Culturing and Measuring Fetal and Newborn Murine Long Bones
Summary
Here, we present a method for ex vivo culture of long murine bones at both fetal and newborn stages, suitable for analyzing bone and cartilage development and homeostasis in controlled conditions while recapitulating the in vivo process.
Abstract
Long bones are complex and dynamic structures, which arise from endochondral ossification via a cartilage intermediate. The limited access to healthy human bones makes particularly valuable the use of mammalian models, such as mouse and rat, to look into different aspects of bone growth and homeostasis. Additionally, the development of sophisticated genetic tools in mice allows more complex studies of long bone growth and asks for an expansion of techniques used to study bone growth. Here, we present a detailed protocol for ex vivo murine bone culture, which allows the study of bone and cartilage in a tightly controlled manner while recapitulating most of the in vivo process. The method described allows the culture of a range of bones, including tibia, femur, and metatarsal bones, but we have focused mainly on tibial culture here. Moreover, it can be used in combination with other techniques, such as time-lapse live imaging or drug treatment.
Introduction
Organ growth has to be tightly tuned to prevent the appearance of growth disorders, and involves the regulation of multiple cell types, molecular pathways and crosstalk among different parts of the body. Imaging techniques are essential to address the changes occurring over time in a growing embryo, both in normal conditions, as well as after a perturbation is induced in the system. Embryos with intrauterine development, such as the widely used rodent models, present an additional challenge for live imaging and drug treatment, which can be partially overcome by using ex vivo culture techniques. To successfully recapitulate the in vivo processes and obtain meaningful results, it becomes crucial to find the right culturing conditions for each organ or tissue.
Most bones of the mammalian skeleton grow through endochondral ossification, where the embryonic cartilage (composed of cells called chondrocytes) drives longitudinal growth and is gradually replaced by bone. This process happens at the growth plates, located at the end of the long bones, where three zones can be distinguished: resting, proliferative, and hypertrophic1,2. First, the round progenitor chondrocytes in the resting zone transition into the cycling columnar chondrocytes in the proliferative zone. During the next stage of differentiation, these chondrocytes become hypertrophic and start secreting type X collagen. Hypertrophic chondrocytes orchestrate the subsequent steps of ossification: they secrete key signaling molecules, such as connective tissue growth factor, bone morphogenetic proteins and Indian hedgehog, and direct the mineralization of the matrix, recruit blood vessels to the central part of the bone, and, upon apoptosis, allow osteoblasts (bone-forming cells) to invade the matrix to form the primary ossification center3,4. The mineralized matrix facilitates the penetration of blood vessels through which osteoblasts migrate to replace this degraded cartilage with a bone matrix5. Most osteoblasts invade the cartilage matrix from the perichondrium, a fibrous layer that wraps the cartilage6. Alternatively, a proportion of hypertrophic chondrocytes are able to survive and transdifferentiate to osteoblasts7,8,9. The final length of the bone is due to the accumulated growth of the transient cartilage, whose growth rate in turn depends on the number and size of the hypertrophic chondrocytes, and their matrix production10. Additionally, it was recently shown that the duration of the last hypertrophy phase correlates with the final length of the bone11. Therefore, tight regulation of the proliferation and differentiation of these cells is required to ensure proper bone size.
Despite of the substantial knowledge acquired over the years on the organization and development of the growth plates, most of these conclusions are based on the observation of fixed histological sections. Tissue sectioning provides valuable information about this process, but can be ridden with technical artifacts, so it cannot be always reliably used to estimate morphological or size changes between different stages. Additionally, as bone growth is a dynamic process, the static two-dimensional (2D) images offer a limited insight into the movement of the cells in the growth plate, while time-lapse imaging on live tissue could offer valuable information on the behavior of the chondrocytes in the growth plate.
All these limitations can be potentially resolved using ex vivo bone cultures. While bone culture protocols have been developed some time ago, they were limitedly applied to murine long bones. Most of the studies use chick bones due to the technical advantages offered by the chick model12,13. Organotypic cultures (air/liquid interface) were applied to chick embryonic femurs, which were maintained in culture for 10 days14. The sophisticated genetic tools available in mouse make this model very appealing to be used in ex vivo bone culture. The studies that used mice to look into bone growth worked mostly with metatarsal bones15, probably due to their small size and greater numbers obtained per embryo16. Although traditionally considered long bones, metatarsi enter senescence (characterized by reduced proliferation and involution of the growth plate17) earlier than other long bones in vivo, and therefore their continuous growth ex vivo does not really recapitulate the in vivo process. For the purposes of this article, we will use the term long bones for bones from the proximal and intermediate limb regions. Several previous studies used long murine bones, such as tibia, in ex vivo cultures and observed a substantial growth of the cartilage but little ossification18. We also used tibial cultures recently, mainly to study chondrocyte dynamics19. Other studies used femoral heads from young mice20 or only the distal part of the femur for culture21. Some more recent works successfully combine the ex vivo culture of full bones with time-lapse imaging to acquire three-dimensional (3D) movies of chondrocytes in living mouse tissue22,23. The authors managed to observe previously unnoticed events in the rearrangement of chondrocytes to the proliferative zone23 in a good example of the potential application of bone ex vivo culture. The alternative, i.e., analyzing static images, requires indirect and complex techniques. This was exemplified by a recent study assessing the importance of transversally-oriented clones for cartilage growth, where genetic tracing with multicolor reporter mouse strains coupled with mathematical modeling were used24. Therefore, ex vivo culture might help gain insight into dynamic processes in a faster and more straightforward way.
