Monitoring Neutrophil Recruitment Dynamics and Bacterial Burden at the Infection Site in a Murine Model
Abstract
Source: Anderson, L. S., et al. A Mouse Model to Assess Innate Immune Response to Staphylococcus aureus Infection. J. Vis. Exp. (2019).
This video demonstrates in vivo imaging to study the neutrophil recruitment dynamics at an infection site in an immunocompromised, transgenic mouse with GFP-expressing neutrophils. It involves injecting luminescent bacteria to cause an infection. This leads to neutrophil recruitment to the infection site, whose tracking is done by imaging.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Mouse Source and Housing
- Derive LysM-eGFP mice on a C57BL/6J genetic background. Derive LysM-EGFP×MyD88-/- mice by crossing LysM-EGFP mice with MyD88-/- mice on a C57BL/6J background.
- House mice in a vivarium. For these studies, animals were housed at the University of California, Davis in groups prior to surgery and single-housed following surgery. Use mice between the ages of 10-16 weeks.
2. Bacterial Preparation and Quantification
- Remove the bioluminescent S. aureus strain of interest from -80 °C storage to thaw on ice. Streak on a 5% bovine blood agar plate. Incubate the streaked plate in a humidified incubator at 37 °C overnight (16 h).
NOTE: In this protocol, the ALC2906 SH1000 strain was used. This strain contains the shuttle plasmid pSK236 with the penicillin-binding protein 2 promoters fused to the luxABCDE reporter cassette from Photohabdus luminescens. - Prepare tryptic soy broth (TSB) by mixing 0.03 g of TSB powder per mL of pure water, and autoclave TSB to sterilize. When cooled, add any necessary antibiotics using a sterile technique. In this protocol, add 10 µg/mL of chloramphenicol to the TSB to select for expression of the pSK236 shuttle plasmid, which contains the bioluminescence luxABCDE cassette.
- Pick 3-4 separate colonies from the S. aureus plate into TSB with 10 µg/mL chloramphenicol to start an overnight culture. Incubate bacteria on an incubating shaker at 37 °C overnight (16 h).
- Start a new bacterial culture from the overnight culture by diluting a sample 1:50 into TSB with 10 µg/mL chloramphenicol. Culture in an incubating shaker at 200 rpm and 37 °C.
- Two hours after splitting the S. aureus, monitor the optical density at 600 nm (OD600) on a spectrophotometer. Observe the OD600 vs. time to find mid-logarithmic phase growth. For the ALC2906 SH1000 strain, an OD600 of 0.5 is mid-logarithmic and corresponds to a concentration of 1 x 108 CFU/mL (Figure 1).
- When OD600 is 0.5, wash bacteria 1:1 with ice-cold DPBS. Centrifuge the bacteria for 10 min at 3,000 x g and 4 °C. Carefully decant the supernatant and add additional chilled DPBS and vortex thoroughly. Centrifuge once more for 10 min at 3,000 x g and 4 °C.
- Carefully decant the supernatant. Resuspend the bacterial pellet at the desired concentration. For these studies, collect 3 mL of ALC2906 SH1000 and resuspend in 1.5 mL of PBS, correlating to a bacteria concentration of about 2 x 108 CFU/mL. Keep bacteria on ice until use.
- To verify bacteria concentration, dilute 100 µL of the bacterial sample 1:10,000 and 1:100,000 in PBS. Plate 20 µL aliquots on an agar plate. Incubate at 37 °C in a humidified incubator for 16 h. Count CFUs by gross examination and calculate a bacterial concentration the following day.
3. Excisional Skin Wounding and Inoculation with S. aureus
- Administer 100 µL of 0.03 mg/mL buprenorphine hydrochloride (~0.2 mg/kg) to each mouse via intraperitoneal injection.
- Twenty minutes post-injection, place 2-4 mice in a chamber with 2-3 LPM oxygen with 2-4% isoflurane. Once mice are fully anesthetized, transfer the mice one at a time to a nose cone connected to 2-3 LPM oxygen with 2-4% isoflurane. Verify mice are fully anesthetized by firmly pinching each rear paw between a thumb and forefinger. Proceed to the next step if the animal does not respond to the pinch.
- Shave a 1-inch by 2-inch section on the back of the mouse with electric clippers and clear the area of fur clippings using a clean wipe or gauze. Avoid using depilatory lotion because it may cause excess inflammation.
