Atmospheric Pressure Fabrication of Large-Sized Single-Layer Rectangular SnSe Flakes
Summary
A protocol is presented demonstrating a two-step fabrication technique to grow large-sized single-layer rectangular shaped SnSe flakes on low-cost SiO2/Si dielectrics wafers in an atmospheric pressure quartz tube furnace system.
Abstract
Tin selenide (SnSe) belongs to the family of layered metal chalcogenide materials with a buckled structure like phosphorene, and has shown potential for applications in two-dimensional nanoelectronics devices. Although many methods to synthesize SnSe nanocrystals have been developed, a simple way to fabricate large-sized single-layer SnSe flakes remains a great challenge. Herein, we show the experimental method to directly grow large-sized single-layer rectangular SnSe flakes on commonly used SiO2/Si insulating substrates using a straightforward two-step fabrication method in an atmospheric pressure quartz tube furnace system. The single-layer rectangular SnSe flakes with an average thickness of ~6.8 Å and lateral dimensions of about 30 µm × 50 µm were fabricated by a combination of vapor transport deposition technique and nitrogen etching route. We characterized the morphology, microstructure, and electrical properties of the rectangular SnSe flakes and obtained excellent crystallinity and good electronic properties. This article about the two-step fabrication method can help researchers grow other similar two-dimensional, large-sized, single-layer materials using an atmospheric pressure system.
Introduction
Research into two dimensional (2D) materials has bloomed in recent years since the successful isolation of graphene, due to the possibility of 2D materials having superior electrical, optical, and mechanical properties over their bulk counterparts1,2,3,4,5. 2D materials show promising applications in optoelectronic and electronic devices6,7, catalysis and water splitting8,9, surface-enhanced Raman scattering sensing10,11, etc. The large family of layered materials which can be exfoliated into 2D materials show great diversity, ranging from the semi-metallic graphene to the semiconducting transition-metal dichalcogenides (TMDs) and black phosphorus (BP) to the insulating hexagonal boron nitride (h-BN). These materials and their heterostructures have been well studied in recent years, and have exhibited many novel properties and applications12. Other less studied, but equally promising 2D layered materials in the IIIA-VIA (GaS, GaSe, and InSe)13,14 and IVA-VIA (GeS, GeSe, and SnS)15,16,17 families have also recently received attention.
SnSe belongs to the IVA-VIA group and crystallizes in an orthorhombic structure, with the atoms arranged in the pnma space group and buckled within the layer, like the crystal structure of phosphorene. SnSe is a narrow gap semiconductor with a band gap of 0.6 eV, but is more well known for its more unique thermoelectric properties, as it is reported to have a very high ZT (thermoelectric figure of merit) value of 2.6 at 923 K18,19, which has been attributed to its unique electronic structure and low thermal conductivity. While bulk SnSe crystals are available commercially and can be grown by known methods, such as the Bridgeman-Stockbarger method20 or the chemical vapor transport method21, growing large sized few-layer and single-layer SnSe on dielectric substrates is more challenging. There are many substrates to support 2D material growth, such as highly oriented pyrolytic graphite (HOPG), mica, SiO2, Si3N4, and glass. Low-cost SiO2 dielectrics are the most commonly used substrate, as these allow the fabrication of field-effect transistors, where the dielectrics serve as part of the electrical back gate. In our experience, unlike graphene and TMDs, it is difficult to obtain few-layer or single-layer SnSe flakes by the micromechanical exfoliation method, as bulk SnSe has a high interlayer binding energy22 of 32 meV/Å2, which leads to thick layers, even along the edges of the exfoliated flakes. Therefore, to study the novel electronic properties of few layer and single layer SnSe, a new, simple, and low-cost synthetic method to prepare high quality large-sized single-layer SnSe crystals on insulating substrates is required, especially since SnSe has shown great promise as a candidate for thermoelectric applications for energy conversion in the low and moderate temperature range19.
