A Facile and Efficient Approach for the Production of Reversible Disulfide Cross-linked Micelles
Summary
To deliver cancer drugs to tumor sites with high specificity and reduced side effects, new methods based on nanoparticles are required. Here, we describe disulfide cross-linked micelles that can be easily prepared by hydrogen peroxide-mediated oxidation and are able to dissociate efficiently under a reducing tumor environment to release payloads.
Abstract
Nanomedicine is an emerging form of therapy that harnesses the unique properties of particles that are nanometers in scale for biomedical application. Improving drug delivery to maximize therapeutic outcomes and to reduce drug-associated side effects are some of the cornerstones of present-day nanomedicine. Nanoparticles in particular have found a wide application in cancer treatment. Nanoparticles that offer a high degree of flexibility in design, application, and production based on the tumor microenvironment are projected to be more effective with rapid translation into clinical practice. The polymeric micellar nano-carrier is a popular choice for drug delivery applications.
In this article, we describe a simple and effective protocol for synthesizing drug-loaded, disulfide cross-linked micelles based on the self-assembly of a well-defined amphiphilic linear-dendritic copolymer (telodendrimer, TD). TD is composed of polyethylene glycol (PEG) as the hydrophilic segment and a thiolated cholic acid cluster as the core-forming hydrophobic moiety attached stepwise to an amine-terminated PEG using solution-based peptide chemistry. Chemotherapy drugs, such as paclitaxel (PTX), can be loaded using a standard solvent evaporation method. The O2-mediated oxidation was previously utilized to form intra-micellar disulfide cross-links from free thiol groups on the TDs. However, the reaction was slow and not feasible for large-scale production. Recently, an H2O2-mediated oxidation method was explored as a more feasible and efficient approach, and it was 96 times faster than the previously reported method. Using this approach, 50 g of PTX-loaded, disulfide cross-linked nanoparticles have been successfully produced with narrow particle size distribution and high drug loading efficiency. The stability of the resulting micelle solution is analyzed using disrupting conditions such as co-incubation with a detergent, sodium dodecyl sulfate, with or without a reducing agent. The drug-loaded, disulfide cross-linked micelles demonstrated less hemolytic activity when compared to their non-cross-linked counterparts.
Introduction
Nanotechnology is a fast-emerging field that has benefited a number of biomedical areas1. Nanoparticles provide opportunities for designing and tuning properties that are not feasible with other types of conventional therapeutics. Nano-carriers enhance the stability of drugs against biodegradation, prolong drug circulation time, overcome drug solubility issues, and can be fine-tuned for targeted drug delivery and for co-delivering imaging agents1,2. Nanoparticle-based delivery systems hold promise in cancer imaging and treatment. Tumor vasculatures are leaky to macromolecules and can lead to preferential accumulation of circulating nanoparticles at tumor sites via the enhanced permeability and retention (EPR) effect3. Among the several nano-carriers (e.g., liposomes, hydrogels, and polymeric micelles) that are being actively pursued as carriers for anti-cancer drugs, polymeric micelles have gained wide popularity over the last decade4,5.
Polymeric micelles are a thermodynamic system that, on intravenous administration, can potentially be diluted below the critical micelle concentration (CMC), leading to their dissociation into unimers. Cross-linking strategies have been employed to minimize micellar dissociation into unimers. However, excessively stabilized micelles may prevent the drug from releasing at the target sites, thereby reducing the overall therapeutic efficacy. Several chemical approaches have been explored to make the cross-linking degradable in response to redox or to external stimuli, such as reducible disulfide bonds6,7 and pH-cleavable8 or hydrolysable ester bonds9,10.
We have previously reported the design and synthesis of micellar nanoparticles consisting of dendritic cholic acid (CA) blocks and linear polyethylene glycol (PEG) copolymers, referred to as telodendrimers (TD)11-15. These TDs are represented as PEGnK-CAy (where n = molecular weight in kilodaltons (K), y = number of cholic acid (CA) units). They are characterized by their small size, long shelf life, and high efficiency in encapsulating drugs such as paclitaxel (PTX) and doxorubicin (DOX) in the hydrophobic core. The building blocks of TD, such as PEG, lysine, and CA, are biocompatible, and the presence of a PEG corona can impart a "stealth" nanoparticle character, preventing non-specific uptake of micellar nanoparticles by the reticuloendothelial systems.
Thiolated linear-dendritic polymers can easily be generated by introducing cysteines into the dendritic oligo-lysine backbone of our standard TDs. This article presents a facile protocol for the production of a reversibly cross-linked micellar drug delivery system by introducing disulfide cross-links into the hydrophobic core of TDs (Figure 1).
