Measuring Diaphragm Thickness and Function Using Point-of-Care Ultrasound
Summary
Diaphragm thickness and function can be assessed in healthy individuals and critically ill patients using point-of-care ultrasound. This technique offers an accurate, reproducible, feasible, and well-tolerated method for evaluating diaphragm structure and function.
Abstract
The diaphragm is the main component of the respiratory muscle pump. Diaphragm dysfunction can cause dyspnea and exercise intolerance, and predisposes affected individuals to respiratory failure. In mechanically ventilated patients, the diaphragm is susceptible to atrophy and dysfunction through disuse and other mechanisms. This contributes to failure to wean and poor long-term clinical outcomes. Point-of-care ultrasound provides a valid and reproducible method for evaluating diaphragm thickness and contractile activity (thickening fraction during inspiration) that can be readily employed by clinicians and researchers alike. This article presents best practices for measuring diaphragm thickness and quantifying diaphragm thickening during tidal breathing or maximal inspiration. Once mastered, this technique can be used to diagnose and prognosticate diaphragm dysfunction, and guide and monitor response to treatment over time in both healthy individuals and acute or chronically ill patients.
Introduction
Ultrasound refers to sound waves beyond the upper audible limits of human hearing. Ultrasound has many applications beyond healthcare, the most famous likely being the development of SONAR (sound navigation and ranging) for military use in World War I1; ultrasound is now routinely used in medical diagnosis and therapy. Medical sonography or diagnostic ultrasound utilizes high frequency sound waves (>20 kHz) to provide images of soft tissue structures within the body. These sound waves are pulsed at frequencies of 1 to 20 million cycles/s (megahertz, MHz), which can be transmitted into the body to examine anatomical structures, such as the liver, heart, and skeletal muscle. Point-of-care ultrasound is increasingly becoming a cornerstone of the evaluation and management of critical illness.
The first application of ultrasound in medicine was in the 1940's by Dr. Karl Dussik, who attempted to locate brain tumors by measuring the transmission of ultrasound beams through the head2. As technology progressed, new techniques were developed, including amplitude mode (A-mode) and brightness mode (B-mode)3, followed by the development of two-dimensional scanners in 19604,5. The field of diagnostic ultrasound has become invaluable in clinical practice, since it avoids exposure to ionizing radiation and can be obtained at the bedside, avoiding the need for in-hospital transport with associated risks. Ultrasound is safe, well-tolerated, reliable, and repeatable in patients6,7.
The diaphragm is a thin, dome-shaped muscular structure that acts as the main respiratory pump driving spontaneous ventilation in humans. The diaphragm separates the thoracic and abdominal cavities and is composed of three separate segments: the central tendon, the costal diaphragm, and the crural diaphragm (Figure 1). The central tendon of the diaphragm is a noncontractile structure that allows major bloods vessels to pass through from the thoracic to the abdominal cavity. The costal diaphragm has fibers running from the rib cage or xiphoid process to the central tendon. The crural diaphragm inserts into the first three lumbar vertebrate. During inspiration, the costal diaphragm contracts, lowering the dome of the diaphragm while expanding the lower rib cage. The costal diaphragm supports the crural diaphragm in the lowering the dome8,9,10.
Transthoracic ultrasound of the diaphragm has gained increasing attention for its ability to monitor diaphragm thickness at the zone of apposition (Figure 1)11,12,13. The diaphragm was first visualized with ultrasound in 1975 by Haber et al.14. Diaphragm contractility and muscular shortening during inspiration can be quantified using M-mode ultrasound to monitor the diaphragm thickness (Tdi) and thickening fraction (TFdi). This assessment of contractility provides a measure of diaphragm muscular performance under a given level of inspiratory drive and effort. Point-of-care ultrasound provides safe, repeatable, and reliable measures of diaphragm function and architecture. In mechanically ventilated patients, changes in diaphragm thickness over time can be used to evaluate the negative impacts of mechanical ventilation, including the effects of myotrauma due to over-assistance (atrophy; decreasing end-expiratory thickness over time) or under-assistance (load-induced injury resulting in inflammation, edema; possibly represented by increasing end-expiratory thickness over time)15. These changes are correlated with adverse clinical outcomes16. Measuring TFdi during tidal breathing permits an assessment of tidal diaphragmatic activity (i.e., inspiratory effort). Measuring TFdi during a maximal inspiratory effort (TFdi,max) provides an assessment of diaphragm strength (since the diaphragm's force-generating capacity is related to its ability to contract and shorten).