Several researchers have developed methods to synthesize high quality SnSe crystals. Liu et al.23 and Franzman et al.24 used a solution-phase method to synthesize SnSe nanocrystals of different shapes, such as quantum dots, nanoplates, single crystalline nanosheets, nanoflowers, and nanopolyhedra using SnCl2 and alkyl-phosphine-selenium or dialkyl diselenium as precursors. Baumgardner et al.25 synthesized colloidal SnSe nanoparticles by injecting bis[bis(trimethylsilyl)amino]tin(II) into hot trioctylphosphine, and they obtained nanocrystals of ~4-10 nm in diameter. Boscher et al.26 used an atmospheric pressure chemical vapor deposition technique to obtain SnSe films on glass substrates using tin tetrachloride and diethyl selenide precursors with a tin tetrachloride ratio 10 larger than diethyl selenide, and their synthesized SnSe films were about 100 nm thick and silver-black in appearance. Zhao et al.27 used vapor transport deposition in a low vacuum system and synthesized single-crystal SnSe nanoplates on mica substrates and obtained square nanoplates of 1-6 µm. However, obtaining single-layer SnSe crystals are not possible using these techniques. Li et al.28 successfully synthesized single-layer single-crystal SnSe nanosheets using a one-pot synthetic method with SnCl4 and SeO2 precursors. However, they were only able to obtain a lateral size of about 300 nm for their nanosheets. We have recently published our method to grow high quality, large-sized single-layer SnSe crystals which are phase pure29. This detailed protocol is intended to help new practitioners to grow other large-sized high-quality ultrathin 2D materials using this methodology.
Protocol
Caution: Some of the chemical agents and gases used in this work are toxic, carcinogenic, flammable, and explosive. Please use all appropriate safety practices when performing a vapor transport deposition including the use of engineering controls (fume hood) and personal protective equipment (safety glasses, professional protective masks, gloves, lab coat, full length pants, and closed-toe shoes).
1. Auto-Tune Function of Temperature Controller Parameters
NOTE: Before the synthesis of SnSe flakes, the heating system of the furnace needs to be calibrated by following the manufacturer's manual.
- Set 80% of the most commonly used temperature as the target temperature. Here, set 560 oC for 1 h and run the furnace.
- When the temperature approaches 560 oC, press the "SET" key for 2 s, note that the parameter "HAL" pops up, and press the "SET" key for 1 s to go next parameter.
- Continue to press the "SET" key. After "Cont=3" appears, set it as 2. The system starts the auto-tune function to work out the value for Int, Pro, and Lt, and then system will go to 3. When re-auto-tune is needed, set it as 2.
2. Pretreatment of Quartz Tubes and Ceramic Boats
NOTE: Before the synthesis of SnSe flakes, a high temperature cleaning process is required, where a new ceramic boat and a new quartz tube are pretreated.
- Position a new ceramic boat inside a new 1-inch diameter quartz tube. Place the 1-inch diameter quartz tube inside a horizontal tube furnace with a new 2 inch diameter quartz tube. Ensure that the both ends of the tubes are firmly fixed and supported.
- Close the furnace lid, and heat the tube furnace to 1,000 oC over 30 min.
- When the temperature at the center of the furnace approaches 1,000 oC, keep the furnace at 1,000 oC for 30 min. Then, gradually move the tube furnace from one end to the other to heat the whole length of the tube for cleaning the quartz tube wall and the ceramic boat.
- After this, allow the tube furnace to cool to room temperature by turning off the furnace. When the furnace has cooled to room temperature, open the furnace lid and take out the new ceramic boat and the new 1-inch diameter quartz tube, which can be used for the subsequent experiments.
3. Pretreatment SiO 2 /Si Substrates
- Cut the SiO2/Si wafer (300 nm thick SiO2 on heavily doped Si) (see Table of Materials) using a diamond scriber into an appropriate size (about 1.5 cm × 2 cm) to be used as growth substrates.
- Clean the SiO2/Si substrates in acetone, isopropanol, and water, followed by a nitrogen blow dry.
