Isolation and Preparation of Bacterial Cell Walls for Compositional Analysis by Ultra Performance Liquid Chromatography
Summary
The bacterial cell wall is composed of peptidoglycan, a macromolecular network of sugar strands crosslinked by peptides. Ultra Performance Liquid Chromatography provides high resolution and throughput for novel discoveries of peptidoglycan composition. We present a procedure for the isolation of cell walls (sacculi) and their subsequent preparation for analysis via UPLC.
Abstract
The bacterial cell wall is critical for the determination of cell shape during growth and division, and maintains the mechanical integrity of cells in the face of turgor pressures several atmospheres in magnitude. Across the diverse shapes and sizes of the bacterial kingdom, the cell wall is composed of peptidoglycan, a macromolecular network of sugar strands crosslinked by short peptides. Peptidoglycan’s central importance to bacterial physiology underlies its use as an antibiotic target and has motivated genetic, structural, and cell biological studies of how it is robustly assembled during growth and division. Nonetheless, extensive investigations are still required to fully characterize the key enzymatic activities in peptidoglycan synthesis and the chemical composition of bacterial cell walls. High Performance Liquid Chromatography (HPLC) is a powerful analytical method for quantifying differences in the chemical composition of the walls of bacteria grown under a variety of environmental and genetic conditions, but its throughput is often limited. Here, we present a straightforward procedure for the isolation and preparation of bacterial cell walls for biological analyses of peptidoglycan via HPLC and Ultra Performance Liquid Chromatography (UPLC), an extension of HPLC that utilizes pumps to deliver ultra-high pressures of up to 15,000 psi, compared with 6,000 psi for HPLC. In combination with the preparation of bacterial cell walls presented here, the low-volume sample injectors, detectors with high sampling rates, smaller sample volumes, and shorter run times of UPLC will enable high resolution and throughput for novel discoveries of peptidoglycan composition and fundamental bacterial cell biology in most biological laboratories with access to an ultracentrifuge and UPLC.
Introduction
The goal of the method described herein is to isolate intact bacterial cell walls (sacculi) and to digest the peptidoglycan (PG) such that Ultra Performance Liquid Chromatography (UPLC) can be used to provide information such as the identity of the muropeptide components and their concentrations, the average length of glycan strands, and the fraction of material involved in crosslinks between strands. For a detailed discussion of PG biochemistry and muropeptide species, there are several excellent reviews that describe PG structure and its role in infection, resistance, morphogenesis, and growth1-6. High Performance Liquid Chromatography (HPLC) for PG analysis was initially developed by Glauner and Schwarz in the 1980s, and more recently has been enhanced and applied extensively in the labs of Miguel de Pedro and Waldemar Vollmer. Previous methods utilized amino acid analysis or paper chromatography, time-consuming and tedious techniques that do not yield accurate or complete assessments of cell-wall components.
UPLC analysis can be easily implemented in any basic research laboratory that has access to an ultracentrifuge and UPLC. The UPLC method that we present below isolates complete sacculi, thereby providing comprehensive, quantitative information on all chemical species therein. This method yields precise quantification of all muropeptides across a population of bacteria, all within a 20 min UPLC run. The implementation of this method involves only basic laboratory skills, without significant financial investment in materials. In order to execute the steps in this method, researchers need only be skilled in pipetting, preparing buffers and enzymes, and adjusting pH, making it accessible to a wide range of scientific disciplines. The choice of enzymes used in this protocol depends on the species of bacterium being analyzed; the protocol described here is useful for Escherichia coli, and has generally been found to be adequate for isolating sacculi from other Gram-negative organisms. Consultation with the literature is recommended when applying this method to Gram-positive bacteria; in these species, sacculus purification has traditionally been more difficult. In particular, this method may have to be altered in terms of enzyme choice and length of digestion times to accommodate the thicker walls and accessory polymers such as teichoic acids of Gram-positive bacteria. The first enzyme in this protocol cleaves outer membrane lipoprotein (such as Braun’s lipoprotein, or Lpp) attachment to the peptidoglycan, thereby releasing all but the C-terminal di- (or tri-) peptide of the Lpp from the cell wall. This step is necessary when examining Enterobacteria, but many other Gram-negative bacteria have no Lpp equivalents, and therefore this step can be skipped. A second enzyme specifically cleaves after the muramic acid component of the peptidoglycan, solubilizing the disaccharide subunit that forms the muropeptide species. To provide an accurate assessment of the architecture of the PG, care should be taken in digesting the sacculi to prevent cleavage of the crossbridges or any other part of the peptide stem.