Protocol
Ethics statement: Female athymic nude mice (Nu/Nu strain), 6-8 weeks old, were purchased and then kept under pathogen-free conditions according to AAALAC guidelines and were allowed to acclimatize for at least 4 days prior to any experiments. All animal experiments were performed in compliance with institutional guidelines and according to protocol No. 07-13119 and No. 09-15584, approved by the Animal Use and Care Administrative Advisory Committee at the University of California, Davis.
1. Synthesis of TD PEG5K-Cys4-Ebes8-CA8
- In a round-bottom flask, dissolve MeO-PEG5K-NH2 (2 g, 0.4 mmol) in 10-20 ml of anhydrous dimethylformamide (DMF) and chill on ice.
- In a glass beaker, dissolve 3 equiv. of 1-hydroxy-6-chloro-benzotriazole (HOBt), 3 equiv. of N,N'-diisopropylcarbodiimide (DIC), and 3 equiv. of (Fmoc)2Lys-OH in anhydrous DMF (10-15 ml). Stir for 15-20 min on a magnetic stir plate.
- Add the mixture to the reaction flask containing the MeO-PEG5K-NH2. Remove the ice bath and stir the reaction mixture overnight at room temperature.
- Confirm the completion of the reaction with thin layer chromatography (TLC)16 and Kaiser's test17 (a yellow color indicates the absence of free NH2). Precipitate the polymeric product 1 (MeO-PEG5K-Lys(NH-Fmoc)2) by adding approximately 200 ml of ice-cold ether to the reaction flask. Separate the precipitated polymer via centrifugation (6 min at 6,000 x g and 4 °C).
- To perform TLC, spot the samples on silica gel-coated TLC plates. Use dichloromethane/methanol (9:1) as the mobile phase. Observe spots under a UV lamp after developing the TLC plates. One can also use ninhydrin staining reagent to visualize amine spots on a hot plate.
- For Kaiser's test, place a little bit of the sample in glass tube containing Kaiser's reagent. Heat it at 100 °C for 5 min and look for the color change (if the color of the solution remains yellow, then the reaction is complete).
- Re-dissolve the product in anhydrous DMF (10-20 ml) and repeat the precipitation and centrifugation (see step 1.4).
- Repeat step 1.5, and then wash the polymer precipitate three times with ice-cold ether.
- Transfer the polymer precipitate 1 to a clean reaction flask and connect the flask to a high vacuum source to remove the residual ether.
- Prepare and add approximately 20-30 ml of 20% (v/v) 4-methylpiperidine in DMF to polymer intermediate 1. Stir until complete dissolution. Run the reaction for 3 hr.
- Perform TLC16 and Kaiser's test (a blue color confirms the presence of free NH2)17 (see step 1.4) to confirm the completion of the reaction. If the reaction is complete, proceed to the ether precipitation, as mentioned for product 1 (steps 1.4-1.6).
- Dry the polymeric product 2 (MeO-PEG5K-Lys(NH2)2) under a vacuum.
- Carry out one more round of (Fmoc)2Lys-OH-coupling onto intermediate 2 (steps 1.1-1.7) to generate product 3 (MeO-PEG5K-Lys(Lys(NH-Fmoc)2)2). De-protect (steps 1.8-1.10) the Fmoc groups (4, MeO-PEG5K-Lys(Lys(NH2)2)2) and couple (steps 1.1-1.7) the (Fmoc)Lys(Boc)-OH to generate a third-generation dendritic polylysine (5, MeO-PEG5k-Lys-Lys2-((Fmoc)Lys(Boc))4) terminated with four Boc and Fmoc groups on one end of the PEG chain.
- Transfer the resulting polymer intermediate 5 to a reaction flask. In a separate reaction flask, prepare 1:1 (v/v) trifluoroacetic acid (TFA) in dichloromethane (DCM). Add 15-20 ml of a 1:1 TFA/DCM (v/v) mixture to the polymer intermediate 5. Stir the mixture until the polymer is completely dissolved. Stir for an additional 3 hr.
- Perform TLC16 and Kaiser's test (a blue color confirms the presence of free NH2)17 (see step 1.4) to confirm the completion of the reaction. If the reaction is complete, evaporate the polymer-in-TFA/DCM mixture with air until a viscous solution is obtained. Proceed to the ether precipitation, as mentioned for product 1 (steps 1.4-1.6). Dry the polymeric product 6 (MeO-PEG5k-Lys-Lys2-((Fmoc)Lys(NH2))4) under a vacuum.