Here, we present a method for murine long bones culture, which can be combined with different molecular treatments and/or with time-lapse live imaging. This protocol adapts the methods used in previous reports15,18,25, but addresses some additional issues and focuses on long bones such as the tibia, rather than metatarsal bones. Finally, it explores the potential of using statistically powerful paired comparisons by culturing left and right bones separately in the presence of different substances.
Protocol
All the experiments should be carried out following the local governmental and institutional guidelines of ethical handling of laboratory animals.
1. Preparations Prior to the Day of Bone Culture
- Set up timed mouse matings to obtain fetuses and pups from embryonic day 14.5 (E14.5) and onward.
NOTE: The culture of long bones can be successfully applied to different mouse strains; in the present protocol, outbred Swiss Webster wild-type mice are used. - Prepare dissection medium (adapted from Houston et al.15): dilute α-minimum essential medium (α-MEM) or Dulbecco’s modified Eagle’s medium (DMEM) 1/13 in phosphate-buffered saline (PBS) and 2 mg/mL bovine serum albumin (BSA) and filter sterilize through a 0.22 µm, 33 mm diameter syringe filter. Store aliquots at -20 °C.
- The day before extraction of the fetuses, prepare the serum-free bone culture medium composed of DMEM containing 0.2% BSA, 0.5 mM L-glutamine, 40 U/mL penicillin/streptomycin, 0.05 mg/mL ascorbic acid, and 1 mM betaglycerophosphate. Filter sterilize through a 0.22 µm, 33 mm diameter syringe filter and store at 4 °C for up to 1 month.
- On the day of the culture and prior to mouse culling, prepare 24-well plates and 60 mm dishes with dissection medium and keep them ice cold. Prewarm the bone culture medium in a 37 °C water bath. Spray 80% (v/v) ethanol on the tools (tweezers and small scissors) to be used for fetus handling. Transfer a binocular scope to a Class II biosafety cabinet.
2. Culture of Fetus and Newborn Tibia and Femur
- Cull the pregnant mouse through cervical dislocation at the desired gestational stage (ranging from E14.5 to E18.5). If newborn pups are used, remove them from the mother one by one and cull by decapitation.
- Place the mouse on her back and sterilize the abdominal region by spraying 80% (v/v) ethanol on its surface.
- Cut the skin and the abdominal muscle with small scissors to access the uterine horns.
- Extract the uterus from the abdominal cavity with the help of tweezers and small scissors, removing the mesometrium and cutting the base of the horns. Place the uterus in a 60 mm Petri dish with ice-cold dissection medium and keep the culture dish on ice during the whole procedure.
- Transfer the Petri dish with the uterus to the biosafety cabinet and work there from now on.
- Separate individual fetuses with scissors by cutting between the sacs.
- Transfer individual sac under a dissection stereomicroscope in a clean 60 mm dish with dissection medium and open them up with tweezers to separate the fetuses from the placentas and clean them from membranes.
NOTE: Work with one embryo at a time, while keeping the rest on ice. - Decapitate the fetuses and transfer the body to a clean new 60 mm dish with a 1 mL cut sterile pipette.
- Remove the skin of the fetuses or pups with tweezers starting from the back and peeling it out till the toes.
- Separate the hindlimbs from the body by cutting with the tweezers close to the spine and transfer them to a clean dish with ice-cold dissection medium.
- Separate the tibia from the femur with tweezers by carefully introducing them between the surface cartilage of distal femur and proximal tibia.
- Remove the hip bones from the proximal femur and the calcaneus bone and the fibula from the tibia.
- Carefully remove the soft tissue from the femur and the tibia by nipping and pulling it off.
NOTE: It is important to remove as much soft tissue as possible, taking special care in removing the tissue that connects the two cartilage poles, but avoiding damaging the cartilage, the perichondrium, and the bones. - Place the four bones (left and right tibias and femurs) in the first well of the 24-well plate with a plastic 1 mL sterile pipette. Alternatively, to compare the effect of different treatments on left and right limbs, place contralateral limbs in different wells.