Although the chemical compositions of peptidoglycan from over 100 strains of ~40 bacterial species have been analyzed by HPLC, no analyses have been performed with UPLC technology. In addition, previous work has characterized peptidoglycan from only a small fraction of the bacterial domain, in part limited by the throughput of HPLC. Therefore, dissemination of this method to as many researchers as possible, and implementation on UPLC platforms, will be critical for driving physiological studies of the large fraction of bacterial species whose peptidoglycan has yet to be categorized.
Protocol
1. Grow Bacterial Cultures in 2.5 ml of Media Overnight
Back-dilute cultures 1:100 into 250 ml of fresh media and grow to OD600 of 0.7-0.8. Prepare a solution of 6% sodium dodecyl sulfate (SDS) in water.
CAUTION: SDS powder is hazardous - avoid inhaling SDS powder; wear a mask over nose and mouth.
2. Day 1 - Lysing Bacterial Cultures is Performed over the Course of One Day and Overnight
- While diluted cultures are growing, set up a boiling water bath on a hot plate in a 1 L beaker. Once the water is boiling, aliquot 6 ml of 6% SDS into 50 ml polypropylene tubes, add one small stir bar to each tube, secure tube lids to finger-tight, place tubes in water bath, and turn on stirring to 500 rpm on the hot plate.
- Harvest the 250 ml cultures at 5,000 × g for 10 min at room temperature and resuspend pellets in 3 ml of media or 1x phosphate-buffered saline. Slowly pipette cell suspensions into 50 ml tubes with 6% boiling SDS to lyse the cells (final concentration 4% SDS) while the tubes are submerged in the boiling water bath, and reclose the lids to finger-tight. Cell suspensions must be quickly transferred into boiling SDS once resuspended. Abrupt environmental changes should be avoided, as this could cause erroneous alteration of cell wall structure.
- Cover boiling water bath and allow cells to boil for 3 hr, checking the water level periodically and refilling the water bath when necessary. After 3 hr, turn off the heating element of the hot plate, and continue to stir overnight at 500 rpm.
3. Day 2 - Enzymatic Digestions are Performed over the Course of One Day
- If SDS has precipitated in the 50 ml tubes overnight, set the water bath to boil for an additional 1-2 hr. Turn on a heat block to 60 °C. Prepare a 1 mg/ml stock of Pronase E in 10 mM Tris-HCl (pH 7.2) + 0.06% w/v NaCl and activate Pronase E at 60 °C for at least 30 min.
- Use an ultracentrifuge set at 400,000 × g to spin samples for 20 min at room temperature in order to pellet the large PG polymers and thereby purify them from other cellular components. Remove supernatant carefully, and then resuspend each pellet in room temperature ultrapure water. NOTE: Resuspension volume depends upon the volume of the ultracentrifuge tubes used; use a volume that fills the tubes at least half way but does not exceed the maximum volume of the tubes. Repeat centrifugation/washing until the water does not form bubbles during resuspension, indicating that the SDS has been fully removed (typically three washes). Stop washing the pellet if a white precipitate forms, as this indicates that the sacculi are clumping together. Clumping is not catastrophic, but clumps bind very strongly to plastic and glassware causing large sample loss. In this case, proceed with the protocol using the clumped sacculi sample.
