Biofilm Removal Using Carbon Dioxide Aerosols without Nitrogen Purge
Summary
Biofilms on surfaces can be effectively and rapidly removed by using a periodic jet of carbon dioxide aerosols without a nitrogen purge.
Abstract
Biofilms can cause serious concerns in many applications. Not only can they cause economic losses, but they can also present a public health hazard. Therefore, it is highly desirable to remove biofilms from surfaces. Many studies on CO2 aerosol cleaning have employed nitrogen purges to increase biofilm removal efficiency by reducing the moisture condensation generated during the cleaning. However, in this study, periodic jets of CO2 aerosols without nitrogen purges were used to remove Pseudomonas putida biofilms from polished stainless steel surfaces. CO2 aerosols are mixtures of solid and gaseous CO2 and are generated when high-pressure CO2 gas is adiabatically expanded through a nozzle. These high-speed aerosols were applied to a biofilm that had been grown for 24 hr. The removal efficiency ranged from 90.36% to 98.29% and was evaluated by measuring the fluorescence intensity of the biofilm as the treatment time was varied from 16 sec to 88 sec. We also performed experiments to compare the removal efficiencies with and without nitrogen purges; the measured biofilm removal efficiencies were not significantly different from each other (t-test, p > 0.55). Therefore, this technique can be used to clean various bio-contaminated surfaces within one minute.
Introduction
Biofilms are complex bacterial community structures in which bacterial cells are embedded within self-produced matrices of extracellular polymeric substances, held together, and protected from the external environment. Biofilms can present a public health risk and cause economic losses because they can form on various surfaces, including medical implant materials and devices, food processing equipment, and heat exchangers. In fact, biofilms have been found to be associated with 65% of all bacterial infectious diseases in humans, according to the Centers for Disease Control and Prevention1.
Autoclaves and disinfectants such as chlorine have generally been used for the inactivation of biofilms. However, the use of an autoclave is limited for surfaces that can neither withstand high temperature steam nor be placed into the autoclave. Disinfectants are not suitable for surfaces sensitive to chemical treatment or prone to collecting toxic oxidation products2. In addition, the biofilm should not only be inactivated, but also removed in order to prevent the attachment of new cells onto the surface, thereby forming a new biofilm3. However, it is difficult to remove biofilms using methods based on viscous fluid shear because the flow velocity near the surface is almost zero and the shear force usually cannot overcome the adhesive forces of micron- and submicron-sized substances. Moreover, the biofilm matrix is known to act as a physical and chemical barrier1.
Many physical and mechanical techniques have been developed to remove biofilms from surfaces, including ultrasonic vibration1, electric currents4, laser irradiation5, and high-pressure water sprays6. Each technique has its own pros and cons. Ultrasonic vibration and electric current can be used to control biofilm formation; however, they require a particular configuration and conductive surfaces, respectively, requiring additional shear stress1, 4. Laser irradiation can be applied to a limited area and to hard surfaces; however, some live and dead cells remain on the surface5. High-pressure water sprays effectively remove biofilms; however, their high momentum can cause damage to soft substrates6.
Biofilm removal using CO2 aerosols has been previously proposed. It has shown promising results, with high removal efficiencies within a short time7-11. CO2 aerosols are generated by adiabatic expansion of a high-pressure CO2 gas through a nozzle, and they are applied to the surfaces contaminated with a biofilm. This cleaning technique utilizes the momentum transfer of solid CO2 particles and the solvent action of the melted CO2 liquids, followed by the aerodynamic shear force of the CO2 gas12. Compared with high-pressure N2 gas jets, CO2 aerosol jets at the same stagnation pressure are much more effective in removing E. coli biofilms7. Moreover, although the momentum of the solid CO2 that is delivered to the bacteria is considerably high, the momentum of the total aerosol jet applied to the solid surface is substantially lower than that of water jets. Therefore, damage-free cleaning is possible using this CO2 aerosol technique.
In this protocol, periodic jets of CO2 aerosols without nitrogen purges were used to remove Pseudomonas putida biofilms from a polished stainless steel surface. In fact, nitrogen purges have been used in many CO2 aerosol studies to increase the removal efficiency by reducing the moisture condensation generated during the cleaning, even though heating with a hot plate or infrared lamps and employing dry boxes have also been adopted12. The surface biofilm formation and the optimized cleaning procedures are described below. The removal efficiency was evaluated by measuring the fluorescence intensity of the biofilm on the surface.
Protocol
1. Preparation of Surface for Biofilm Formation
- Cut 1-mm-thick 304 stainless steel plates into chips (10 × 10 mm2) with a mechanical cutter.
- Perform ultrasonic cleaning of the chips in acetone, methanol, and deionized (DI) water sequentially for 10 min each. Use a solvent-resistant container, made of substances such as glass, to remove organic contamination.