NOTE: Extra care should be taken when the bones are transferred to the wells, as they can easily stick to the pipette. - Proceed the same way with as many fetuses as necessary.
NOTE: Change the dish with the dissection medium as soon as it gets too clouded, as it is important to see clearly the dissected bones. - When all the desired bones are transferred to the wells, remove the dissection medium with a plastic 1 mL sterile pipette and take extra care not to aspirate the bones.
- Depending on the purpose of experiment, pictures can be taken of the bones before removing the dissection medium, as timepoint zero of the experiment. Take pictures with a microscope attached to a digital camera and annotate the exposure and scale used. To ensure easy and reliable measurements, take pictures with good contrast to distinguish the mineralized part.
- Add 1 mL of culture medium to each well. If any treatment is intended on the bones (doxycycline, tamoxifen, growth factors, etc.), it should be added now.
- To observe the effect of growth inhibition in the culture conditions, treat the left tibias with retinoic acid (RA, 500 nM), while incubating the right tibias with an equivalent volume of vehicle (dimethyl sulfoxide [DMSO], final concentration 0.1%) as a control.
- Leave the bones to grow for two days or more in a cell culture incubator under standard cell culture conditions (at 37 °C in a 5% CO2 incubator).
- To assess proliferation, add 5-ethynyl-2’-deoxyuridine (EdU) or 5-bromo-2’-deoxyuridine (BrdU) to the medium at a final concentration of 10 µM 1−2 h before fixation.
NOTE: The stock concentration of EdU is 20 mM.
CAUTION: EdU and BrdU are thymidine analogues and can be toxic and mutagenic. - Thaw 4% paraformaldehyde (PFA) and fix the bones by immersion in PFA in individual 2 mL tubes.
CAUTION: PFA is toxic and designated as a probable human carcinogen. Avoid breathing paraformaldehyde powder and vapors. EdU and BrdU are thymidine analogues and can be toxic and mutagenic. - After a brief 10 min fixation in PFA at room temperature, transfer bones to PBS for picture acquisition at final timepoint. Then place bones back into PFA for overnight fixing at 4 °C.
- After fixation bones can be processed for desired downstream applications.
3. Measurement and Analysis of the Full Length of the Bone and of the Mineralized Region
- Use an image editing software to measure the length of the bones, taking into account the scale of the image. Measure both total length of the bone and the mineralized region. Start the measurements from the first dark cells at one end until the last ones at the other end.
NOTE: The mineralized region is characterized by the darker color and is easily distinguished from the cartilage. - To calculate the growth rate, defined as the average increase in length per day, divide the difference between the final length of the bone and the initial one by the number of days in culture.
Representative Results
Bone culture can be performed starting from different stages. In Figure 1A-D, a comparison between cultured tibia and freshly extracted ones at equivalent stages is shown. The first observation is that up to two days of culture the size achieved is comparable to the in vivo bone growth for both cartilage and mineralized bone (Figure 1A,B,D). Longer culture periods lead to bigger differences between the cultured bones and the freshly extracted (Figure 1C). Additionally, as mentioned in the protocol, it is crucial to remove the soft tissue connecting both ends of the bones, as otherwise the bones will bend. Figure 1E shows an example of a tibia grown with incomplete removal of soft tissue versus a tibia without soft tissue.
Next, tibias were cultured for 2 days and their length was measured. As can be seen in Figure 2A,B, the measurement of the total length and of the mineralized part can be performed with an image analysis software. As shown by De Luca et al.26, treatment with RA has a strong effect on the growth of the tibias already after 2 days of treatment and a similar result was observed in our cultures (Figure 2B-D). Importantly, the experiment was performed using paired bones, with the right bone as control and the left treated with RA (Figure 2A,B,E), which helps overcome the natural variability in bone size between different specimens. Thus, the culturing method described is suitable for assessing the effect of different compounds on bone growth.
Next, the growth rate of the bones after culture was assessed. Bones were extracted, measured and cultured at E15.5, 16.5 or P1, and fixed and measured again two days later. Both the total increase in length and the length of the mineralized part were measured (Figure 3C). An example of E15.5 tibia and femur before culture (Figure 3A) and at the end point of the experiment (Figure 3B) are shown. As can be observed from the graph and the table (Figure 3C,D), there is a consistent increase in the total length of the tibia, corresponding to an approximate increase of 9−29% from the initial length. This is less increase than the one observed in vivo; the main difference is likely due to the level of the proximal cartilage, bigger than the distal and less accessible to nutrients. Indeed, EdU labeling showed fewer positive cells in this region after culture compared to freshly extracted bones of equivalent stage (Figure 4A,C). The EdU incorporation in the distal tibia was similar in the cultured and freshly extracted bones (Figure 4B,D) The distal cartilage contributes approximately one third to the total growth of the tibia in vivo at this stage27, comparable to the growth rate observed in culture, so we propose that the analysis should be focused on this part of the bone. Additionally, we assessed the mineralization of the cultured bones, and observe almost no increase in length of this region. The difference might be due to the absence of vessel and osteoblasts invasion in the ex vivo culture. This suggests that studies of cartilage growth can extend for several days, while if the region of interest is the ossified part of the bone, the period of culture should be shorter. This observation was confirmed by keeping tibia in culture for a longer period (up to 6 days). As can be seen in Figure 5, the total length of the skeletal element increases substantially, while almost no increase in the mineralized region is observed (Figure 5C).
