Bone Marrow Aspiration: A Method to Obtain Bone Marrow to Examine Cell Morphology
Abstract
Source: Cloos, J. et al. Comprehensive Protocol to Sample and Process Bone Marrow for Measuring Measurable Residual Disease and Leukemic Stem Cells in Acute Myeloid Leukemia. J. Vis. Exp. (2018).
This video describes the technique of aspirating bone marrow from iliac spine and smear preparation for biopsy. In the example, we perform bone marrow aspiration from an acute myeloid leukemia (AML) patient and examine the cells to check for measurable residual disease (MRD), an important prognostic biomarker.
Protocol
All procedures involving human participants have been performed in compliance with the institutional, national, and international guidelines for human welfare and have been reviewed by the local institutional review board.
1. Bone marrow aspiration and sample preparation
- Patient and material preparation
- Fill one or two 10 mL syringes with 1% lidocaine using a 16-gauge needle. Replace the needle for a 21-gauge needle.
- Put two drops of 5% EDTA on a watch-glass.
- Set up glass slides (n=15 with consistent patient numbering and date) for the smear preparations.
- Place patient in lateral decubitus position. Locate the superior posterior iliac spine and mark with pen.
NOTE: In general, the posterior superior iliac spine is located one hand width distal to the iliac crest and one hand width lateral to the midline. In females, the actual spine may be a bit more lateral, in some men a bit more medial. - Disinfect the skin with chlorhexidine 0.5-1% in ethanol from the intended biopsy area outward in circles.
- Open the package of sterile gloves, put on the sterile gloves and lay down the package on the table to create a sterile field. Open the package with the aspiration needle and place it onto the sterile field.
- Infiltrate the skin and subcutaneous tissue and finally periosteum. At the periosteum, administer the lidocaine in such a way that an area of 1 cm diameter has been anesthetized.
NOTE: Adequate administration of lidocaine to the periosteum is one of the most important factors for patient comfort. Test whether the periosteum has been adequately anesthetized by tapping the intended biopsy location with the introduced needle and ask if the patient feels any pain. Of note: Children are fully anesthetized during the whole procedure. - Hold the aspirate needle (15 Ga x 2.8") with the proximal end in the palm and index finger against the side of the needle's metal shaft near the tip; this position allows better control.
- Introduce the needle with a rotating movement (by quickly alternating pronating/supinating movement) through the skin toward the iliac spine and bring the needle into contact with the posterior iliac spine.
- Ensure that is needle is introduced to the anesthetized area of the periosteum; the patient should feel only pressure and no pain. If the patient feels pain, either reposition the needle, or administer more lidocaine.
- Using gentle but firm pressure, advance the needle while rotating it in an alternating clockwise-counter clockwise motion. Entrance into the marrow cavity is generally detected by decreased resistance.
- Remove the stylet from the needle. Attach a 10 mL empty syringe to the needle.
- Apply negative pressure by withdrawing the syringe plunger with a gentle pull. Warn the patient that they may feel a cramping sensation and pain when marrow is being aspirated. If not enough bone marrow spicules are released, another aspiration should be performed with one quick draw. Most spicules will be in the first 1-2 mL obtained from the initial pull. Aspirate only 1-2 mL to avoid diluting the sample.
NOTE: Dilution will result in hemodilution and may confound MRD results. - Remove the syringe and replace the stylet into the aspiration needle. Eject part of the marrow into a watch-glass and the rest into an 8 mL tube coated with heparin.
NOTE: Invert each tube immediately after placing the marrow aspirate into the specimen tube to ensure adequate anticoagulation. Bone marrow coagulation is often the cause of inappropriate material. Additional bone marrow can be aspirated from the same spot, but preferably, the needle is advanced 5-10 mm before a new aspirate is taken. Preferably not more than 2 draws per insertion level should be taken. Make sure to mark the tubes with increasing numbers representing the first, second and/or consecutive draws. It is common rule to use the first draw for the most relevant diagnostic analysis. - Alternatively, retract the needle from the insertion place and reintroduce it into the marrow cavity a few millimeters away from the original insertion place and repeat the procedure.
NOTE: Do not aspirate more than 1-2 mL per pull, to avoid significant blood contamination. - Repeat as often as material is needed. When sufficient material for the specific clinical study protocol is aspirated remove the bone marrow needle.
NOTE: In case of slow or otherwise difficult bone marrow aspirations the use of syringes that were pre-flushed with anticoagulant may be helpful. In case of a dry tap, perform a trephine biopsy which is outside the scope of this manuscript.
2. Preparation of smears for morphologic examination
- For optimal morphological assessment, pick out spicules (e.g. using a plastic spatula) from the aspirate in the watch-glass and place them on a glass slide.
- Gently place another glass slide over the slide with the marrow and gently slide; avoid any pressure.
NOTE: Only in case of very large bone marrow spicules slight pressure may be exerted, to decrease the thickness of the cell spreading. The procedure benefits from help from an assistant who can handle the specimen tubes. Accurate labelling of the tubes with the patient number and number of the sequential bone marrow draw is crucial. - Dry the slide thoroughly, and then perform May Giemsa Grünwald staining (see table of materials). Examine under the light microscope for morphology (see Figure 1).
NOTE: Figure 1A shows the smears of healthy bone marrow consisting of different functional cell types while Figure 1B an AML patient with predominantly leukemic blasts. To better define the residual leukemic burden, immunophenotyping needs to be performed.
Representative Results
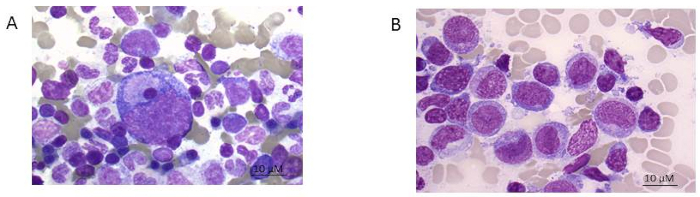
Figure 1: Bone marrow smear. A) healthy donor and B) AML patient (FAB 5 subtype). Giemsa stain shown at 100x magnification. Please click here to view a larger version of this figure.
Offenlegungen
The authors have nothing to disclose.