- Transfer polymer intermediate 6 into a reaction flask. Use approximately 40 ml of anhydrous DMF containing 8 equiv. of N,N-diisopropylethylamine (DIEA) to dissolve the polymer intermediate 6. In a glass beaker, dissolve 12 equiv. of HOBt, 12 equiv. of DIC, and 12 equiv. of (Fmoc)Cys(Trt)-OH in 20-25 ml of anhydrous DMF. Shake for 10-15 min, and then add the reaction mixture to the reaction flask containing 6. Run the reaction overnight.
- Confirm the completion of reaction with TLC16 and Kaiser's test (see step 1.4; a yellow color indicates the absence of free NH2)17. If the reaction is complete, proceed to the ether precipitation, as mentioned for product 1 (step 1.4-1.6), to isolate product 7 (MeO-PEG5K-Lys-Lys2-((Fmoc)Lys-((Fmoc)Cys(Trt)))4.
- Perform Fmoc de-protection on 7, as outlined in step 1.8, to obtain product 8 (PEG5K-Lys-Lys2-(Lys(NH2)-(Cys(NH2)(Trt)))4). Couple (Fmoc)-PEG2-Suc-OH ("Ebes" linker, 24 equiv.) on a polymer intermediate using the procedure outlined above for HOBt/DIC-mediated coupling to obtain intermediate 9 (MeO-PEG5K-Lys-Lys2-Lys4-(Cys(Trt))4(Ebes(NH-Fmoc))8).
- Perform one more round of Fmoc de-protection (steps 1.6-1.8) to get intermediate 10 (MeO-PEG5K-Lys-Lys2-Lys4-(Cys(Trt))4(Ebes(NH2))8).
- Transfer the polymer intermediate 10 into a reaction flask and add anhydrous DMF (approximately 30-40 ml) to dissolve it. In another reaction flask, dissolve 24 equiv. of CAOSu (prepared according to the previously published procedure) in anhydrous DMF (20-30 ml)18. Add 48 equiv. of N,N-diisopropylethylamine and let it stir for 10-15 min. Transfer the contents into the reaction flask containing 10 and let the reaction run overnight.
- Confirm the completion of the reaction with TLC16 and Kaiser's test (see step 1.4; a yellow color indicates the absence of free NH2)17. If the reaction is complete, proceed to the ether precipitation, as mentioned for product 1 (steps 1.4-1.6) to isolate product 11 (MeO-PEG5K-Lys-Lys2-Lys4-(Cys(Trt))4-Ebes8-CA8). Dialyze it in de-ionized water and lyophilize the sample to yield a white powder.
- Place the polymer intermediate 11 into a reaction flask. Prepare and add 20 ml of the TFA/1,2-ethanedithiol (EDT)/triethylsilane (TIS)/H2O (94/2.5/1/2.5, v/v) mixture into the polymer solution. Stir the mixture until complete dissolution. Run the reaction for 4 hr. Confirm the completion of the reaction by TLC16.
- Under the fume hood, blow air into the polymer-TFA/EDT/TIS/H2O mixture until the solution becomes viscous. Proceed to the ether precipitation, as mentioned for product 1 (steps 1.4-1.6), to isolate the final product, 12 (PEG5K-Cys4-Ebes8-CA8). Dissolve it in acetonitrile and lyophilize it to yield a white powder.
2. Preparation of PTX-loaded Micelles
- Prepare a PTX-loaded PEG5K-Cys4-Ebes8-CA8 micelle using the standard evaporation method.
- Dissolve 20 mg of TD with a different amount of PTX (1-9 mg) in 1 ml of chloroform (CHCl3). Remove the solvent using a rotary evaporator to obtain a homogeneous, dry polymer film. Reconstitute the film with 1 ml of phosphate-buffered saline (PBS) by vortex, followed by sonication for 30 min at 40 kHz, if necessary, to allow the formation of drug-loaded micelles.
- Add 6 µl of 3% (w/w) H2O2 (1 equiv. to free the thiol groups) to oxidize the thiol groups on the TD. Use the micelle solution for further characterization once the level of free thiol groups remains at constant low values, as indicated by Ellman's test19.
- Filter the solution with a 0.22-µm filter to sterilize the sample. Analyze the amount of drug loaded in the micelles on a HPLC20 system after releasing the drugs from the micelles by adding 9 times acetonitrile and performing 10 min of sonication. Use a C18 column for HPLC with acetonitrile/water as the mobile phase.
- Calculate the drug loading according to the calibration curve between the HPLC area values and the concentrations of the drug standard11.
NOTE: The loading efficiency is defined as the ratio of drug loaded into the micelles to the initial drug content.
3. Characterizations of Micelles
- Measure the size and size distribution of the micelles with a dynamic light scattering (DLS) instrument29. Perform the measurements at room temperature and keep the micelle concentration at 1 mg/ml.