Overall, these results suggest that the culture of long bones can be used to analyze the effect of different factors on overall bone growth and particularly to assess cartilage dynamics. While well-established metatarsal cultures can also be used with these purposes, we submit that both types of cultures complement each other, given the intrinsic differences between metatarsals and the rest of long bones.
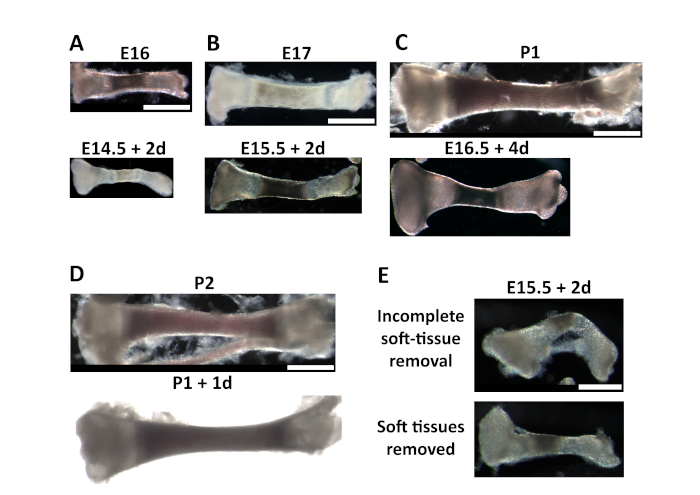
Figure 1: Example of cultured tibia for different time periods. (A-D) Comparison between freshly extracted tibia (top) and tibia extracted at E14.5 (bottom) and cultured for 2 days (A), extracted at E15.5 and cultured for 2 days (B), extracted at E16.5 and cultured for 4 days (C) and freshly extracted at postnatal day 2 (P2) and extracted at postnatal day 1 (P1) and cultured for 1 day (D). Note that, while cartilage growth remains quite physiological after different culturing periods, the ossified part shows a growth delay after culture time longer than 2 days compared to the freshly extracted bone at the corresponding stage. Scale bar = 1 mm. (E) Tibia cultured for 2 days starting at E15.5; note that the incomplete removal of the soft tissue between the two ends of the bones leads to the bending of the bone. Scale bar = 600 µm. Please click here to view a larger version of this figure.
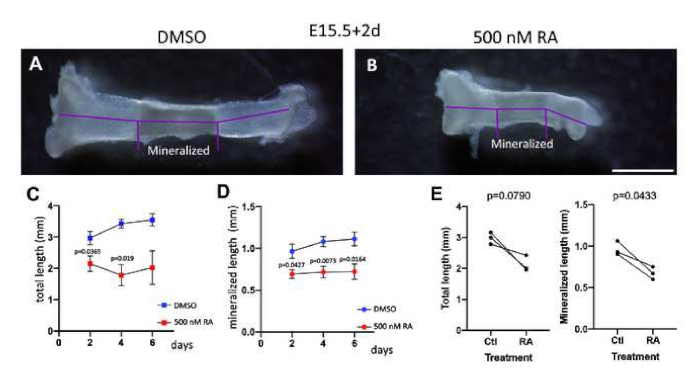
Figure 2: Measurement of the length of the bones upon retinoic acid (RA) treatment. (A-B) Tibias extracted at E15.5 and grown for 2 days with 0.1% DMSO (A) and RA (B). Note the difference in both total length and of the mineralized part. Scale bar = 1 mm. (C-D) Changes in total length (C) or the mineralized part (D) of the tibia over a period of 6 days in control situation and in presence of RA. Results are shown as mean ± standard deviation (SD); comparison is done by two-way analysis of variance (ANOVA) with the type of treatment as variable (p values are shown in the graph). (E) Comparison of the length of paired bones cultured for 2 days with either DMSO (right tibia) or RA (left tibia); each dot represents one of the 3 biological replicates per condition. Paired two-tailed Student’s t-test was used. Please click here to view a larger version of this figure.