4. Synthesis of Bulk Rectangular Shaped SnSe Flakes
- Place 0.010 g SnSe powder (see Table of Materials) in the clean ceramic boat. Place a clean SiO2/Si substrate (about 1.5 cm × 2 cm) onto the ceramic boat, growth side facing the SnSe powder. Position the ceramic boat inside a clean 1-inch diameter quartz tube.
- Place the 1-inch diameter quartz tube inside a horizontal tube furnace with a 2-inch diameter quartz tube on the outside, and ensure that the ceramic boat is located upstream of the heating zone of the tube furnace. Tighten the flanges at both ends of the tube, and close the vent valve, which seals the 2-inch diameter quartz tube.
- Turn on the pump that connects to the quartz tube, pump the tube to a pressure of ~1×10-2 mbar to remove air and moisture in the tube. After that pressure is achieved, turn off the pump.
- Then open the carrier gas valves, using the gas flow meter to control the gas flows. Introduce 40 standard cubic cm per min (sccm) Ar and 10 sccm H2 (purity: 99.9%) into the quartz tube until atmospheric pressure was achieved. Open the vent valves to allow a continuous flow of gas in the quartz tubes.
- Close the furnace lid and rapidly heat the tube furnace with a 35 oC per minute heating-rate.
- When the temperature at the center of the furnace approaches 700 oC, quickly move the tube furnace to position the SnSe powders at the center of the furnace. The SnSe powder will evaporate, and the bulk SnSe flakes will deposit on the SiO2/Si surface.
- After 15 min growth time, open the furnace lid to quickly cool the tube furnace to room temperature. Meanwhile, adjust the flow of Ar/H2 carrier gas to maximum, which will help to drive the unreacted gas or particles out of the tubes. When the growth process is completed, bulk SnSe flakes will be obtained on the surface of the SiO2/Si substrates.
5. Fabrication of Single-Layer Rectangular Shaped SnSe Flakes
- Place the as-grown bulk SnSe/SiO2/Si sample face up onto a new clean ceramic boat. Position the ceramic boat inside a new clean 1-inch diameter quartz tube.
- Put the 1-inch diameter quartz tube inside the horizontal tube furnace with a 2-inch diameter quartz tube, with the ceramic boat located upstream of the heating zone of the tube furnace. Tighten the flanges at both ends of the tube, and close the vent valve to seal the 2-inch diameter quartz tube.
- Turn on the pump that connects to the quartz tube, pump down the tube to a pressure of ~1×10-2 mbar to remove air and moisture in the tube. After that is achieved, turn off the pump.
- Open the carrier gas valves, using the gas flow meter to control the gas flows. Introduce 50 sccm N2 (purity: 99.9%) into the quartz tube until atmospheric pressure is achieved. Open the vent valves to allow a continuous flow of gas in the quartz tubes.
- Close the furnace lid and rapidly heat the tube furnace to 700 oC in 20 min.
- When the temperature at the center of the furnace approaches 700 oC, quickly move the tube furnace to position the bulk SnSe/SiO2/Si sample at the center of the furnace.
- Maintain the furnace at 700 oC for ~5-20 min to complete the etching process. After that, open the furnace lid, and quickly cool the tube furnace to room temperature. Meanwhile, keep the flow of N2 gas to a maximum, which will help to drive the unreacted gas or particles out of the tubes. When the etching process is completed, observe the single-layer rectangular shaped SnSe flakes obtained on the surface of the SiO2/Si substrates.
NOTE: The etching gas and etching time are the main controlling factors in this process. The etching mechanism is investigated in reference 29, so see reference 29 for more details.
Representative Results
Schematic diagrams of the experimental apparatus, optical images, atomic force microscopy (AFM) images, scanning electron microscopy (SEM) images, and transmission electron microscopy (TEM) images of the fabricated SnSe flakes are shown in Figure 1, Figure 2, and Figure 3. The optical images are performed by a traditional optical microscope. The eyepiece lens is 10X, and the objective lens is 20X, 50X, and 100X. The exposure time is about 0.3 seconds. The resolution of the obtained optical image is 1,376×1,038. The scan size is 30 µm with an aspect ratio of 1. The X and Y offsets and angle are both set as 0. The scan rate is 3.92 Hz with a 512 sample/line. The integral gain and proportional gain are set as 1.000 and 5.000, respectively. The amplitude setpoint, drive frequency, and amplitude set as 208.9 mV, 1400.789 KHz, 85.14 mV, respectively. SEM and TEM images were performed in an electron microscope operated at 30 kV and 200 kV, respectively.