There is substantial consensus on the optimal protocol for acquiring and analyzing measurements17. Competency in diaphragm ultrasound imaging involves a moderately steep learning curve; thorough training in the technique and its potential pitfalls is essential. Studies have shown that proficiency in diaphragm ultrasound expertise can be acquired in a short period of time through remote, web-based training18. Therefore, this protocol has been optimized to provide a consistent measurement of diaphragm thickness and thickening fraction that can be applied to both healthy and patients with suspected respiratory pathology19
Protocol
Studies employing this technique have received ethical approval from the Research Ethics Board at the University Health Network, Toronto, Canada.
1. Evaluating diaphragm thickness and thickening fraction during tidal breathing
- Identifying the diaphragm
- Place the patient in a semi-recumbent position (30°-45° from parallel) on their back. Remove any article of clothing from the right side of the chest.
NOTE: A similar procedure may be used to visualize the left hemidiaphragm; the left side is generally more difficult to visualize, and measurement precision is reported to be much lower19. - Power on the tablet powering the portable ultrasound unit and initiate the appropriate application (see Table of Materials). Initiate a musculoskeletal exam with a high-frequency linear array transducer (minimum of 12 MHz).
NOTE: Any ultrasound system may be used to perform this technique. - Cover the tip of the linear array transducer with a sufficient amount of ultrasound gel and ensure the ultrasound is in B-mode for positioning. Hold the probe by enclosing the tip of the probe with the thumb and index finger (Figure 2A).
- Palpate the chest wall surface to locate the right eighth, ninth, or 10th intercostal spaces between the mid- and anterior axillary lines, as shown in Figure 1C and Figure 2A, and place the probe in the zone of apposition (typically around the eighth intercostal space).
- Angle the transducer in the sagittal plane so that it is situated entirely between the ribs (Figure 2A) and no rib artifacts are visible in the image (Figure 2B). If a rib appears in the image, adjust the angle of the probe by tilting up or down. If a rib is still visible, rotate the probe until only the diaphragm is visible. If visualization of the diaphragm continues to be problematic, slide the probe up or down to a new intercostal space.
- On the ultrasound monitor, identify two bright white parallel lines immediately superior to the liver, indicating the pleural and peritoneal membranes (Figure 2B). The relatively hypoechoic costal diaphragm can be visualized between these lines.
- Adjust the depth of the image by clicking on the increase or decrease depth button to optimize the size of the diaphragm. Ensure the diaphragm is centered on the display monitor. This will ensure maximum resolution of the pleural and peritoneal lines from surrounding structures.
- If the image remains suboptimal (i.e., the lungs or the ribs are visible in the image or the pleural and peritoneal membranes are not clearly visualized), adjust the probe for better visualization by shifting the probe up and down along the rib space, back and forth from the base, or rotate. See Table 1 for examples of common issues in transdiaphragmatic ultrasonography.
- Place the patient in a semi-recumbent position (30°-45° from parallel) on their back. Remove any article of clothing from the right side of the chest.
- Optimizing images
- Once the transducer is in the correct location, optimize the image quality by altering the following components prior to data collection.
NOTE: On different ultrasound unit software, there are model and software differences. In this software, we have performed the following button clicks to achieve the goal. - On the ultrasound unit software, click on the gain button to alter the brightness of the image. Increase the gain by clicking the increase button, to make the image appear brighter. Conversely, click the decrease button to darken the image. If the gain is too low, structures may be difficult to ascertain. If the gain is too high, extraneous echoes can appear and the image will appear too bright.