- On the last centrifugation/washing step, resuspend the samples in 900 µl of 10 mM Tris-HCl (pH 7.2) + 0.06% w/v NaCl and transfer to 2 ml tubes previously poked with holes in the tops with a small needle. Add 100 µl of 1 mg/ml activated Pronase E (100 µg/ml final concentration) to each sample and incubate at 60 °C for 2 hr. Set a different heat block to 100 °C.
- Stop the Pronase E digestion by adding 200 µl of 6% SDS to each sample and boil the samples in the 100 °C heat block for 30 min. Set a different heat block to 37 °C and make a 1 mg/ml stock of muramidase (mutanolysin) in 50 mM phosphate buffer (pH 4.9).
- As in step 3.2, use an ultracentrifuge set at 400,000 × g to spin samples for 20 min at room temperature and wash with room temperature ultrapure water until SDS is fully removed (typically three washes). Resuspension volume depends upon the volume of the ultracentrifuge tubes used; use a volume that fills the tubes at least half way but does not exceed the maximum volume of the tubes.
- On the last centrifugation/washing step, resuspend samples in 200 µl of 50 mM sodium phosphate buffer (pH 4.9). This volume can be adjusted according to the amount of peptidoglycan in the sample, and may be species dependent. If there are previously published reports of HPLC analysis for the species of interest, this volume can be estimated based on these quantitations (a compilation of HPLC PG studies can be found in the Supplemental Information of reference7). For other species, the sacculus prep can be executed up to this step and then different amounts of resuspension volumes can be added to replicate samples in order to determine the minimal volume that allows the PG to remain in solution (see Discussion for further estimates). If the sample contains more peptidoglycan, increase the resuspension volume; if the sample has little peptidoglycan, reduce the resuspension volume to a minimum of 50 µl.
- Transfer samples to 1.5 ml tubes and add 1 mg/ml muramidase to give a final concentration of 40 µg/ml. Incubate 6-8 hr or overnight at 37 °C.
4. Day 3 - Preparation of Samples for UPLC is Performed on the Last Day
- Turn on a heat block to 100 °C. Boil the samples without SDS for 5 min to stop the muramidase digestion. Centrifuge samples for 10 min at 16,000 × g at room temperature, then transfer the supernatant (muropeptides are now soluble) to 13 mm x 100 mm glass tubes. Try to recover as much supernatant as possible, getting very close to the pellet without disturbing it.
- Adjust the pH by adding 500 mM borate buffer (pH 9) to the sample for a final concentration of 100 mM borate buffer. Borate buffer is compatible with the reducing agent sodium borohydride. Add 1-2 grains of sodium borohydride to reduce each sample and let the reaction proceed for at least 30 min at room temperature. CAUTION: Sodium borohydride is highly reactive and dangerous to handle - avoid contact with skin (wear gloves) and avoid contact with eyes (wear safety glasses).
- Adjust samples to pH 3-4 (the muropeptide isoelectric point is ~3.5) with 50% v/v orthophosphoric acid using 20 µl increments until pH 6, as measured with pH indicator paper, then using 2 µl increments. CAUTION: Orthophosphoric acid is corrosive and dangerous to handle - avoid contact with skin (wear gloves) and avoid contact with eyes (wear safety glasses). The sample should bubble in response to addition of orthophosphoric acid; when the sample stops bubbling, this typically indicates a pH of 6 has been reached. If no bubbling occurs in the sample, this may indicate that the amount of sodium borohydride added was too small. In this case, stop lowering pH, carefully add one or two grains of sodium borohydride, let react for 5-10 min, then resume pH adjustment.
- Filter sample through a 0.22 µm syringe filter directly into a UPLC vial. If a precipitate has formed, heat the tube with several passes through a flame before filtering. If the sample will not be injected into the UPLC instrument within the day, freeze at -20 °C overnight. Samples can be stored for up to a year at -80 °C. The sample can be thawed by passing through a flame several times.