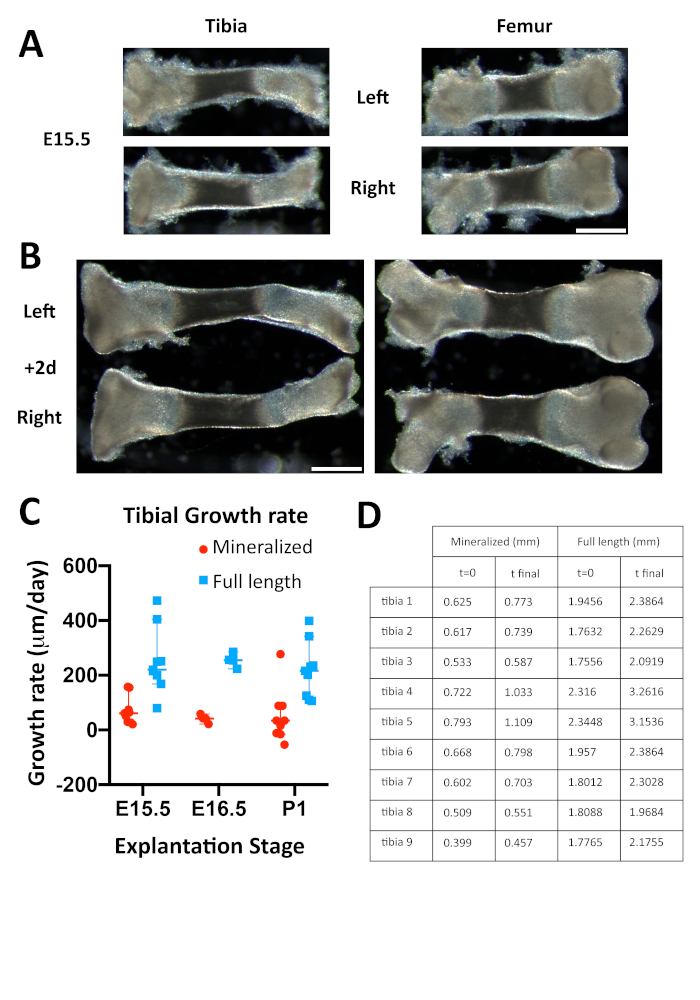
Figure 3: Comparison of total growth and mineralization of the tibia after 48 hours of ex vivo culture. (A) E15.5 tibias and femurs from left (top) and right (bottom) limbs prior to culturing. (B) The same tibias and femurs followed up after 2-day culture. Scale bar = 600 µm. (C) Graph representing the growth rate of tibias cultured at different developmental stages. Both the total growth and the growth of the mineralized part were assessed. (D) Table showing the initial and final length of the whole bone and the mineralized part before culturing at E15.5 and after 2 days in culture. Please click here to view a larger version of this figure.
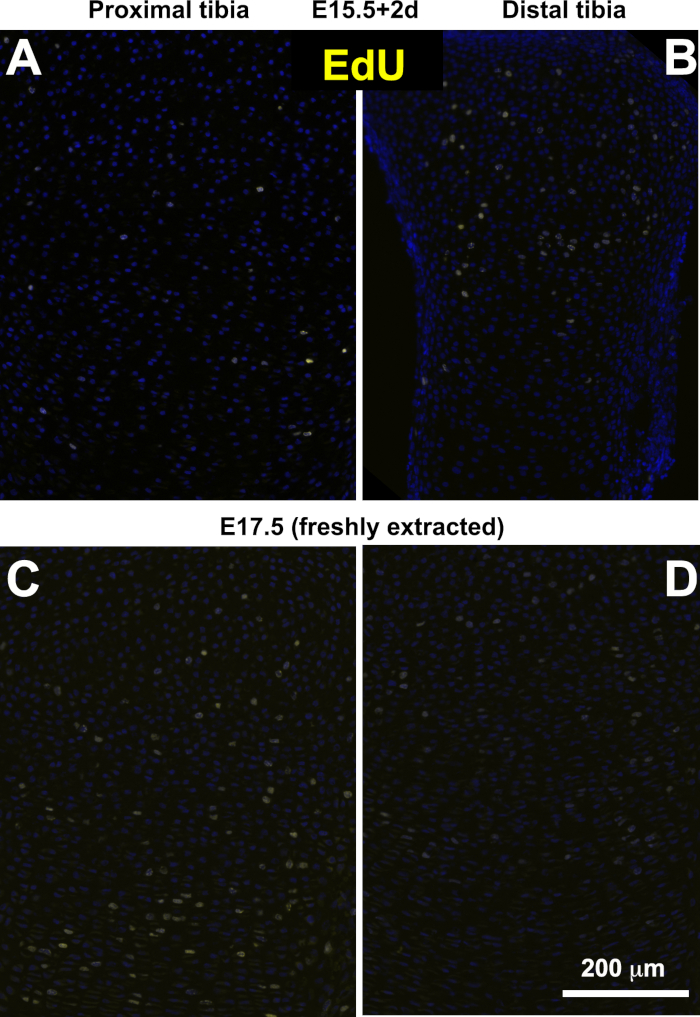
Figure 4: Bulky growth plates do not show much proliferation in culture. (A-B) EdU staining for proximal (A) and distal (B) tibial growth plates cultured from E15.5 for 2 days. (C-D) EdU staining for proximal (C) and distal (D) tibial growth plates freshly extracted at E17.5. Note the difference in the number of EdU(+) cells in the proximal tibia. Data include 6 cultured pairs of limbs and 3 freshly extracted. Please click here to view a larger version of this figure.