- If available on the ultrasound unit, click on the focus button to adjust the focus to alter the image quality. Click the increase button to increase the focus or the decrease button to lower the focus.
- Once the transducer is in the correct location, optimize the image quality by altering the following components prior to data collection.
- Acquiring images
- Once placement and image quality have been optimized, place the ultrasound in M-mode by clicking the M-mode button on the ultrasound software.
- A single vertical scan line will appear on the imaging screen. Place the line between the section where the pleural and peritoneal lines are clearest.
NOTE: There may be some variability between ultrasound devices in obtaining M-mode images. Ensure a clear area where well defined pleural and peritoneal membranes are visualized prior to the initiation of M-mode. Place the scan line in a location where pleural and peritoneal membranes are well defined throughout the respiratory cycle and no lungs or ribs enter the field of view. - Run M-mode over a full cycle of inspiration and expiration during tidal breathing and then click on the freeze and then save buttons to capture the actual state and save the image. If available, adjust the sweep speed by clicking on the sweep speed button to adjust the rate of collection to ensure two respiratory cycles are obtained. Repeat this process to obtain another image.
- With a skin safe marker, mark the location of the probe on the patient's body, to help ensure the exact same position of the diaphragm is measured over time. This is essential to maintain the reproducibility of the measure, as diaphragm thickness varies over its surface area19.
- From these images, the diaphragm thickness (Tdi) and thickening fraction (TFdi) can be measured. If the values from the second M-mode image are not within 10% of the first image, repeat the M-mode image acquisition until two images with a set of values within 10% of each other are obtained. See details on image analysis below.
- Once the exam is complete, click the end exam button on the ultrasound software.
- To export files, click on Export images and ensure the files are exported in DICOM format.
- Wipe the patient's side if there is any remaining gel and sanitize the ultrasound equipment with appropriate disinfectant wipes.
- Analyzing images
- Open the necessary DICOM files in MicroDicom DICOM viewer or similar software.
- Click on the "distance" tool (may be called calipers or straight line) and draw a straight line from the inner edge of the pleural membrane to the inner edge of the peritoneal membrane at end expiration (Tdi,ee).
- Ensure both membranes are not included in this measurement and that both ends of the straight line are placed directly across (vertically) from each other such that there is no time difference between the markers, which may artificially increase the distance, as per Figure 2B17.
- Record this value as diaphragm thickness (Tdi,ee).
- Repeat step 4.2 at peak inspiration of the same breath to obtain the diaphragm thickness at peak inspiration (Tdi,pi).
- If the patient does not appear to be breathing, and no diaphragm thickening fraction is evident during inspiration, measure the Tdi,pi at a location representative of diaphragm thickness during the inspiratory phase (in this case, it will be approximately the same as Tdi,ee), as seen in Figure 3.
- Both Tdi,ee and Tdi,pi should be analyzed from the same breath, as seen in Figure 2C, to assess the diaphragm thickening fraction during tidal breathing (TFdi).
- Using Tdi,pi and Tdi,ee, calculate the TFdi for each breath:
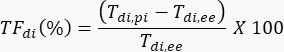
- Obtain a second pair of measurements from the same M-mode image (see Figure 2C).
- Repeat steps 1.4.1-1.4.9 on the second M-mode image. At this point, four measurements of Tdi,ee and four measurements of TFdi have been obtained.
- If the values from the second M-mode image are not within 10% of the first image, repeat the M-mode image acquisition until two images with a set of values within 10% of each other are obtained.