- Inject 10 µl of each sample onto a UPLC instrument equipped with a C18 1.7 µm reversed-phase column and an absorbance detector set to monitor 202-208 nm. Samples are injected sequentially, but autosampler capabilities allow up to 96 samples to be processed in batch. Use 50 mM sodium phosphate (pH 4.35) + 0.4% v/v sodium azide for solvent A, and 75 mM sodium phosphate (pH 4.95) + 15% v/v methanol for solvent B. NOTE: Sodium azide is added to compensate for the 205 nm absorption of methanol to avoid baseline drift. Set the flow to 0.25 ml/min and use a linear gradient over 25 min to achieve 100% solvent B and sequential elution of muropeptides within 30 min. If mass spectrometry will be used to characterize muropeptides after UPLC, collect fractions of the peak of interest in a fraction collector and dry the fractions using a centrifugal evaporator.
Representative Results
Using the procedure outlined in Figure 1, the final sample should consist of at least 200 µl of clear solution that has been filtered directly into a UPLC vial (step 4.4). UPLC separation of the various muropeptides in a bacterial sample relies upon their relative solubility between the liquid mobile phase and the column’s stationary phase. Reversed-phase C18 columns provide a strongly hydrophobic matrix to separate the muropeptide species based on hydrophobicity and size8; polar, low molecular-weight monomers elute first, and apolar, higher molecular-weight oligomers elute after (Figure 2). A typical UPLC result is shown in Figure 2, with detection via UV absorbance at 202-208 nm as a function of time that establishes a particular muropeptide’s retention time. This representative result shows clear resolution between most muropeptide species and strong signal strength across the spectrum, which enables the analysiz, and average glycan strand length.
The final step outlined in Figure 1 involves adjusting pH and filtering any fine particulates that may clog the small dimensions of the tubing used in UPLC. If a sample is superconcentrated, for instance by resuspending in 100 µl of sodium phosphate buffer in step 3.5 instead of 200 µl, the sample may turn cloudy, indicating that a critical concentration has been achieved and the muropeptides have precipitated. This loss of solubility will result in clogging of the syringe filter used in step 4.4, preventing the deposition of muropeptides into the UPLC vial and/or clogging of the UPLC machine conduits and column, which are costly items to replace. An example chromatogram reflecting this aberrant sample processing is shown in Figure 3; no peaks eluted, resulting in the absence of any data on PG composition. Misadjusting the pH to well below the isoelectric point of the muropeptides (e.g. to a pH of 2) may also result in the precipitation of muropeptides and hence the absence of discernable peaks from UPLC analysis.
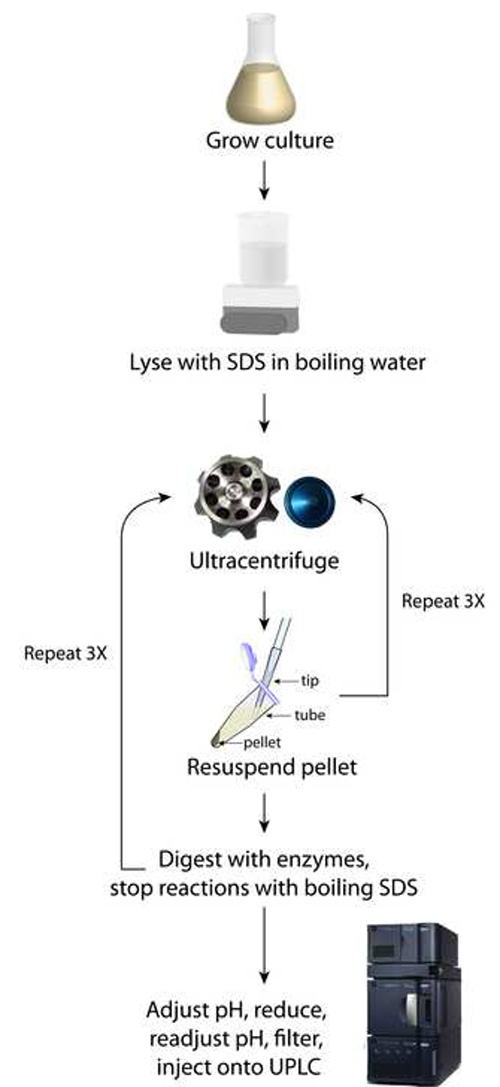
Figure 1. Schematic of sacculi preparation. This method relies upon iterative rounds of ultracentrifugation to purify SDS away from the pelleted sacculi.