NOTE: To perform particle size analysis, use PBS as blank, and then record the particle size for actual samples. Take the readings in triplicate for samples, and then average the readings. - Use fluorescence spectra to measure the critical micellar concentration (CMC) of PEG5K-Cys4-Ebes8-CA8 before and after cross-linking with pyrene as a hydrophobic fluorescent probe, as described previously13,21.
NOTE: Typically, the micelle concentration ranges from 5×10-7 to 5×10-4 M.
4. Stability of Micelles in SDS with or without Reducing Agents
- Prepare stock solutions of sodium dodecyl sulfate (SDS) solution (7.5 mg/ml) and disulfide cross-linked micelles (1.5 mg/ml) in PBS. Next, using the stock solutions, make a solution mixture in which the final SDS concentration is at 2.5 mg/ml and the micelle concentration is at 1.0 mg/ml.
- Measure the size and size distribution (as mentioned in step 3.1) of the micelle solutions at predetermined time intervals with or without the presence of 10 mM glutathione (GSH).
5. Hemolysis Assay
- Evaluate the hemolytic potential of PTX-loaded, non-cross-linked micelles (PTX-NCMs) prepared according to previously published procedure11 and PTX-loaded cross-linked micelles (PTX-DCMs) using fresh citrated blood from nude mice collected from the tail veil.
- Collect red blood cells by centrifugation of blood sample (1.0 ml) at 1,000 x g for 10 min, wash them three times with PBS, and then re-suspend the cell pellets with PBS to a final concentration of 2%.
- Mix 200 µl of erythrocyte suspension with different concentrations (0.2 and 1.0 mg/ml) of PTX-NCMs and PTX-DCMs, respectively, and incubate for 4 hr at 37 °C in an incubator shaker.
- Centrifuge the micelle-erythrocyte mixtures at 3,000 x g for 5 min. Transfer 100 µl of the supernatant of all samples to a 96-well plate. Measure the absorbance of free hemoglobin in the supernatant at 540 nm using a micro-plate reader.
NOTE: RBCs incubated with Triton-100 (2%) and PBS are to be used as the positive and negative controls, respectively. The percent hemolysis of the RBCs is calculated with the following formula: RBC hemolysis = (ODsample – ODnegative control)/(ODpositive control – ODnegative control) × 100%.
Representative Results
Preparation and Characterization of Drug-loaded, Disulfide Cross-linked Micelles
Amphiphilic polymer PEG5K-Cys4-Ebes8-CA8 is a dendritic polymer capable of forming a disulfide cross-linked micellar system for cancer drug delivery. Structurally, it is defined as a dendritic oligomer of cholic acids (hydrophobic domain) linked to one end of the linear PEG molecule (hydrophilic domain, molecular weight 5K) through a branched poly(lysine-cysteine-Ebes) backbone. There are several advantages of using these block copolymers over other reported micellar systems. First, it can be readily synthesized via stepwise solution phase condensation reactions. Second, compared to several other reported amphiphilic polymers, the thiolated polymer system, prepared through well-established solution phase stepwise Fmoc peptide chemistry, has a well-defined structure. It can be stored in lyophilized powder form and has an extended shelf life.
Several techniques can be utilized to characterize the end product. The amount of cholic acid attached to TD can be detected by comparing the signal ratio of the protons on PEG to those on the three methyl groups of cholic acid in the 1H NMR spectra. The molecular weights of the TD can be confirmed by MALDI-TOF mass spectrometry and gel permeation chromatography (GPC). The quantitative Ellman's test can be used to decipher the number of free cysteine residues present per molecule.
PEG5K-Cys4-Ebes8-CA8 can self-assemble to form micelles in aqueous media. Using a standard solvent evaporation method, a variety of hydrophobic drugs, such as PTX and vincristine, have been successfully encapsulated in the micelles13,22. Oxygen was previously utilized to oxidize the free thiol groups of PEG5K-Cys4-Ebes8-CA8 and to form intra-micellar disulfide cross-linkages. As monitored by Ellman's test, the conversion rate from free thiol groups to disulfide bonds reached 85% after 48 hr of oxidation. A H2O2-mediated oxidization was recently employed as an alternative method. The conversion rate reached 88% in 30 min (Figure 2), which was 96 times faster than the previous approach. Fifty grams of PTX-loaded, disulfide cross-linked nanoparticles have been successfully produced using this more efficient approach (Figure 3). The particle size was around 27 nm, with a narrow size distribution (Figure 3). The drug loading efficiency approached 100% with PTX loading of 4.0 mg/ml. Disulfide cross-linking had a dramatic effect on the CMC value. Compared to standard TD PEG5K-CA8 that lack disulfide bonds, the cross-linked TD showed a 10-fold decrease in the observed CMC values (5.53 µM versus 0.67 µM)13.