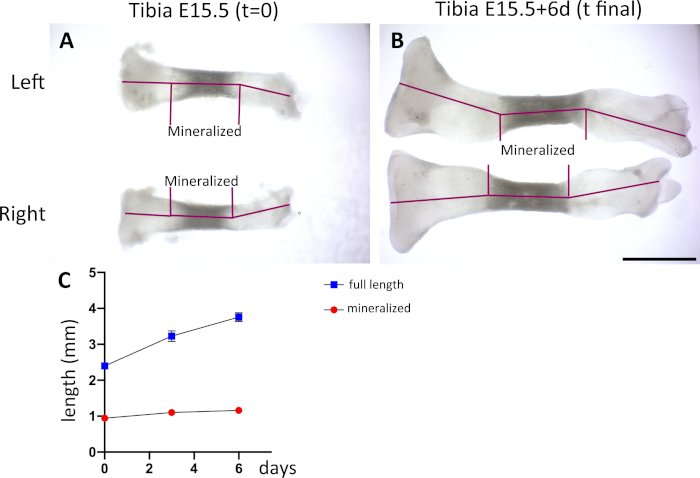
Figure 5: Longer periods of tibia culture show substantial cartilage growth but little mineralization. Freshly extracted tibia at E15.5 (t = 0) (A) and after 6 days in culture (B). Note the differences between cartilage growth and the growth of the mineralized part. Scale bar = 1 mm (C) Graph showing the changes in the total length and the mineralized region over a period of 6 days. n = 5 cultured tibias; SD at each time point is as follows: t = 0, full length = 0.0777, mineralized = 0.0213; t = 3 d, full length = 0.1495, mineralized = 0.056; t = 6 d, full length = 0.1193, mineralized = 0.0521. Please click here to view a larger version of this figure.
Discussion
Bone ex vivo culture methods have been used for some time to assess the biology of bone growth28, but have been seldom applied to murine long bones. With the development of imaging techniques, ex vivo bone culture offers an attractive way to study bone growth in real time in a setting closely resembling the in vivo conditions. In this scenario, it is important to define the conditions in which the growth of long bones is comparable to their growth in vivo.
In the present study, we describe a simple and affordable protocol for long bone culture, addressing the limitations and possible applications. The most critical step of this method is the isolation of the bones, which have to remain intact and with as less soft tissue between the ends of the bone as possible. Leaving soft tissue between the ends of the bones will prevent normal growth of the bone and induce bending, as is exemplified in Figure 1E. Soft tissue at the ends of the bones can be left, as it will eventually disappear. Another step that requires extra care is the transfer of the bones to the culturing plate, as they can easily stick to the plastic pipette. One possible solution is pipetting up and down dissection medium containing blood and tissue pieces, as it creates a coating that helps to preclude the bones from sticking to the pipette.
Once extracted, bones reach comparable size to the corresponding in vivo stage when cultured for up to 48 h. Apart from measuring the length of the bone, the growth rate (average increase in length per day) can be estimated easily in these conditions. However, this approach for growth rate calculation is not valid over longer culturing periods, where other methods, such as calcein labeling29, should be used. Treatment with retinoic acid, which promotes premature chondrocyte differentiation, leads to a substantial reduction in the growth of the bones, suggesting that this time for culturing is enough to observe the effect that different substances might have on bone growth. Importantly, the comparison was also done on paired bones from the same specimen, so that the left tibia received the RA treatment and the right was the control. This is an advantage of the bone culture model, as it allows performing paired comparisons, which are statistically more powerful.
Culturing for longer time shows significant growth of the cartilage part, but a delay in the growth of the mineralized one, and it is important to consider this result when choosing the application of the technique. Similar results were observed in chick femurs cultured for up to 10 days which show an enlarged epiphyseal region and a reduced diaphyseal bone collar14, as well as in mouse tibia cultured for 6 days18. Additionally, it is important to mention that in the cartilage region the growth is also not homogenous: the bulky distal region of the femur and proximal region of the tibia do not receive nutrients efficiently by simple diffusion and cannot grow properly. In this context, the studies should focus on the opposite growing plates, which were estimated to contribute to one-third of total growth27, similar to the observed growth in culture. The delay in growth of cultured bones was also described for metatarsal cultures15.