- Rinse the chips with flowing DI water for 3-5 sec.
- Dry the chips using N2 gas flow for 3-5 sec.
2. Preparation of Bacterial Culture
- Take a P. putida KT2440 (kindly provided by Prof. Sung Kuk Lee, UNIST, South Korea) stock stored in a -80 °C deep-freezer.
- Thaw at room temperature after 1 min, and the top layer of the frozen stock solution turns to slush. Immerse a loop into the melted layer of the stock solution.
- Use this loop to streak the bacteria onto a Luria-Bertani (LB) plate containing 1.5% agar.
- Incubate the plate overnight at 30 °C to grow the bacterial colonies.
- Pick a single colony from the plate using a fresh loop.
- Inoculate 10 ml of LB broth in a 50 ml conical tube with the loop containing the single bacterial colony.
- Incubate the broth in a shaking incubator for 16 hr at 30 °C and 160 rpm.
3. Biofilm Formation on the Surface
- Pick up each of the prepared chips with tweezers and dip them in 70% ethanol 5 times for 1-2 sec each, to sterilize the surface of each chip. Ensure that the chips are held with tweezers during the dipping.
- Dip each chip in autoclaved DI water and in LB broth sequentially, 5 times for 1-2 sec each, to remove remaining ethanol.
- Place these chips in 6-well culture plates with 2 chips and 5 ml LB broth per well.
- Dilute the bacterial culture until the concentration in the LB broth reaches 8 × 108 cells/ml (optical density at 600 nm wavelength: ~0.8).
- Inoculate each well with 50 µl of the diluted bacterial culture.
- Incubate the plates at 30 °C without shaking for 24 hr for formation of biofilms.
4. CO2 Aerosol Cleaning
- Dip the biofilm-formed chips in 10 mM ammonium acetate buffer (volatile) 5 times to remove loosely attached and planktonic bacteria.
- Dry these chips in a biosafety cabinet, where air flows mildly.
- Immediately after drying, place a chip on the loading place, which is 20 mm from the CO2 nozzle along the axis of the jet. Tilt the jet axis to a 40° angle.
- Set the stagnation pressures of CO2 and N2 gas to 5.3 MPa and 0.7 MPa, respectively, using gas-pressure regulators.
- Apply the aerosol jet onto the central part of the chip. White aerosols including the solid CO2 should be visible. Turn "on" the solenoid valve for CO2 for 5 sec, and then turn it "off" for 3 sec (cycle: 8 sec) periodically using a manually controlled switch. If a nitrogen purge needs to be used, turn on the solenoid valve for a continuous supply of N2.
- Treat the chips with CO2 aerosols for 16, 40, and 88 sec with and without nitrogen purges. Retain chips without treatment as negative controls.
5. Analysis for Removal Efficiency
- Prepare 1 µM green fluorescence nucleic acid stain (excitation/emission wavelength: 480/500 nm) in DI water to stain bacterial cells on the control and aerosol-treated chips.
- Place the chips in the staining solution.
- Incubate the chips in an incubator without light at 37 °C for 30 min.
- Following the incubation, gently rinse the chips with flowing DI water to remove excessive fluorescent dye.
- Dry the chips with N2 gas flow.
- Take fluorescence microscopic images of 5 random fields of view (321 × 240 µm2) for each chip using an epifluorescence microscope, a 40X objective lens, and a CCD camera.
- Obtain the fluorescence intensity for each image using an image processing software such as ImageJ. In ImageJ, use the "Subtract Background" function in the "Process" menu, and select "Integrated density" in the "Set Measurements" window in the "Analyze" menu. Do the "Measurement" in the "Analyze" menu to obtain the fluorescence intensity.
- Calculate the biofilm removal efficiency according to the following formula: 100% × (I of control chips – I of treated chips)/(I of control chips), where I is the calculated fluorescence intensity.
- Obtain the average removal efficiencies and standard deviations. Use at least 4 chips for each case.
Representative Results
CO2 aerosols were used to remove the P. putida biofilms from SUS304 surfaces (Figure 1). Most of the surfaces were covered with the biofilm after 24 hr of growth. Most of the biofilm was removed using the CO2 aerosols (Figure 2). As expected, Figure 3 shows an increase in biofilm removal efficiency as the CO2 aerosol treatment time increased. For the treatment time of 88 sec, the removal efficiency was determined to be as high as 97.74% and 98.29% with and without a nitrogen purge, respectively. The air temperature was 23-24 °C and the relative humidity was 26-50% when the cleaning experiments were conducted.