Stability Studies
We further investigated the stability of PTX-loaded, disulfide cross-linked micelles against severe micelle-disrupting conditions. Sodium dodecyl sulfate (SDS) is a strong ionic detergent that is routinely used for assessing micellar stability, as it can efficiently break down polymeric micelles. The stability of drug-loaded, disulfide cross-linked micelles (1.0 mg/ml) was monitored by DLS in the presence of the micelle-disrupting SDS (2.5 mg/ml). The particle size of the micelles remained steady over time, indicating that such cross-linked micelles remained intact (Figure 4, left panels). Reductive conditions are expected to cleave disulfide bonds in the hydrophobic core, thereby making micelles susceptible to destabilization. Glutathione (GSH), an endogenous reducing agent, is often employed for such studies. There is stark contrast in the intracellular level of GSH compared to the extracellular level (10 mm versus 2 µm). This difference in concentrations is often used to generate stimuli-responsive systems. After the addition of GSH at an intracellular concentration (10 mm)23, the size of the drug-loaded, disulfide cross-linked micelles remained intact for 30 min. They abruptly decreased to 1 nm, signifying the reduction of a critical number of disulfide bonds, a prerequisite of the rapid dissociation of the micelles (Figure 4, right panel). On the contrary, the system remained stable in the presence of extracellular concentrations of GSH (data not shown).
Hemolysis Study
Figure 5 shows differences in the observed hemolytic activity of PTX-loaded micelles, with or without disulfide cross-linking. Hemolysis of blood cells must be avoided when administering these particles into the blood stream, as it undermines their therapeutic advantages. Hemato-compatibility is very crucial for the in vivo application of polymer-based drug carriers, such as the amphiphilic TDs that have the potential to solubilize lipids or to insert themselves into phospholipid membranes, leading to the rupturing of plasma membranes. As shown in Figure 5, the PTX-NCMs were found to have a dose-dependent red blood cell (RBC) lysis count, with the percentage of hemolysis increasing from 8.1% to 14.2% as the concentration of PTX-NCMs increased from 0.2 mg/ml to 1.0 mg/ml. However, PTX-DCMs that have disulfide cross-linking showed no observable hemolytic activities (< 1.0%) in the RBCs under the same experimental conditions. This difference in the hemolysis trend can be attributed to the intra-micellar disulfide bridges present at the hydrophobic core, which prevent PTX-DCMs from dissociating to form amphiphilic TDs.
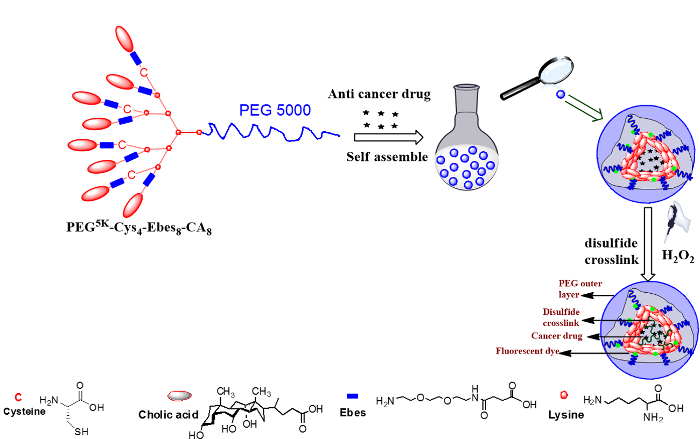
Figure 1: Steps for forming cross-linked micelles. Schematic representation of the disulfide cross-linked micelles formed by oxidization of the thiolated telodendrimer PEG5K-Cys4-Ebes8-CA8 after self-assembly. Please click here to view a larger version of this figure.
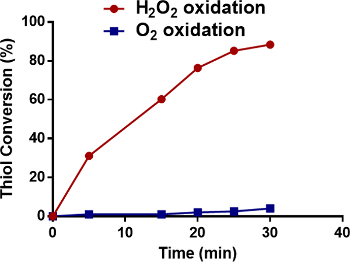
Figure 2: Oxidation methods for cross-linked micelle formation. The conversion rate of the thiol groups on the TDs to disulfide bonds as a function of oxidation time for the two oxidation methods. The total concentration of the micelles was kept at 20 mg/ml. Please click here to view a larger version of this figure.