An important consideration of this type of culture is the absence of growth of the ossified part of the bone. It is well established that the osteoblasts invade the cartilage matrix from the periosteum together with blood vessels3,4 and this process is obviously disrupted when bone is isolated. This might explain the absence of ossification under culture conditions. Hypertrophic chondrocyte transdifferentiation was also shown as an important source of osteoblasts7,8,9, but whether this process also requires in part the presence of blood vessels, as it seems to do during fracture healing30, or some other tissues not present in ex vivo cultures, needs further investigation. Additionally, it is well described the importance of the mechanical load in shaping bone growth31,32, which influences long bones and metatarsal bones differently, but is absent in an ex vivo culturing setup. Nevertheless, the described culturing method is suitable for measuring chondrocyte dynamics and changes in longitudinal growth and, thus, can be used for certain applications.
Overall, the described protocol provides a simple and cheap method to culture long bones starting from different stages, which can be coupled with additional techniques to address key cellular and molecular mechanisms, such as live time-lapse imaging13,23 or drug treatment.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
We would like to thank Alexandra Joyner for her support when this protocol was being established, Edwina McGlinn and Yi-cheng Chang for sharing retinoic acid. The Australian Regenerative Medicine Institute is supported by grants from the State Government of Victoria and the Australian Government.
Materials
| 5-Ethynyl-2'-deoxyuridine | Santa Cruz | CAS 61135-33-9 | |
| 5-Bromo-2′-deoxyuridine | Sigma | B5002 | |
| 50mL Conical Centrifuge Tubes | Falcon | 352070 | |
| 60 mm TC-treated Center Well Organ Culture Dish, 20/Pack, 500/Case, Sterile | Falcon | 353037 | |
| Adobe Photoshop | Adobe | CS4 | |
| Ascorbic acid | Sigma | A92902 | |
| Base unit for the scope | Zeiss | 435425-9100-000 | |
| Betaglycerophosphate | Sigma | G9422 | |
| Binocular scope | Zeiss | STEMI-2000 | |
| Bovine Serum Albumin (BSA) fraction v | Roche/Sigma | 10735086001 | |
| DigiRetina 500 camera | Aunet | ||
| Dissection kit | Cumper Robbins | PFS00034 | |
| DMEM | Gibco | 11960044 | |
| DMSO | Sigma | D8418 | |
| Eppendorf 2-mL tubes | Eppendorf | 0030120094 | |
| Ethanol 96% | Merk | 159010 | |
| Forceps Dumont#5 Inox08 | Fine Science Tools | T05811 | |
| Heracell 150 CO2 incubator | Thermo Fisher | 51026282 | |
| Minimum Essential Medium Eagle | Sigma | M2279 | |
| Multiwell 24 well | Falcon | 353047 | |
| Paraformaldehyde | Sigma | 158127 | |
| Penicillin-Streptomycin (10,000 U/mL) | Gibco | 15140-122 | |
| Plastic pipettes 1mL Sterile Individually wrapped | Thermo | 273 | |
| Syringe filter 0.2 um | Life Sciences | PN4612 | |
| Terumo syringe 20 mL | Terumo | DVR-5174 | |
| Tretinoin (retinoic acid) | Sigma | PHR1187-3X | |
| Trinocular scope | Aunet | AZS400T |
Referenzen
- Long, F., Ornitz, D. M. Development of the endochondral skeleton. Cold Spring Harbor Perspectives in Biology. 5 (1), a008334 (2013).
- Mackie, E. J., Ahmed, Y. A., Tatarczuch, L., Chen, K. S., Mirams, M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. The International Journal of Biochemistry & Cell Biology. 40 (1), 46-62 (2008).
- Goldring, M. B., Tsuchimochi, K., Ijiri, K. The control of chondrogenesis. Journal of Cellular Biochemistry. 97 (1), 33-44 (2006).
- Kronenberg, H. M. Developmental regulation of the growth plate. Nature. 423 (6937), 332-336 (2003).
- Mackie, E. J., Tatarczuch, L., Mirams, M. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. The Journal of Endocrinology. 211 (2), 109-121 (2011).
- Maes, C., et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Developmental Cell. 19 (2), 329-344 (2010).
- Park, J., et al. Dual pathways to endochondral osteoblasts: a novel chondrocyte-derived osteoprogenitor cell identified in hypertrophic cartilage. Biology Open. 4 (5), 608-621 (2015).