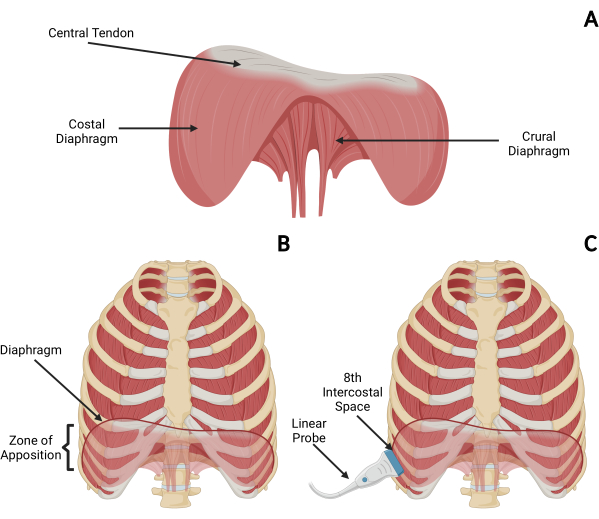
Figure 1: Overview of diaphragm anatomy and placement of ultrasound probe. (A) Anatomical structures for ultrasound of the costal diaphragm. The diaphragm consists of the central tendon, costal diaphragm, and crural diaphragm. (B,C) To visualize the the costal diaphragm at the zone of apposition on ultrasound, the patient is placed in the semirecumbent position and the eighth, ninth, or 10th intercostal space is located. A high frequency (>12 MHz) linear array ultrasound probe is placed parallel to the ribs in the intercostal space along the midaxillary line to visualize the costal diaphragm as a cross-section. Please click here to view a larger version of this figure.
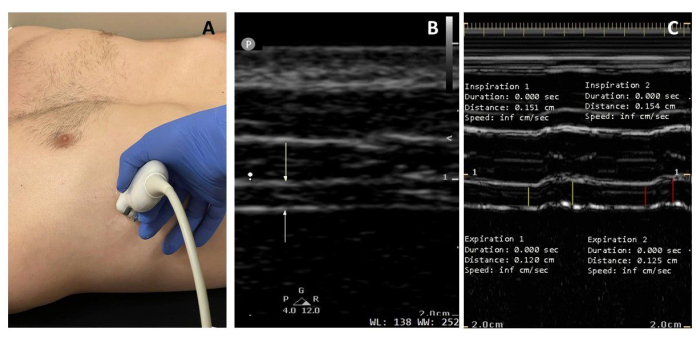
Figure 2: Ultrasound diaphragm thickness and thickening during tidal breathing. (A) The probe is placed at the eighth, ninth, or 10th intercostal space to visualize the diaphragm as a cross-section. (B) In the B-mode image, the white arrows demonstrate the hyperechoic pleural and peritoneal membranes. (C) The M-mode image projects variation in diaphragm thickness at a particular point over time. From left to right, the yellow lines measure diaphragm thickness at end expiration (Tdi,ee) and diaphragm thickness at peak inspiration (Tdi,pi) of the first breath, and red lines denote that of the second breath. Diaphragm thickness (Tdi,ee) measures 1.20 and 1.25 mm, and TFdi 26% and 23%, respectively, in a healthy male subject. Please click here to view a larger version of this figure.
Table 1: Common issues in transdiaphragmatic ultrasonography Please click here to download this Table.
2. Evaluating the maximal diaphragm thickening fraction
NOTE: The maximal diaphragm thickening fraction may be assessed during the same experimental session as diaphragm thickness.
- Acquiring images
- Utilizing the same methodology as described above, identify the diaphragm using the B-mode ultrasound and optimize accordingly.
- In mechanically ventilated patients, ensure that there is adequate respiratory drive for diaphragm functional assessment by measuring the airway occlusion pressure (P0.1) on the ventilator. The P0.1 should be at least 2 cm H2O to proceed. If it is less than 2 cm H2O, consider having sedation or ventilatory support reduced to increase respiratory drive prior to ultrasound imaging.
- Once the respiratory drive is adequate in mechanically ventilated patients, reduce the ventilatory support to a minimum level (e.g., pressure support ventilation (PSV): 0 cm H2O; positive end-expiratory pressure (PEEP): 0 cm H2O; modest levels of PSV or PEEP may be maintained if needed for gas exchange) to augment diaphragmatic contractility temporarily.