Figure 1 shows the process of evaporating the precursor SnSe powder, which deposited on the SiO2/Si surface to grow large-sized rectangular bulk SnSe flakes through a vapor transport deposition technique in the atmospheric pressure quartz tube system. To fabricate single-layer SnSe flakes, we transferred the as-grown bulk SnSe/SiO2/Si sample into an adjacent tube furnace for nitrogen etching. We did not employ any thermal/chemical treatment methods, nor were they necessary after the growth processes.
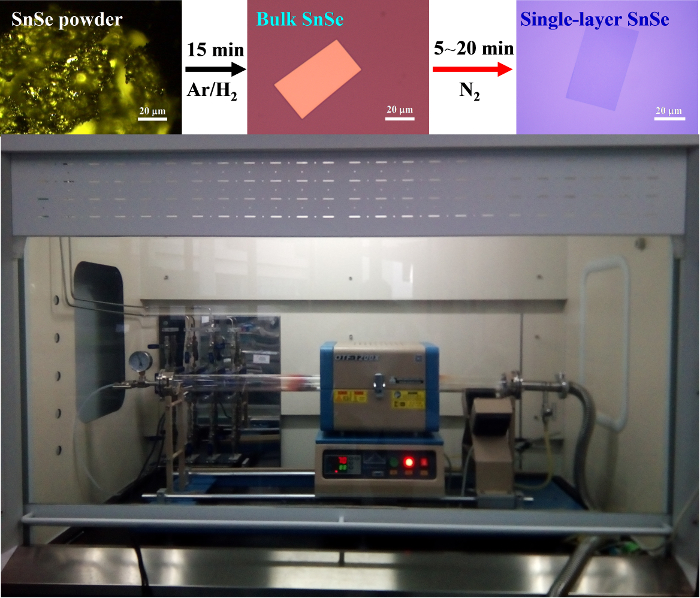
Figure 1: Synthesis. Schematic diagrams showing the experimental apparatus and process of synthesizing bulk rectangular SnSe flakes and fabrication of single-layer rectangular SnSe flakes. Please click here to view a larger version of this figure.
Figure 2 shows the optical microscopy and AFM characterization of the morphology of the as-synthesized bulk and single layer SnSe flakes. We found that the bulk and single layer SnSe flakes are approximately rectangular and grow randomly on the SiO2/Si substrates. Figure 2a-d and Figure 2f-i: We obtained SnSe flakes that are about 30 µm × 50 µm in size, about 200 times larger than single-layer single-crystalline SnSe nanosheets obtained by Li et al.28 Figure 2e shows an AFM image with the corresponding line profile of a typical synthesized bulk SnSe flake, revealing a flat surface with a thickness of about 54.9 ± 5.6 nm. We measured a thickness of ~6.8 ± 1.4 Å for the ultra-thin rectangular SnSe flakes (Figure 2j), near to the theoretical value of single-layer SnSe of 5.749 Å18.