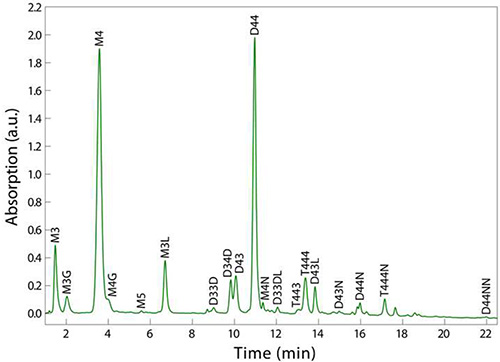
Figure 2. Example UPLC chromatogram of PG from sacculi digested from E. coli MG1655 cells. Note that comparable resolution of all muropeptides is achieved in 10% of the time of a typical HPLC run. Muropeptide labels - M = monomer, D = dimer, T = trimer; (2,3,4,5) indicate the number of amino acid stem peptides; modifications - G = glycine replacing L-alanine, L = two additional amino acids from Pronase E cleavage, D = 3,3-diaminopimelic acid (DAP)-DAP crossbridge, N = terminating anhydro-muropeptide. For example, D33DL is a dimer with 3-aa stem peptides, linked through a DAP-DAP crossbridge, containing an additional two amino acids from Pronase E cleavage.
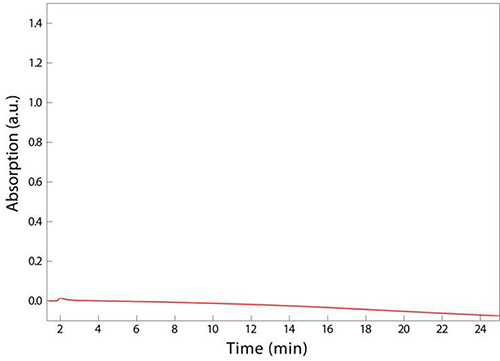
Figure 3. Unsuccessful UPLC analysis of sacculi digested from E. coli MG1655 cells. The absence of peaks, indicating that there were no muropeptides present in the sample, is due to muropeptides crashing out of solution before extraction into a UPLC vial (step 4.4). This precipitation may have been due to overconcentration of the muropeptides or to the pH being too low.
Discussion
A critical step in this procedure is step 3.1 of the second day of sample preparation. If the SDS has precipitated overnight, or if the samples have been stored in 4% SDS for several weeks at room temperature, the samples must be reboiled for at least 1 hr to redissolve the SDS. A common cause for SDS precipitation is the use of media with potassium salts, so potassium should be avoided in media if possible. As mentioned in the Representative Results section, it is also critical to adjust the pH to within the isoelectric point of the muropeptides (~3.5), but not too much lower than pH 3, or the material may precipitate. Finally, resuspension of the sample must occur in the appropriate volume of sodium phosphate buffer (step 3.5), which must be judged by the researcher. When choosing the resuspension volume of sodium phosphate buffer, it is important to consider how much muropeptide material is likely to be generated from a particular bacterial culture. For instance, if the final culture volume is 250 ml, as outlined above, the sample must be resuspended in at least 200 µl of sodium phosphate buffer. If the researcher modifies the method to 50 ml cultures, samples may be resuspended in 100 µl of sodium phosphate buffer or less. Consideration of the predicted amounts of peptidoglycan in a species is also important; for example, E. coli spheroplasts contain substantially less peptidoglycan (approximately 7% of the amount in wild-type E. coli cells10), and so resuspension in 100 µl or less of sodium phosphate buffer is appropriate. If it is difficult to grow a certain bacterial species or strain to large quantities (250 ml), the final culture volume can be reduced and/or the dilution of the overnight culture can be reduced. Finally, injection onto an HPLC system requires a minimum volume of 200 µl, whereas 10 µl is sufficient for a UPLC system.