NOTE: The removal of ventilatory support increases respiratory drive and effort to facilitate the assessment of diaphragm function.
- Place the ultrasound into M-mode by clicking the M-mode button.
- While running M-mode, coach the participant to perform a maximal volitional inspiratory effort against a non-occluded airway (i.e., inspiratory capacity maneuver), instructing the participant to "take a big breath in" if able.
- If the patient is unable to follow commands to make maximal inspiratory efforts, apply a brief airway occlusion maneuver (the Marini maneuver)20 for up to 20 s to stimulate increased respiratory effort. Then, release the occlusion and measure the TFdi,max after releasing the occlusion.
- Freeze the recording and save the image.
- Repeat steps 2.1-2.4 twice more to obtain a total of three M-mode images for analysis, or until the sonographer is confident that the patient has made maximal volitional efforts.
- Export M-mode images in DICOM format for careful offline blinded analysis.
- Wipe the patient's side to clean any remaining gel and sanitize the ultrasound equipment with appropriate disinfectant wipes.
- Analyzing images
- Open the necessary DICOM files in MicroDicom DICOM viewer or similar software.
- Click on the distance tool (may be called calipers or straight-line) and draw a straight line from the from inner edge of the pleural membrane to the inner edge of the peritoneal membrane at end expiration (Tdi,ee) and peak inspiration (Tdi,pi) during a maximal inspiratory trial, as seen in Figure 3B.
- Ensure all measurements exclude the pleural and peritoneal membranes and both ends of the straight line are placed directly across (vertically) from each other, such that there is no time difference.
- TFdi,max for each breath is computed as:
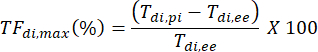
- Record the highest value of at least three consistent attempts as TFdi,max.
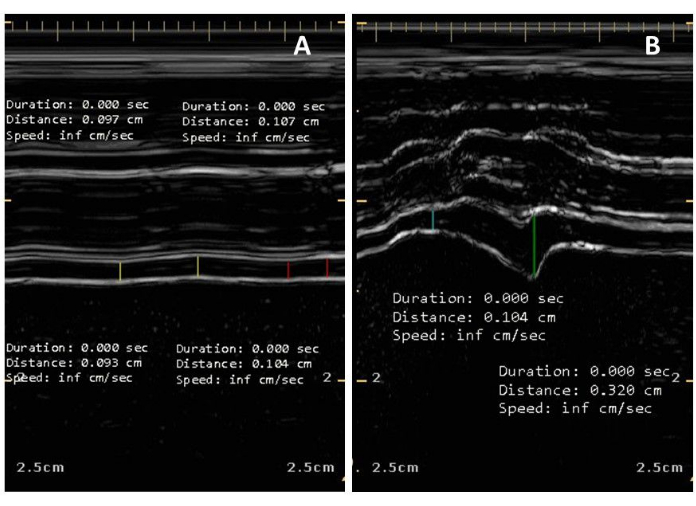
Figure 3: Examples of minimal and maximal diaphragm thickening fraction. (A) Ultrasound diaphragm thickness (Tdi) and thickening fraction (TFdi) were measured in the presence of minimal diaphragmatic contraction. If necessary, adjust the sweep speed; two breaths are used to assess for TFdi. In the absence of clear peak inspiratory thickness, the timing of inspiratory effort is determined clinically at the bedside. TFdi here is calculated as 11%, but would be averaged over a further two breaths (total of four breaths captured in two images). (B) Maximal diaphragm thickening fraction measured during maximal inspiratory efforts (TFdi,max) is stimulated either by coaching the patient to make maximal volitional efforts, or following a Marini mauver if the patient is unable to be coached and there is a P0.1 >2 cm H2O. TFdi,max is calculated here as 208%, however the largest value obtained after several (at least three) attempts would be recorded as the TFdi,max. There are pronounced difference in TFdi and Tdi during a maximal inspiration (B) compared to a minimal inspiratory effort (A). Please click here to view a larger version of this figure.