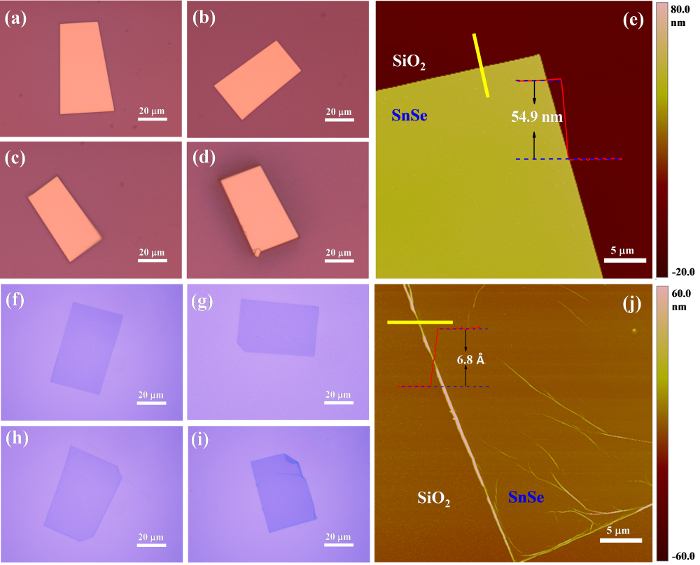
Figure 2: Images of SnSe flakes. Optical images of as-synthesized bulk (a-d) and single-layer (f-i) rectangular shaped SnSe flakes. Typical AFM images of the bulk (e) and single-layer (j) rectangular shaped SnSe flakes at the flake edges of (a) and (f), respectively. Copyright: IOP publishing (permission to reproduce required).This figure has been modified from Jiang et al.29 Please click here to view a larger version of this figure.
To analyze the micro-structure and chemical composition of the as-synthesized samples, we characterized the bulk and single-layer SnSe flakes by SEM and energy dispersive X-ray spectrometry (EDX). Figure 3a-b shows the typical SEM images of bulk and single-layer SnSe flakes, which are randomly distributed on the surface of the SiO2/Si wafer. We can see that both the bulk and single-layer SnSe flakes are approximately rectangular with dimensions of about 30 µm × 50 µm, in excellent agreement with the results obtained from optical microscopy images (Figure 2). The EDX spectrum (Figure 3c) shows a 1:0.92 atomic ratio of Sn and Se in the as-synthesized bulk sample, which confirms stoichiometric SnSe and not SnSe2. Figure 3d shows a typical TEM image of the transferred SnSe fragment. The selected area electron diffraction pattern (SAED) of a single-layer SnSe fragment clearly exhibits an orthogonally symmetric diffraction pattern (Figure 3e), indicating that our sample is single-crystal in nature. The single-layer SnSe flakes are normally orientated along the [100] plane direction, as the SAED also shows a spot pattern of 0kl reflection. Figure 3f shows the high resolution TEM (HR-TEM) image of the transferred SnSe fragment with two apparent orthogonal lattice fringes from the and planes and lattice spacings of about 0.30 nm. The angle between the lattice fringes is approximately 86.5o, which corresponds to an orthorhombic crystal structure, in agreement with theory18.
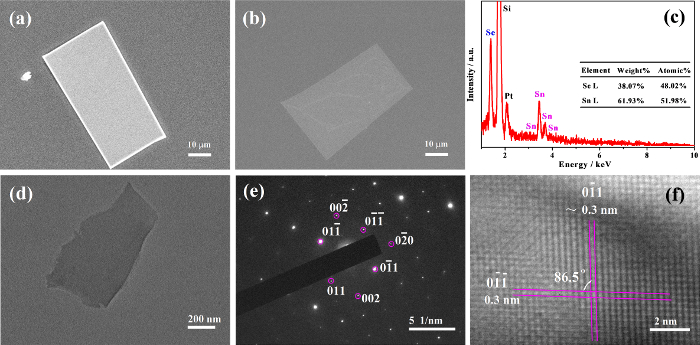
Figure 3: SEM image (a) and EDX spectrum (c) of the bulk SnSe flakes; SEM image (b), TEM image (d), SAED pattern (e), and high-resolution TEM image (f) of the single-layer rectangular shaped SnSe flakes fragment, respectively. Copyright: IOP publishing (permission to reproduce required). This figure has been modified from Jiang et al.29 Please click here to view a larger version of this figure.
Discussion
Here, the combination of a vapor transport deposition method and a nitrogen etching technique in an atmospheric pressure system is first reported. In this protocol, the critical steps are the section of the fabrication of single-layer SnSe flakes.
Although the bulk samples can be etched to form a high-quality single-layer sample, the thickness of the bulk samples should be uniform and the decomposition temperature of bulk samples should be higher than the etching temperature. The resulting sample has a low coverage density, due to most bulk samples being completely etched.