The purification of PG components in different organisms or for different applications can be tuned by varying the type of mobile phase, column, and gradient on the UPLC instrument. Mobile phases that are solvent-based, such as acetonitrile, can be used to desalt samples prior to mass spectrometry. Different column chemistries may also be required to thoroughly desalt samples for subsequent analysis. Figure 2 shows the common elution profile of muropeptides for the Gram-negative rod-shaped bacterium E. coli MG1655; the analysis of PG from Gram-positive bacteria and/or species with other shapes may require fine-tuning of the gradient outlined in step 4.5. Although UPLC spectra such as the one shown in Figure 2 generate many classes of information concerning peptidoglycan, such as muropeptide identity, crosslinking percentage, and glycan strand length, the method does have several limitations, including the inability to map the spatial distribution of structural features across sacculi. For example, neither the locations of crossbridging along a glycan chain nor the locations of bound lipoproteins can be discerned via HPLC or UPLC.
The advantages of analyzing the chemical composition of PG with HPLC include higher resolution, shorter analysis time, and accurate quantitation11 compared with traditional techniques such as amino acid analysis12-14 or paper chromatography15,16. UPLC offers higher speed and sensitivity than HPLC due to higher pressures that enable faster flows and therefore shorter run times. Resolution is not sacrificed with UPLC, as the sub-2 µm particle size columns, high sampling rate detectors, and low-volume injectors are able to withstand very high pressures and thus function accurately at high speeds17,18. This results in the ability to analyze sample volumes on the order of 1 µl in tens of minutes, compared with 200 µl and hours for HPLC.
Techniques that are complementary to UPLC include muropeptide mass analysis by mass spectrometry. UPLC is not a destructive technique; the eluate from the column can be collected after UV detection and dried using a centrifugal evaporator commonly available in most laboratories. Although the sample can be desalted to prepare it for mass spectrometry (step 4.5), Matrix Assisted Laser Desorption/Ionization - Time Of Flight Mass Spectrometry enables analysis without much sensitivity to salt concentration19, and yields mass data capable of definitive identification of muropeptides. The cost of preparing one cell wall sample for UPLC is approximately $6-7, including the costs of enzymes, chemicals, and supplies used. Given its inexpensive running costs, accessibility, and demonstrated utility for studies of the bacterial cell wall, UPLC should become the method of choice for high-resolution, high-throughput, accurate quantification of peptidoglycan composition across the bacterial kingdom.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIH Director's New Innovator Award DP2OD006466 (to K.C.H.). The authors thank Russell Monds for a practical demonstration of the method and for scientific discussions.
Materials
| Pronase E | Amresco | E629 | |
| Mutanolysin from Streptomyces | Sigma-Aldrich | M9901 | |
| Sodium borohydride (NaBH4) | Sigma-Aldrich | 452882 | Sodium borohydride is highly reactive and dangerous to handle |
| Orthophosphoric acid | Sigma-Aldrich | 79607 | Orthophosphoric acid is corrosive and dangerous to handle |
| Boric acid | Sigma-Aldrich | 31146 | |
| Sodium azide | Sigma-Aldrich | S2002 | Sodium azide is a poison |
| Sodium tetraborate | Sigma-Aldrich | 221732 | |
| Millex 0.22 μm syringe filters | Fisher | SLGVR04NL | |
| pH strips (pH range 0-6) | Fisher | M95863 | |
| 50 ml polypropylene Falcon tubes | VWR | 21008-951 | |
| 13 mm x 100 mm glass tubes | Kimble Chase | 60CM13 | |
| 12 mm x 32 mm screw neck glass recovery vial | Waters | 186000327C | |
| Sodium Dodecyl Sulfate | Ambion | AM9820 | SDS powder is hazardous |
| Instrumentation | |||
| Waters Acquity UPLC H-Class system, including: | |||
| Acquity UPLC H-Class Sample Manager FTN | |||
| Acquity UPLC H-Class Quaternary Solvent Manager | |||
| Acquity UPLC BEH C18 1.7 µm column | |||
| Acquity UPLC PDA Detector | |||
| Waters Fraction Collector III | |||
| Acquity UPLC 30 cm Column Heater/Cooler | |||
Referenzen
- den Blaauwen, T., de Pedro, M. A., Nguyen-Disteche, M., Ayala, J. A. Morphogenesis of rod-shaped sacculi. FEMS Microbiol. Rev. 32, 321-344 (2008).
