chemistry. 56 (13), 4943-4954 (2008).
- Lum, T. -. S., Leung, K. . S. -. Y. Strategies to overcome spectral interference in ICP-MS detection. Journal of Analytical Atomic Spectrometry. 31 (5), 1078-1088 (2016).
- Agatemor, C., Beauchemin, D. Matrix effects in inductively coupled plasma mass spectrometry: A review. Analytica Chimica Acta. 706 (1), 66-83 (2011).
- Melaku, S., Dams, R., Moens, L. Determination of trace elements in agricultural soil samples by inductively coupled plasma-mass spectrometry: Microwave acid digestion versus aqua regia extraction. Analytica Chimica Acta. 543 (1), 117-123 (2005).