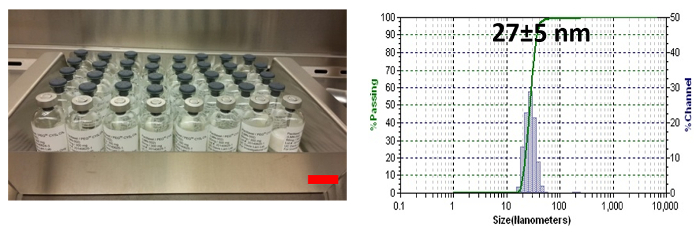
Figure 3: Drug-loaded, cross-linked micelle. Left panel: a picture of a batch of PTX-loaded, disulfide cross-linked micelles. Scale bar: 1 inch. Right panel: the particle size, as determined by dynamic light scattering (DLS). The total concentration of micelles was kept at 20 mg/ml. The loading of PTX was 4 mg/ml. The particle size data was shown as the average particle size ± SD based on three measurements. SD: standard deviation, describes the width of the measured particle size distribution. Please click here to view a larger version of this figure.
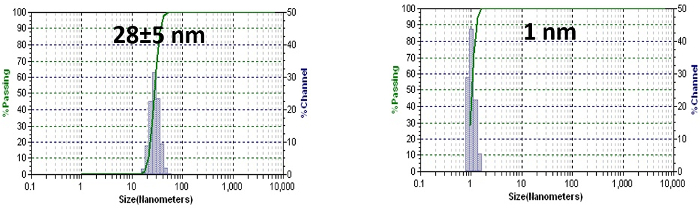
Figure 4: Stability study. The particle size of PTX-loaded, disulfide cross-linked micelles in the presence of 2.5 mg/ml SDS without (left) or with (right) GSH (10 mM), as measured by DLS. The particle size data was shown as the average particle size ± SD based on three measurements. SD: standard deviation, describes the width of the measured particle size distribution. Please click here to view a larger version of this figure.
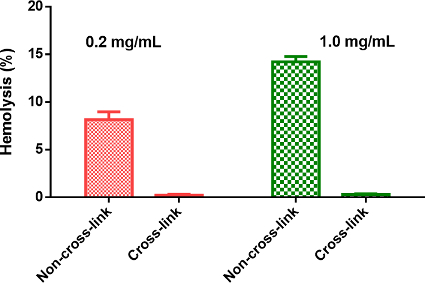
Figure 5: Hemolysis assay. The in vitro hemolytic activities of PTX-loaded, disulfide cross-linked micelles in comparison to non-crosslinked micelles on red blood cells (RBCs). Triton-100 (2%) and PBS were used as the positive and negative controls, respectively. The reported values are the mean ± SD of triplicate samples. Please click here to view a larger version of this figure.
Discussion
Several nanoparticles have been investigated for their potential use in drug delivery. Liposomal doxorubicin and paclitaxel (PTX)-loaded human serum albumin nano-aggregates are among the nanotherapeutics approved by the FDA for cancer treatment. However, although clinically effective, both of these nanotherapeutics are relatively "large" in size, and they tend to accumulate in the liver and lungs. Polymeric micelles with relatively smaller particle sizes and higher drug loading capacities are emerging nanocarriers for drug delivery. Their unique core-shell structure ''solubilize'' the hydrophobic drug molecules under aqueous conditions by physical encapsulation. Polymeric micelles composed of well-defined TDs prepared in our lab form monodispersed nanoparticles (10-50 nm particle size) with a long shelf-life and high efficiency in encapsulating drugs. One of the many advantages of the robust TD platform is the versatility of the polymer. Numerous drugs, fluorescent dyes, and targeting ligands can easily be incorporated into the TD platform13,15,24.
Critical Steps within the Protocol
We have developed a robust, reversible, disulfide cross-linked micelle system for PTX delivery, which is well-defined, with a particle size around 27 nm, and has a narrow size distribution. The TDs were synthesized via stepwise solution phase peptide chemistry. The well-defined chemical structure of these TDs is the key factor for generating nanoparticles with the desired properties. Therefore, confirming the completion of the reaction with TLC and Kaiser's test is very critical. The H2O2 oxidization is also a critical step, the thiol groups on the TD are oxidized to form disulfide cross-links, which greatly enhance the stability of the micelles. Compared to our previous oxygen-based oxidation method, the H2O2-based oxidization method is 96 times faster, thus providing cross-linked micelles in shorter time periods. Furthermore, GSH, a thiol-containing tripeptide, is an important anti-oxidant produced by the cells, and it plays an important role in scavenging free radicals and reactive oxygen compounds. It makes major contributions by maintaining the cellular redox homeostasis. The cellular concentration of GSH is an interesting aspect of drug design. The intracellular concentration of GSH is now firmly established to be substantially higher than the extracellular concentration (10 mM versus 2 µM, respectively)25. More importantly, an elevated intracellular GSH level has often been reported in many drug-resistant human and murine tumor cells26. The high intracellular distribution of GSH facilitates the breakage of the disulfide cross-linking of the micelles and results in the release and accumulation of the drug payload. The disulfide cross-linked nanoparticles are stable in the presence of SDS. However, a GSH-rich intracellular environment triggers the release of the drug by breaking down the disulfide bonds, thereby destabilizing the system. Polymer-induced hemolysis resulted from the detrimental interaction of polymeric micelles with blood constituents, such as red blood cells. Cross-linked micelles showed much less hemolysis compared to non-cross-linked analogues.