For the application of scanning tunneling microscopy (STM), the coverage density of single-layer samples is not enough. However, for the application of optoelectronic devices, the coverage density is satisfactory. As there has been a recent increase in interest in novel 2D group-IV monochalcogenides materials, we think that this simple two-step fabrication technique can be extended to and will be helpful for others in the preparation of other large-sized high-quality ultrathin 2D materials.
The investigation of the long-term stability, XRD analysis, and Raman characterization of SnSe flakes can be found elsewhere29.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This research was supported by the 1,000 Talents Program for Young Scientists of China, National Natural Science Foundation of China (Grant No. 51472164), the A*STAR Pharos Programme (Grant No. 152 70 00014), and facility support from the NUS Center for Advanced 2D Materials.
Materials
| SnSe powder | Sigma-Aldrich | 1315-06-6 | (99.999%) toxic, carcinogenic |
| Ar gas | explosive | ||
| H2 gas | flammable, explosive | ||
| SiO2/Si wafer | 300 nm thick SiO2 on heavily doped Si | ||
| Acetone | Sigma-Aldrich | 67-64-1 | toxic, flammable |
| Isopropanol | Sigma-Aldrich | 67-63-0 | flammable |
| Quartz tube | Dongjing Quartz Company, China | ||
| Ceramic boat | Dongjing Quartz Company, China | ||
| Optical microscope | Olympus, BX51 | ||
| Atomic force microscopy | Bruker | Using FastScan-A probe type and ScanAsyst-air | |
| Scanning electron microscopy | JEOL JSM-6700F | ||
| transmission electron microscopy | FEI Titan | ||
| Tube furnace | MTI Corporation |
Referenzen
- Geim, A. K., Novoselo, K. S. The Rise of Graphene. Nature Mater. 6, 183-191 (2007).
- Chhowalla, M., Shin, H. S., Eda, G., Li, L. -. J., Loh, K. P., Zhang, H. The Chemistry of Two-Dimensional Layered Transition Metal Dichalcogenide Nanosheets. Nat. Chem. 5, 263-275 (2013).
- Zhang, W., Wang, Q., Chen, Y., Wang, Z., Wee, A. T. S. Van der Waals Stacked 2D Layered Materials for Optoelectronics. 2D Mater. 3 (1-17), 02200 (2016).
- Li, M. -. Y., et al. Epitaxial Growth of a Monolayer WSe2-MoS2 Lateral p-n Junction with an Atomically Sharp Interface. Science. 349, 524-528 (2015).
- Wang, H., Yuan, H., Hong, S. S., Li, Y., Cui, Y. Physical and Chemical Tuning of Two-Dimensional Transition Metal Dichalcogenides. Chem. Soc. Rev. 44, 2664-2680 (2015).
- Wang, Q. H., Kalantar-Zadeh, K., Kis, A., Coleman, J. N., Strano, M. S. Electronics and Optoelectronics of Two-Dimensional Transition Metal Dichalcogenides. Nat.Nanotechnol. 7, 699-712 (2012).
- Kim, K. S., et al. Large-Scale Pattern Growth of Graphene Films for Stretchable Transparent Electrodes. Nature. 457, 706-710 (2009).
- Shalom, M., Gimenez, S., Schipper, F., Herraiz-Cardona, I., Bisquert, J., Antonietti, M. Controlled Carbon Nitride Growth on Surfaces for Hydrogen Evolution Electrodes. Angew. Chem. 126, 3728-3732 (2014).
- Liu, J., et al. Metal-Free Efficient Photocatalyst for Stable Visible Water Splitting via a Two-Electron Pathway. Science. 347, 970-974 (2015).
- Jiang, J., Zou, J., Wee, A. T. S., Zhang, W. Use of Single-Layer g-C3N4/Ag Hybrids for Surface-Enhanced Raman Scattering (SERS). Sci.Rep. 6 (1-10), 34599 (2016).