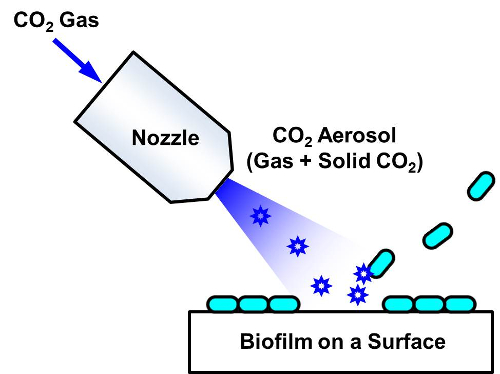
Figure 1. Schematic showing the removal of a biofilm using CO2 aerosols. The aerosols are generated when high pressure CO2 gas is adiabatically expanded through a venturi nozzle (0.4 mm) and the gas temperature drops rapidly. These high-speed CO2 aerosols are applied to a surface covered with a biofilm. When a nitrogen purge is used, N2 gas is supplied through 8 nozzles surrounding the central CO2 nozzle. Please click here to view a larger version of this figure.
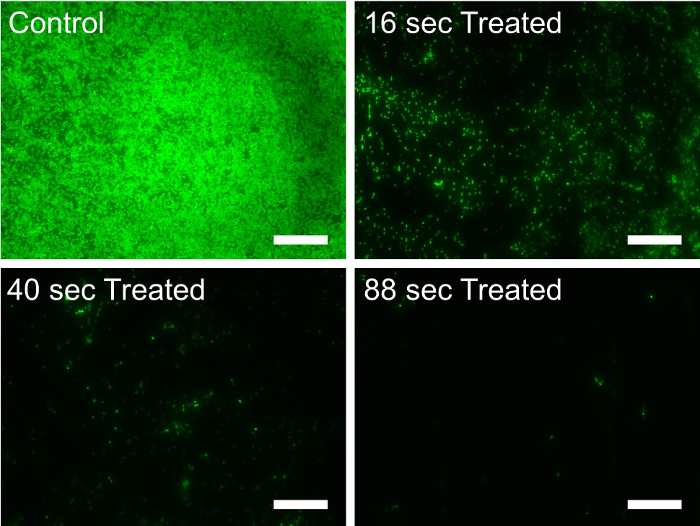
Figure 2. Fluorescence microscopic images for 24-hr-grown P. putida biofilms on 304 stainless steel chips. The chips were treated with CO2 aerosols for different treatment times (0, 16, 40, and 88 sec) without nitrogen purges. Monochrome images were colored green, and the white scale bars indicate 50 µm. Please click here to view a larger version of this figure.
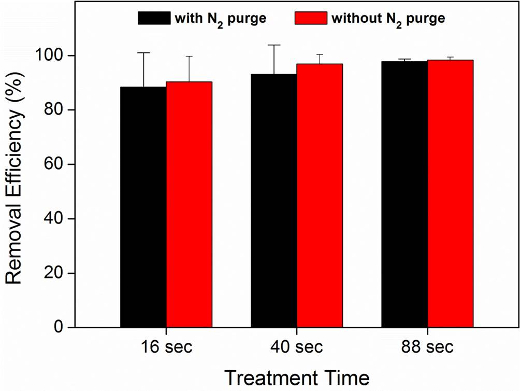
Figure 3. Removal efficiencies for the P. putida biofilms from the 304 stainless steel surfaces with different CO2 aerosol treatment times, with and without nitrogen purges. The removal efficiencies were calculated based on the fluorescence intensities of the biofilms. The error bars represent the standard deviations. Please click here to view a larger version of this figure.
Discussion
Previously, we conducted optimization studies on CO2 gas pressure, jet angle, and distance to the solid surface in CO2 aerosol cleaning7. Unlike our previous studies, in the present study, a nitrogen purge was not included in the aerosol (Figure 1). Moreover, 304 stainless steel was used in this protocol, since it is one of the most common stainless steels and is widely used in the food industry. The polished surface is beneficial for fluorescence analysis because of a uniform focal length and reduced light scattering. The bacterial species P. putida is also commonly used in the field of biofilm research. Fluorescence intensity was employed for the quantification of the amount of biofilm attached to the surface. Two-dimensional fluorescent images were used to quantify the amount of biofilm on the surface. This method may be sufficient for 24-hr-grown thin biofilms; however, confocal 3D images should be used for thick biofilms. This method is simple and enables biological recognition of bacterial cells. Since the stain penetrates the bacterial membrane and stains nucleic acids irrespective of cell viability, the green fluorescence shows all live and dead bacterial populations. In addition, fluorescence intensity was higher if the bacterial cells were overlapping (Figure 2), which could not have been taken into account if the methods were solely based on the coverage area of the cells 5, 7, 8, 10.