Representative Results
Following this protocol, diaphragm thickness and thickening fraction can be measured as noninvasive and reproducible means of evaluating diaphragm structure and function. Measurements can be made at the bedside and saved for blinded offline analysis. These measures can be obtained repeatedly over time to assess changes in diaphragm structure and function longitudinally.
In healthy adults, resting end-expiratory diaphragm thickness can range from 1.5 mm to 5.0 mm, depending on height, sex, and, probe position21. In healthy adults breathing at rest, tidal TFdi typically ranges between 15%-30%. During maximal inspiratory efforts, TFdi,max typically ranges between 30% and 130%13,21,22. Maximal TFdi <20% is diagnostic for severe diaphragm dysfunction13,21. Table 2 summarizes healthy and critically ill diaphragm thickness and thickening fraction.
Table 2: Reference values for diaphragm thickness and thickening fraction11,13,19,21,22,23,24,25,26,27,28,29,30,31,32. Please click here to download this Table.
In critically ill patients receiving invasive mechanical ventilation, baseline diaphragm thickness measured at the outset of respiratory failure is correlated to clinical outcome (higher baseline Tdi predicts lower mortality and faster liberation from mechanical ventilation). In these patients, the subsequent evolution of Tdi over time varies widely between patients. About 40%-50% of patients develop atrophy (a decrease in Tdi from baseline by more than 10%) within the first week of mechanical ventilation15. A small subset of patients exhibit a rapid early increase in Tdi exceeding 10% of baseline, possibly indicative of injury, inflammation, or edema in the muscle (but not muscle hypertrophy, since hypertrophy takes weeks to occur). TFdi,max <30% predicts a higher risk of failed weaning from mechanical ventilation23.
In the example shown in Figure 2A, diaphragm thickness in the first breath (in yellow) was 1.20 mm at end expiration and 1.51 mm at peak inspiration. The thickening fraction can then be calculated using the formula below and expressed as a percentage.
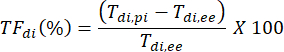
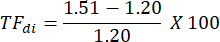
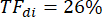
Discussion
Diaphragm ultrasound provides a noninvasive, reliable, and valid technique to monitor diaphragm structure and function in healthy subjects and critically ill patients. Diaphragm thickening fraction provides a bedside measure of diaphragm contractile activity and function that is much more feasible than magnetic twitch transdiaphragmatic pressure measurements, the traditional gold standard method for evaluating diaphragm function33. Monitoring diaphragm function and thickness by point-of-care ultrasound provides a means of detecting diaphragm atrophy. As such, experts recommend a minimum of 15 separate transdiaphragmatic ultrasounds be performed and analyzed to develop competency17.
To ensure reproducible and precise measurements, it is imperative to mark probe placement19. The B-mode image should be optimized by adjusting the probe placement, as well as the depth, gain, and focus of the instrument. The sweep speed of the ultrasound used should be adjusted to obtain a minimum of two breaths within a captured image if possible. Lastly, measurements should be repeated until consistent values (within 10%) are obtained.
Some of the difficulties associated with obtaining Tdi and TFdi are the placement and orientation of the linear probe. Table 1 highlights some common scenarios and the associated troubleshooting measures users should undertake.
Some limitations of this ultrasound technique need to be noted. First, diaphragm thickness varies widely between patients, and changes in thickness over time need to be referenced to the baseline value (for example, to diagnose atrophy). Second, despite the simplicity of the technique, training is required to ensure competency. A web-based online training platform has been validated to achieve competency in the technique18. Third, the ultrasound technique described provides limited data on muscle structure (mass) and function (contractility). New techniques, such as shear ultrasonography and ultrasound elastography, can provide additional information concerning muscle stiffness and fibrosis34,35,36,37,38.
In summary, transdiaphragmatic ultrasonography provides key measures of diaphragm structure and function that can easily be performed in healthy and critically ill patients. This technique is reliable and valid, considering a competent user with sufficient training. This article outlines how to perform transdiaphragmatic ultrasound and cautions users to undergo sufficient training before data acquisition.