- Clean the back of the mouse first with 10% povidone-iodine-soaked gauze and then with 70% ethanol-soaked gauze. Clean the area in a spiral pattern, moving outward from the center of the surgical area. Wait approximately 1 min for the surgical area to dry prior to surgery.
- Hold the shaved back of the mouse loosely between two fingers and firmly press a sterile 6 mm punch biopsy at the center of the prepared surgical area. Do not pull the skin taut.
- Twist the punch biopsy to create a circular outline on the skin that fully cuts through the skin in at least one section of the outline. Be careful not to cut into the underlying fascia or tissue.
- Use sterile scissors and forceps to cut through the epidermis and dermis following the circular pattern imprinted by the punch biopsy.
4. S. aureus Inoculation
- Fill a 28 G insulin syringe with the desired bioluminescent bacterial inoculant. In this study, administer a concentration of 1 x 108 CFU/mL (50 µL). Do not administer more than 100 µL of volume.
- Inject 50 µL of inoculant between the fascia and tissue in the center of the wound on the back of the mouse. Ensure that the inoculant forms a bubble at the center of the wound with minimal leakage or dispersion.
- Pull the dermis to the side, hold the syringe nearly parallel to the tissue, and slowly push the syringe into the tissue until a sudden decrease in resistance is felt, which indicates piercing of the fascia. Carefully lead the syringe into the center of the wound and dispense the inoculant slowly. Remove the syringe slowly from the animal.
- Inject the same volume of sterile PBS into the wounds of uninfected animals as described above.
- Return the animal to its cage. Place the cage under a heat lamp or on top of a heating pad, and monitor the animal until recovery from anesthesia.
5. In Vivo BLI and FLI
- Initialize the whole animal imager through the instrument software. Anesthetize mice in a chamber receiving 2-3 LPM oxygen with 2-4% isoflurane. Deliver anesthesia to the nosecones inside the imager.
- Place the wounded and infected mouse into the imager. Position the mouse such that the wound is as flat as possible. Use the following sequence set-up to image the mice.
- Select Luminescence and Photograph as the imaging mode. The exposure time is 1 min at small binning and F/stop 1 (luminescence) and F/stop 8 (photograph). The emission filter is Open. Click the Acquire button to record the image.
- Select Fluorescence and Photograph as the imaging mode. The exposure time is 1 s at small binning and F/stop 1 (Fluorescence) and F/stop 8 (photograph) with an excitation wavelength of 465/30 nm and an emission wavelength of 520/20 nm with a high lamp intensity. Click the Acquire button to record the image.
- Return the animal to its cage and monitor it until recovery from anesthesia.
- Image mice daily as described above.
6. Image Analysis
- Open images to be quantified.
- Place a large circular region of interest (ROI) over the entire wound area including the surrounding skin for each mouse in the image. The neutrophil in vivo FLI EGFP signals and in vivo BLI S. aureus signals extend beyond the wound edge after several days and were included in these studies (Figures 2A and 2B). Click Measure ROI and record values for mean flux for each mouse. Plot the mean flux of each signal (p/s) versus time.
- If absolute numbers of neutrophils or S. aureus in the wound are desired, perform the following experiments.
- To correlate neutrophil numbers to the in vivo FLI EGFP signals, wound C57BL/6J mice as described above, and transfer a range of bone marrow-derived neutrophils (5 x 105 to 1 x 107) from LysM-EGFP or LysM-EGFPxMyD88-/- donors directly on top of different wounds. Image as described above and correlate the in vivo FLI EGFP signals to the known quantity of neutrophils.
- To correlate S. aureus CFU to the in vivo BLI signals, wound and infect mice as described above. On day 1 post-infection record in vivo BLI signals from the mice and immediately euthanize and chill carcasses. Excise the wound, homogenize the tissue, and plate bacterial dilutions on agar for overnight incubation. The next day, count colonies to determine CFU per wound.
- To measure wound healing fit a circular ROI over the wound edge and measure the area of the wound (cm2) and plot the fractional change from baseline vs. time (Figure 2C).