- Holtje, J. V. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol. Mol. Biol. Rev. 62, 181-203 (1998).
- Typas, A., Banzhaf, M., Gross, C. A., Vollmer, W. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat. Rev. Microbiol. 10, 123-136 (2011).
- Vollmer, W., Blanot, D., de Pedro, M. A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 32, 149-167 (2008).
- Davis, K. M., Weiser, J. N. Modifications to the peptidoglycan backbone help bacteria to establish infection. Infect. Immun. 79, 562-570 (2011).
- Healy, V. L., Lessard, I. A., Roper, D. I., Knox, J. R., Walsh, C. T. Vancomycin resistance in enterococci: reprogramming of the D-ala-D-Ala ligases in bacterial peptidoglycan biosynthesis. Chem. Biol. 7, 109-119 (2000).
- Desmarais, S. M., De Pedro, M. A., Cava, F., Huang, K. C. Peptidoglycan at its peaks: how chromatographic analyses can reveal bacterial cell wall structure and assembly. Mol. Microbiol. 89, 1-13 (2013).
- Glauner, B., Holtje, J., Schwarz, U. The Composition of the murein of Escherichia coli. J. Biol. Chem. 263, 10088-10095 (1988).
- Glauner, B. Separation and quantification of muropeptides with high-performance liquid chromatography. Anal. Biochem. 172 (2), 451-464 (1988).
- Joseleau-Petit, D., Liebart, J. C., Ayala, J. A., D’Ari, R. Unstable Escherichia coli L forms revisited: growth requires peptidoglycan synthesis. J. Bacteriol. 189, 6512-6520 (2007).
- Hakenbeck, R., Holtje, J. V., Labischinski, H. . The target of penicillin: the murein sacculus of bacterial cell walls architecture and growth. , (1983).
- Boylen, C. W., Ensign, J. C. Ratio of teichoic acid and peptidoglycan in cell walls of Bacillus subtilis following spire germination and during vegetative growth. J. Bacteriol. 96, 421-427 (1968).
- Sutow, A. B., Welker, N. E. Chemical composition of the cell walls of Bacillus stearothermophilus. J. Bacteriol. 93, 1452-1457 (1967).
- Wang, W. S., Lundgren, D. G. Peptidoglycan of a chemolithotrophic bacterium, Ferrobacillus ferrooxidans. J. Bacteriol. 95, 1851-1856 (1968).
- de la Rosa, E. J., de Pedro, M. A., Vazquez, D. Penicillin binding proteins: role in initiation of murein synthesis in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 82, 5632-5635 (1985).
- Kandler, O., Schleifer, K. H., Dandl, R. Differentiation of Streptococcus faecalis Andrewes and Horder and Streptococcus faecium Orla-Jensen based on the amino acid composition of their murein. J. Bacteriol. 96, 1935-1939 (1968).
- Kumar, A., Saini, G., Nair, A., Sharma, R. UPLC: a preeminent technique in pharmaceutical analysis. Acta Poloniae Pharmaceutica. 69, 371-380 (2012).
- Wilson, I. D., et al. High resolution "ultra performance" liquid chromatography coupled to oa-TOF mass spectrometry as a tool for differential metabolic pathway profiling in functional genomic studies. J. Proteome Res. 4, 591-598 (2005).
- Wieser, A., Schneider, L., Jung, J., Schubert, S. MALDI-TOF MS in microbiological diagnostics-identification of microorganisms and beyond (mini review). Appl. Microbiol. Biotechnol. 93, 965-974 (2012).