Offenlegungen
The authors have nothing to disclose.
Materials
| 10-15 MHz linear array transducer | Philips | L12-4 | Any 10-15MHz linear array transducer may be used |
| Any DICOM viewer software Example: MicroDicom DICOM viewer | MicroDicom | Free for non-commerical use analysis software: https://www.microdicom.com/company.html | |
| Lumify Ultrasound Application | Philips | Other systems will use their own software | |
| Lumify Ultrasound System | Philips | Any ultrasound system may be used | |
| Skin Safe Marker | Viscot | 1450XL | Used for marking location of probe |
| Ultrasound Gel | Wavelength | NTPC201X | Any ultrasound gel may be used |
Referenzen
- Hagen-Ansert, S. L. . Textbook of Diagnostic Sonography-E-Book. , (2017).
- Dussik, K. T. On the possibility of using ultrasound waves as a diagnostic aid. Neurol Psychiat. 174, 153-168 (1942).
- Shampo, M. A., Kyle, R. A. John Julian Wild-pioneer in ultrasonography. Mayo Clinin Proceedings. 72 (3), 234 (1997).
- Kurjak, A. Ultrasound scanning – Prof. Ian Donald (1910-1987). European Journal of Obstetrics, Gynecology, and Reproductive Biology. 90 (1910-1987), 187-189 (2000).
- Donald, I., Macvicar, J., Brown, T. G. Investigation of abdominal masses by pulsed ultrasound. Lancet. 1 (7032), 1188-1195 (1958).
- Fowlkes, J. B. American Institute of Ultrasound in Medicine consensus report on potential bioeffects of diagnostic ultrasound: executive summary. Journal of Ultrasound in Medicine. 27 (4), 503-515 (2008).
- Jenssen, C., et al. European federation of societies for ultrasound in medicine and biology (EFSUMB) policy document development strategy – clinical practice guidelines, position statements and technological reviews. Ultrasound International Open. 5 (1), E2-E10 (2019).
- Pickering, M., Jones, J. F. X. The diaphragm: two physiological muscles in one. Journal of Anatomy. 201 (4), 305-312 (2002).
- De Troyer, A., Sampson, M., Sigrist, S., Macklem, P. T. The diaphragm: two muscles. Science. 213 (4504), 237-238 (1981).
- Mittal, R. K. The crural diaphragm, an external lower esophageal sphincter: a definitive study. Gastroenterology. 105 (5), 1565-1567 (1993).
- Boussuges, A., Rives, S., Finance, J., Brégeon, F. Assessment of diaphragmatic function by ultrasonography: Current approach and perspectives. World Journal of Clinical Cases. 8 (12), 2408-2424 (2020).
- Ueki, J., De Bruin, P. F., Pride, N. B. In vivo assessment of diaphragm contraction by ultrasound in normal subjects. Thorax. 50 (11), 1157-1161 (1995).
- Gottesman, E., McCool, F. D. Ultrasound evaluation of the paralyzed diaphragm. American Journal of Respiratory and Critical Care Medicine. 155 (5), 1570-1574 (1997).
- Haber, K., Asher, M., Freimanis, A. K. Echographic evaluation of diaphragmatic motion in intra-abdominal diseases. Radiology. 114 (1), 141-144 (1975).
- Goligher, E. C., et al. Evolution of diaphragm thickness during mechanical ventilation. impact of inspiratory effort. American Journal of Respiratory and Critical Care Medicine. 192 (9), 1080-1088 (2015).
- Goligher, E. C., et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. American Journal of Respiratory and Critical Care Medicine. 197 (2), 204-213 (2018).
- Haaksma, M. E., et al. EXpert consensus On Diaphragm UltraSonography in the critically ill (EXODUS): a Delphi consensus statement on the measurement of diaphragm ultrasound-derived parameters in a critical care setting. Critical Care. 26 (1), 99 (2022).
- Dugar, S., et al. Validation of a web-based platform for online training in point-of-care diaphragm ultrasound. ATS Scholar. 3 (1), 13-19 (2022).