It should be noted that the stagnation pressure of the CO2 gas is a critical factor for removal efficiency. The white CO2 aerosol jet was not visible, and the removal efficiency was dramatically decreased, when the gas pressure was below 4 MPa7. This decrease is attributed to the lower proportion of solid CO2 generated. The measured biofilm removal efficiencies with and without nitrogen purges were not significantly different (t-test, p > 0.55) (Figure 3), even though dry nitrogen gas is commonly used to increase the removal efficiency. In fact, for the removal of submicron particles, dry nitrogen gas has often been used to reduce the moisture condensation caused by cryogenic CO2 aerosols12. In this study, however, the moisture might positively affect the removal of the biofilm.
Although CO2 aerosols can significantly inactivate bacteria11, the dispersal of viable bacteria during the cleaning process can result in secondary contamination and infection through aerosol inhalation. Therefore, this removal method needs to be utilized in conjunction with other disinfection methods such as ultraviolet irradiation, especially if the bacteria are pathogenic. In addition to providing damage-free and residue-free cleaning12, this technique can potentially be applied to the removal of biofilms from surfaces with complicated topographies or narrow gaps and protrusions.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, and Future Planning (# 2015R1A2A2A01006446).
Materials
| 304 stainless steel | Steelni (South Korea) |
Polished and diced ones | |
| Ultrasonic cleaner | Branson (USA) |
5510E-DTH | |
| Luria-Bertani (LB) | Becton, Dickinson and Company (USA) |
244620 | 500 g |
| Agar | Becton, Dickinson and Company (USA) |
214010 | 500 g |
| 6-well culture plate | SPL Life Sciences (South Korea) |
32006 | |
| Ammonium acetate buffer | Sigma-Aldrich (USA) |
667404 | 10 mM |
| Dual gas unit | Applied Surface Technologies (USA) |
K6-10DG | One nozzle for CO2 gas & 8 nozzles for N2 gas |
| SYTO9 | Thermo Fissher Scientific (USA) |
Invitrogen | Excitaion: 480 nm Emission: 500 nm |
| Epifluorescence microscope | Nikon (Japan) | Eclipse 80i | |
| 40× objective lens | Nikon (Japan) |
Plan Fluor | NA: 0.75 |
| CCD camera | Photometrics (USA) |
Cool SNAP HQ2 | Monochrome |
Referenzen
- Jain, A., Gupta, Y., Agrawal, R., Khare, P., Jain, S. K. Biofilms – A microbial life perspective: A critical review. Crit. Rev. Ther. Drug. 24 (5), 393-443 (2007).
- Bott, T. R. Biofouling control with ultrasound. Heat Transfer Eng. 21 (3), 43-49 (2000).
- Meyer, B. Approaches to prevention, removal and killing of biofilms. Int. Biodeterior. Biodegradation. 51 (4), 249-253 (2003).
- Hong, S. H., et al. Effect of electric currents on bacterial detachment and inactivation. Biotechnol. Bioeng. 100 (2), 379-386 (2008).
- Nandakumar, K., Obika, H., Utsumi, A., Ooie, T., Yano, T. In vitro laser ablation of laboratory developed biofilms using an Nd:YAG laser of 532 nm wavelength. Biotechnol. Bioeng. 86 (7), 729-736 (2004).
- Gibson, H., Taylor, J. H., Hall, K. E., Holah, J. T. Effectiveness of cleaning techniques used in the food industry in terms of the removal of bacterial biofilms. J. Appl. Microbiol. 87 (1), 41-48 (1999).
- Kang, M. Y., Jeong, H. W., Kim, J., Lee, J. W., Jang, J. Removal of biofilms using carbon dioxide aerosols. J. Aerosol Sci. 41 (11), 1044-1051 (2010).
- Cha, M., Hong, S., Kang, M. Y., Lee, J. W., Jang, J. Gas-phase removal of biofilms from various surfaces using carbon dioxide aerosols. Biofouling. 28 (7), 681-686 (2012).
- Dwidar, M., Hong, S., Cha, M., Jang, J., Mitchell, R. J. Combined application of bacterial predation and carbon dioxide aerosols to effectively remove biofilms. Biofouling. 28 (7), 671-680 (2012).
- Cha, M., Hong, S., Lee, S. Y., Jang, J. Removal of different-age biofilms using carbon dioxide aerosols. Biotechnol. Bioprocess Eng. 19 (3), 503-509 (2014).
- Singh, R., Monnappa, A. K., Hong, S., Mitchell, R. J., Jang, J. Effects of Carbon Dioxide Aerosols on the Viability of Escherichia coli during Biofilm Dispersal. Sci. Rep. 5, 13766 (2015).
- Sherman, R. Carbon Dioxide Snow Cleaning. Particul. Sci.Technol. 25 (1), 37-57 (2007).