Representative Results
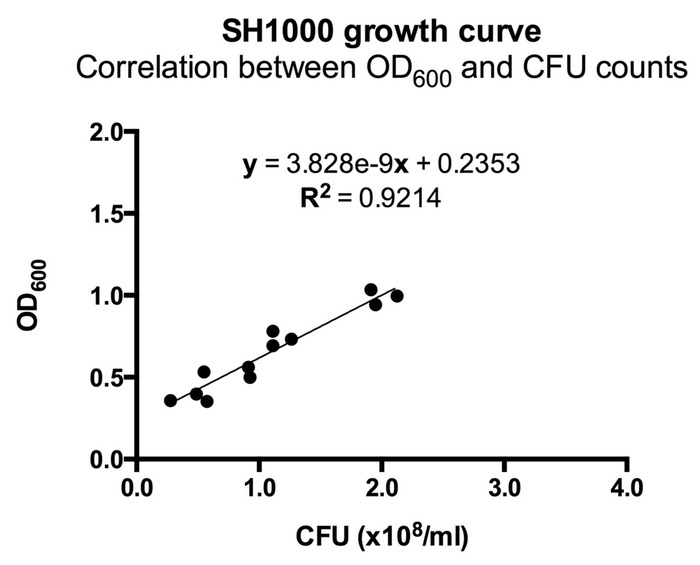
Figure 1: Correlation between OD600 and CFU counts for ALC2906. 3-4 ALC2906 SH1000 colonies were picked from an agar plate and transferred into TSB with 10 µg/mL chloramphenicol for overnight culture. The next day, the suspension was split 1:50 into TSB with 10 µg/mL chloramphenicol and cultured. Optical density at 600 nm (OD600) was measured in regular intervals after 2 hours using a spectrophotometer. At each measurement, the bacteria were diluted 1:100,000 in ice-cold PBS and aliquoted onto an agar plate for overnight incubation. CFUs were counted the following day to calculate the initial concentration and correlated to OD600. N = 3 with 4 different OD600 measurements per experiment.
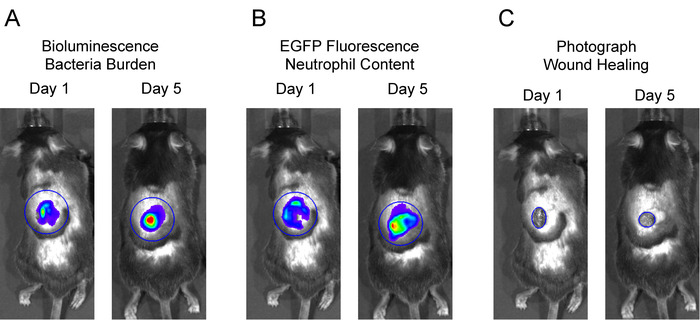
Figure 2: Representative regions of interest (ROIs) for data analysis. To analyze in vivo BLI and in vivo FLI signals, large, equivalent-sized ROIs were centered over the wound, and total flux (photons per second) was measured. To measure wound closure, an ROI was fit to the wound edge, and the area (cm2) was measured.
Offenlegungen
The authors have nothing to disclose.
Materials
| 14 mL Polypropylene Round-Bottom Tube | Falcon | 352059 | |
| 6mm Disposable Biopsy Punch | Integra Miltex | 33-36 | |
| Bioluminescent S. aureus | Lloyd Miller, Johns Hopkins | ALC 2906 SH1000 | |
| Bovine Blood Agar, 5%, Hardy Diagnostics | VWR | 10118-938 | |
| Buprenoprhine hydrochloride injectable | Western Medical Supply | 7292 | 0.3 mg/mL |
| C57BL/6J Mice | Jackson Labratory | 664 | |
| Chloramphenicol (crystalline powder) | Fisher BioReagents | BP904-100 | |
| DPBS (1X) | Gibco | 14190-144 | |
| Insulin Syringes | Becton, Dickson and Company | 329461 | .35 mm (28 G) x 12.7 mm (1/2'') |
| IVIS Spectrum In Vivo Imaging System | Perkin Elmer | 124262 | |
| Living Image Software – IVIS Spectrum Series | Perkin Elmer | 128113 | |
| LysM-eGFP Mice | Thomas Graff Albert Einstein College of New York | NA | |
| Microvolume Spectrophotometer | ThermoFisher Scientific | ND-2000 | |
| MyD88 KO Mice | Jackson Labratory | 9088 | |
| Non-woven sponges | AMD- Ritmed Inc | A2101-CH | 5 cm x 5 cm |
| Povidone Iodine 10% Solution | Aplicare | 697731 | |
| Tryptic Soy Broth | Becton, Dickson and Company | 211825 |