- Goligher, E. C., et al. Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: feasibility, reproducibility and validity. Intensive Care Medicine. 41 (4), 642-649 (2015).
- Truwit, J. D., Marini, J. J. Validation of a technique to assess maximal inspiratory pressure in poorly cooperative patients. Chest. 102 (4), 1216-1219 (1992).
- Boon, A. J., et al. Two-dimensional ultrasound imaging of the diaphragm: quantitative values in normal subjects. Muscle & Nerve. 47 (6), 884-889 (2013).
- Harper, C. J., et al. Variability in diaphragm motion during normal breathing, assessed with B-mode ultrasound. The Journal of Orthopaedic and Sports Physical Therapy. 43 (12), 927-931 (2013).
- DiNino, E., Gartman, E. J., Sethi, J. M., McCool, F. D. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax. 69 (5), 423-427 (2014).
- Carrillo-Esper, R., et al. Standardization of sonographic diaphragm thickness evaluations in healthy volunteers. Respiratory Care. 61 (7), 920-924 (2016).
- Schepens, T., et al. The course of diaphragm atrophy in ventilated patients assessed with ultrasound: a longitudinal cohort study. Critical Care. 19, 422 (2015).
- Haaksma, M. E., et al. Anatomical variation in diaphragm thickness assessed with ultrasound in healthy volunteers. Ultrasound in Medicine and Biology. 48 (9), 1833-1839 (2022).
- Farghaly, S., Hasan, A. A. Diaphragm ultrasound as a new method to predict extubation outcome in mechanically ventilated patients. Australian Critical Care. 30 (1), 37-43 (2017).
- Vivier, E., et al. Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Medicine. 38 (5), 796-803 (2012).
- Pirompanich, P., Romsaiyut, S. Use of diaphragm thickening fraction combined with rapid shallow breathing index for predicting success of weaning from mechanical ventilator in medical patients. Journal of Intensive Care. 6, 6 (2018).
- Scarlata, S., Mancini, D., Laudisio, A., Raffaele, A. I. Reproducibility of diaphragmatic thickness measured by M-mode ultrasonography in healthy volunteers. Respiratory Physiology & Neurobiology. 260, 58-62 (2019).
- van Doorn, J. L. M., et al. Association of diaphragm thickness and echogenicity with age, sex, and body mass index in healthy subjects. Muscle & Nerve. 66 (2), 197-202 (2022).
- Ferrari, G., et al. Diaphragm ultrasound as a new index of discontinuation from mechanical ventilation. Critical Ultrasound Journal. 6 (1), 8 (2014).
- Cattapan, S. E., Laghi, F., Tobin, M. J. Can diaphragmatic contractility be assessed by airway twitch pressure in mechanically ventilated patients. Thorax. 58 (1), 58-62 (2003).
- Drakonaki, E. E., Allen, G. M., Wilson, D. J. Ultrasound elastography for musculoskeletal applications. The British Journal of Radiology. 85 (1019), 1435-1445 (2012).
- Şendur, H. N., Cerit, M. N., Şendur, A. B., Özhan Oktar, S., Yücel, C. Evaluation of diaphragm thickness and stiffness using ultrasound and shear-wave elastography. Ultrasound Quarterly. 38 (1), 89-93 (2022).
- Tuinman, P. R., et al. Respiratory muscle ultrasonography: methodology, basic and advanced principles and clinical applications in ICU and ED patients-a narrative review. Intensive Care Medicine. 46 (4), 594-605 (2020).
- Bachasson, D., et al. Diaphragm shear modulus reflects transdiaphragmatic pressure during isovolumetric inspiratory efforts and ventilation against inspiratory loading. Journal of Applied Physiology. 126 (3), 699-707 (2019).
- Fossé, Q., et al. Ultrasound shear wave elastography for assessing diaphragm function in mechanically ventilated patients: a breath-by-breath analysis. Critical Care. 24 (1), 669 (2020).