Modifications and Troubleshooting
Utilizing H2O2 as an oxidizing agent allows for a more efficient disulfide cross-link formation when compared to the previously reported, oxygen-based method13. H2O2 is a strong oxidizing agent27 that is inexpensive and highly soluble in water and gives high-quality products compared to some other known oxidizing agents (e.g., chromate or permanganate). However, the oxidization time may need to be further optimized based on the scale of the synthesis.
Limitations of the Technique
H2O2 is a strong oxidizer. Even though a low concentration of H2O2 is used for carrying out the disulfide cross-link formation, care has to be taken when using with TDs loaded with "oxidation-sensitive" drugs to prevent undesirable drug degradation.
Significance of the Technique with Respect to Existing/Alternative Methods
The technique described is an easy experimental setup that can be readily replicated in a standard laboratory. For instance, drug loading using a standard evaporation method is less time consuming compared to the dialysis method. The disulfide formation in the core using H2O2 as the oxidizing agent is 96 times faster than the previously reported oxidization method using oxygen13. Reproducibility of the drug-loading step can be easily assessed by measuring the particle size (DLS method) and drug content (HPLC).
Future Applications or Directions after Mastering this Technique
Compared to small-molecule drugs, one of the major obstacles that needs to be overcome before nano-medicine can enter mainstream cancer care settings is the technical challenges of scaling up the production28. After mastering this newly developed technique, one can easily synthesize disulfide cross-linked micelles on a large scale. This is extremely desirable for clinical studies of this nano-formulation in human patients.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
The authors thank Ms.Alisha Knudson for the editorial help. They would also like to acknowledge the financial support from the NIH/NCI (3R01CA115483, to K.S.L.), the DoD PRMRP Award (W81XWH-13-1-0490, to K.S.L.), the NIH/NCI (1R01CA199668, to Y.L.), and the NIH/NICHD (1R01HD086195, to Y.L.).
Materials
| MeO-PEG5K-NH2 | Rapp Polymere | 125000-2 | |
| Fmoc-Lys(Fmoc)-OH | Aaptec | AFK107 | |
| Fmoc-Lys(Boc)-OH | Anaspec | AS-20132 | |
| Fmoc-Cys(Trt)-OH | Aapptec | AAC105 | |
| Dimethylformamide | Fisher Scientific | BP1160-4 | |
| Ethyl ether | Fisher Scientific | E134-20 | |
| N,N-Diisopropylethylamine | Sigma Aldrich | D125806 | |
| Trifluoroacetic acid | Sigma Aldrich | T6508 | Corrosive, handle with care |
| 4-methyl piperidine | Alfa-Aesar | L-02709 | |
| Ebes linker | Anaspec | AS-61924 | |
| Cholic acid | Sigma Aldrich | C1129 | |
| 1,2-Ethanedithiol | Sigma Aldrich | 02390 | Handle inside fume hood. Bleach gloves after usage |
| Triisopropylsilane | Sigma Aldrich | 233781 | |
| Chloroform (anhydrous) | Sigma Aldrich | 288306 | |
| Hydrogen peroxide solution 30% | Aaron Industries | NA | |
| HoBt-Cl | Aaptec | CXZ096 | |
| DIC | Sigma Aldrich | D125407 | |
| Female athymic nude mice (Nu/Nu strain), 6–8 weeks age | Harlan (Livermore, CA) | ||
Referenzen
- Zhang, L., et al. Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Ther. 83, 761-769 (2008).
- Wang, A. Z., Langer, R., Farokhzad, O. C. Nanoparticle Delivery of Cancer Drugs. Annu. Rev. Med. 63, 185-198 (2012).
- Iyer, A. K., Khaled, G., Fang, J., Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Disc. Today Targets. 11, 812-818 (2006).
- Morachis, J. M., Mahmoud, E. A., Almutairi, A. Physical and chemical strategies for therapeutic delivery by using polymeric nanoparticles. Pharmacol. Rev. 64, 505-519 (2012).