- Yang, L., Tsang, K. Y., Tang, H. C., Chan, D., Cheah, K. S. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proceedings of the National Academy of Sciences of the United States of America. 111 (33), 12097-12102 (2014).
- Zhou, X., et al. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genetics. 10 (12), e1004820 (2014).
- Wilsman, N. J., Bernardini, E. S., Leiferman, E., Noonan, K., Farnum, C. E. Age and pattern of the onset of differential growth among growth plates in rats. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society. 26 (11), 1457-1465 (2008).
- Cooper, K. L., et al. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature. 495 (7441), 375-378 (2013).
- Smith, E. L., Rashidi, H., Kanczler, J. M., Shakesheff, K. M., Oreffo, R. O. The effects of 1alpha, 25-dihydroxyvitamin D3 and transforming growth factor-beta3 on bone development in an ex vivo organotypic culture system of embryonic chick femora. PloS One. 10 (4), e0121653 (2015).
- Li, Y., Li, A., Junge, J., Bronner, M. Planar cell polarity signaling coordinates oriented cell division and cell rearrangement in clonally expanding growth plate cartilage. eLife. 6, (2017).
- Kanczler, J. M., Smith, E. L., Roberts, C. A., Oreffo, R. O. A novel approach for studying the temporal modulation of embryonic skeletal development using organotypic bone cultures and microcomputed tomography. Tissue Engineering. Part C, Methods. 18 (10), 747-760 (2012).
- Houston, D. A., Staines, K. A., MacRae, V. E., Farquharson, C. Culture of Murine Embryonic Metatarsals: A Physiological Model of Endochondral Ossification. Journal of Visualized Experiments. (118), (2016).
- Abubakar, A. A., Noordin, M. M., Azmi, T. I., Kaka, U., Loqman, M. Y. The use of rats and mice as animal models in ex vivo bone growth and development studies. Bone & Joint Research. 5 (12), 610-618 (2016).
- Lui, J. C., et al. Differential aging of growth plate cartilage underlies differences in bone length and thus helps determine skeletal proportions. PLoS Biology. 16 (7), e2005263 (2018).
- Agoston, H., et al. C-type natriuretic peptide regulates endochondral bone growth through p38 MAP kinase-dependent and -independent pathways. BMC Developmental Biology. 7, 18 (2007).
- Rosello-Diez, A., Madisen, L., Bastide, S., Zeng, H., Joyner, A. L. Cell-nonautonomous local and systemic responses to cell arrest enable long-bone catch-up growth in developing mice. PLoS Biology. 16 (6), e2005086 (2018).
- Marino, S., Staines, K. A., Brown, G., Howard-Jones, R. A., Adamczyk, M. Models of ex vivo explant cultures: applications in bone research. BoneKEy Reports. 5, 818 (2016).
- Okubo, N., et al. Prolonged bioluminescence monitoring in mouse ex vivo bone culture revealed persistent circadian rhythms in articular cartilages and growth plates. PloS One. 8 (11), e78306 (2013).
- Hirota, K., et al. Live imaging analysis of the growth plate in a murine long bone explanted culture system. Scientific Reports. 8 (1), 10332 (2018).
- Romereim, S. M., Conoan, N. H., Chen, B., Dudley, A. T. A dynamic cell adhesion surface regulates tissue architecture in growth plate cartilage. Development (Cambridge, England). 141 (10), 2085-2095 (2014).
- Kaucka, M., et al. Oriented clonal cell dynamics enables accurate growth and shaping of vertebrate cartilage. eLife. 6, (2017).
- Alvarez, J., et al. TGFbeta2 mediates the effects of hedgehog on hypertrophic differentiation and PTHrP expression. Development (Cambridge, England). 129 (8), 1913-1924 (2002).
- De Luca, F., et al. Retinoic acid is a potent regulator of growth plate chondrogenesis. Endocrinology. 141 (1), 346-353 (2000).
- Stern, T., et al. Isometric Scaling in Developing Long Bones Is Achieved by an Optimal Epiphyseal Growth Balance. PLoS Biology. 13 (8), e1002212 (2015).
- Minkin, C., et al. Skeletal development and formation of osteoclast-like cells from in situ progenitors in fetal mouse metatarsals cultured in chemically defined medium. Bone and Mineral. 12 (3), 141-155 (1991).
- Erben, R. G. . Handbook of histology methods for bone and cartilage. , 99-117 (2003).
- Hu, D. P., et al. Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes. Development (Cambridge, England). 144 (2), 221-234 (2017).
- Fritton, S. P., McLeod, K. J., Rubin, C. T. Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. Journal of Biomechanics. 33 (3), 317-325 (2000).
- Duncan, R. L., Turner, C. H. Mechanotransduction and the functional response of bone to mechanical strain. Calcified Tissue International. 57 (5), 344-358 (1995).