- Jiang, J., Zhu, L., Zou, J., Ou-yang, L., Zheng, A., Tang, H. Micro/Nano-Structured Graphitic Carbon Nitride-Ag Nanoparticle Hybrids as Surface-Enhanced Raman Scattering Substrates with Much Improved Long-Term Stability. Carbon. 87, 193-205 (2015).
- Jariwala, D., Marks, T. J., Hersam, M. C. Mixed-dmensional van der Waals Heterostructures. Nature Mater. 16, 170-181 (2017).
- Late, D. J., et al. GaS and GaSe Ultrathin Layer Transistors. Adv. Mater. 24, 3549-3554 (2012).
- Klein, A., Lang, O., Schlaf, R., Pettenkofer, C., Jaegermann, W. Electronically Decoupled Films of InSe Prepared by van der Waals Epitaxy: Localized and Delocalized Valence States. Phys. Rev. Lett. 80, 361-364 (1998).
- Gomes, L. C., Carvalho, A. Phosphorene Analogues: Isoelectronic Two-Dimensional Group-IV Monochalcogenides with Orthorhombic Structure. Phys. Rev. B. 92 (1-8), 085406 (2015).
- Xue, D., Tan, J., Hu, J., Hu, W., Guo, Y., Wan, L. Anisotropic Photoresponse Properties of Single Micrometer-Sized GeSe Nanosheet. Adv. Mater. 24, 4528-4533 (2012).
- Antunez, P. D., Buckley, J. J., Brutchey, R. L. Tin and Germanium Monochalcogenide IV-VI Semiconductor Nanocrystals for Use in Solar Cells. Nanoscale. 3, 2399-2411 (2011).
- Zhao, L. D., et al. Ultralow Thermal Conductivity and High Thermoelectric Figure of Merit in SnSe Crystals. Nature. 508, 373-377 (2014).
- Zhao, L. D., et al. Ultrahigh Power Factor and Thermoelectric Performance in Hole-Doped Single-Crystal SnSe. Science. 351, 141-144 (2016).
- Bhatt, V. P., Gireesan, K., Pandya, G. R. Growth and Characterization of SnSe and SnSe2 Single Crystals. J. Cryst. Growth. 96, 649-651 (1989).
- Yu, J. G., Yue, A. S., Stafsudd, O. M. Growth and Electronic Properties of the SnSe Semiconductor. J. Cryst. Growth. 54, 248-252 (1981).
- Zhang, L., et al. Tinselenidene: a Two-dimensional Auxetic Material with Ultralow Lattice Thermal Conductivity and Ultrahigh Hole Mobility. Sci. Rep. 6 (1-9), (2016).
- Liu, X., Li, Y., Zhou, B., Wang, X., Cartwright, A. N., Swihart, M. T. Shape-Controlled Synthesis of SnE (E=S, Se) Semiconductor Nanocrystals for Optoelectronics. Chem. Mater. 26, 3515-3521 (2014).
- Franzman, M. A., Schlenker, C. W., Thompson, M. E., Brutchey, R. L. Solution-Phase Synthesis of SnSe Nanocrystals for Use in Solar Cells. J. Am. Chem. Soc. 132, 4060-4061 (2010).
- Baumgardner, W. J., Choi, J. J., Lim, Y. -. F., Hanrath, T. SnSe Nanocrystals: Synthesis, Structure, Optical Properties, and Surface Chemistry. J. Am. Chem. Soc. 132, 9519-9521 (2010).
- Boscher, N. D., Carmalt, C. J., Palgrave, R. G., Parkin, I. P. Atmospheric Pressure Chemical Vapour Deposition of SnSe and SnSe 2 Thin Films on Glass. Thin Solid Films. 516, 4750-4757 (2008).
- Zhao, S., et al. Controlled Synthesis of Single-Crystal SnSe Nanoplates. Nano Res. 8, 288-295 (2015).
- Li, L., et al. Single-Layer Single-Crystalline SnSe Nanosheets. J. Am. Chem. Soc. 135, 1213-1216 (2013).
- Jiang, J., et al. Two-Step Fabrication of Single-Layer Rectangular SnSe Flakes. 2D Mater. 4 (1-9), 021026 (2017).