- Kamaly, N., Xiao, Z., Valencia, P. M., Radovic-Moreno, A. F., Farokhzad, O. C. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem. Soc. Rev. 41, 2971-3010 (2012).
- Li, Y. L., et al. Reversibly stabilized multifunctional dextran nanoparticles efficiently deliver doxorubicin into the nuclei of cancer cells. Angew. Chem. Int. Ed. Engl. 48, 9914-9918 (2009).
- Miyata, K., et al. Block catiomer polyplexes with regulated densities of charge and disulfide cross-linking directed to enhance gene expression. J. Am. Chem. Soc. 126, 2355-2361 (2004).
- Chan, Y., Wong, T., Byrne, F., Kavallaris, M., Bulmus, V. Acid-labile core cross-linked micelles for pH-triggered release of antitumor drugs. Biomacromolecules. 9, 1826-1836 (2008).
- Rijcken, C. J., Snel, C. J., Schiffelers, R. M., van Nostrum, C. F., Hennink, W. E. Hydrolysable core-crosslinked thermosensitive polymeric micelles: synthesis, characterisation and in vivo studies. Biomaterials. 28, 5581-5593 (2007).
- Talelli, M., et al. Core-crosslinked polymeric micelles with controlled release of covalently entrapped doxorubicin. Biomaterials. 31, 7797-7804 (2010).
- Xiao, K., et al. A self-assembling nanoparticle for paclitaxel delivery in ovarian cancer. Biomaterials. 30, 6006-6016 (2009).
- Li, Y., et al. A novel size-tunable nanocarrier system for targeted anticancer drug delivery. J. Control. Release. 144, 314-323 (2010).
- Li, Y., et al. Well-defined, reversible disulfide cross-linked micelles for on-demand paclitaxel delivery. Biomaterials. 32, 6633-6645 (2011).
- Li, Y., et al. Well-defined, reversible boronate crosslinked nanocarriers for targeted drug delivery in response to acidic pH values and cis-diols. Angew. Chem. Int. Ed. Engl. 51, 2864-2869 (2012).
- Li, Y., et al. A smart and versatile theranostic nanomedicine platform based on nanoporphyrin. Nat. Commun. 5, (2014).
- Belenki, B. G., Gankina, E. S. Thin-Layer chromatography of polymers. J. Chromatogr. A. 141, 13-90 (1977).
- Kaiser, E., Colescott, R. L., Bossinger, C. D., Cook, P. I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 34, 595-598 (1970).
- Pandey, P. S., Rai, R., Singh, R. B. Synthesis of cholic acid-based molecular receptors: head-to-head cholaphanes. J. Chem. Soc., Perkin Trans 1. , 918-923 (2002).
- Riddles, P. W., Blakeley, R. L., Zerner, B. Reassessment of Ellman’s reagent. Methods Enzymol. 91, 49-60 (1983).
- Ahuja, S., Rasmussen, H. Overview of HPLC method development for pharmaceuticals. HPLC Method Development for Pharmaceuticals. , 1-11 (2007).
- Li, Y., Pan, S., Zhang, W., Du, Z. Novel thermo-sensitive core-shell nanoparticles for targeted paclitaxel delivery. Nanotechnology. 20 (6), 065104 (2009).
- Kato, J., et al. Disulfide cross-linked micelles for the targeted delivery of vincristine to B-cell lymphoma. Mol. Pharm. 9, 1727-1735 (2012).
- Lu, S. C. Regulation of glutathione synthesis. Mol. Aspects Med. 30, 42-59 (2009).
- Xiao, K., et al. “OA02” peptide facilitates the precise targeting of paclitaxel-loaded micellar nanoparticles to ovarian cancer in vivo. Cancer Res. 72, 2100-2110 (2012).
- Koo, A. N., et al. Disulfide-cross-linked PEG-poly(amino acid)s copolymer micelles for glutathione-mediated intracellular drug delivery. Chem. Commun. 28, 6570-6572 (2008).
- McLellan, L. I., Wolf, C. R. Glutathione and glutathione-dependent enzymes in cancer drug resistance. Drug. Resist. Update. 2, 153-164 (1999).
- Karala, A. R., Lappi, A. K., Saaranen, M. J., Ruddock, L. W. Efficient peroxide-mediated oxidative refolding of a protein at physiological pH and implications for oxidative folding in the endoplasmic reticulum. Antioxid. Redox Signal. 11, 963-970 (2009).
- Gabizon, A., et al. Cancer nanomedicines: closing the translational gap. Lancet. 384, 2175-2176 (2014).

