Establishment of an Oronasal Fistula Mice Model
Summary
This article outlines a step-by-step procedure for establishing a mice model with an oronasal fistula. The oronasal fistula was created by employing heated ophthalmologic cautery to damage the midline portion of the hard palate, resulting in the formation of an opening between the oral and nasal cavities.
Abstract
This study presents a method utilizing heated ophthalmologic cautery to develop a viable model for investigating oronasal fistulas. C57BL/6 mice were used to establish the oronasal fistula (ONF) model. To create the ONF, the mice were anesthetized, immobilized, and their hard palates were exposed. During the surgical procedure, a 2.0 x 1.5 mm full-thickness mucosal injury was induced in the midline of the hard palate using ophthalmologic cautery. It was crucial to control the size of the ONF and minimize bleeding in order to ensure the success of the experiment. Verification of the ONF model’s effectiveness was conducted on the 7th-day post-operation, encompassing both anatomical and functional assessments. The presence of the nasal septum within the oral cavity and the outflow of sterile water from the nostrils upon injection into the oral cavity confirmed the successful establishment of the ONF model. The model demonstrated a practical and successful oronasal fistula, characterized by a low mortality rate, significant weight changes, and minimal variation in ONF size. Future studies may consider adopting this methodology to elucidate the mechanisms of palate wound healing and explore novel treatments for oronasal fistulas.
Introduction
Oronasal fistula (ONF), an abnormal opening between the oral and nasal cavities, clinically manifests as a defect in a structural area from the alveolar process to the uvula, which commonly occurs as a complication following cleft palate repair1. Patients with ONF experience food reflux, articulation disorders, and impaired velopharyngeal function, significantly impacting their quality of life2,3,4. The rate of post-operative ONF ranges from 2.4% to 55% due to factors such as cleft width, Veau type, and surgical method5,6,7,8. Additionally, the recurrence rate after ONF repair is high, ranging from 0% to 43%9.
Several novel treatments have recently shown promise in the field of ONF, including different materials, drugs, and novel techniques10,11,12,13,14,15,16,17. Accurate evaluation of therapeutic effects is essential as it provides the basis for selecting and further developing ONF treatments. However, obtaining a valid assessment in the short term for therapeutic effects other than surgery is challenging, as the characteristics of ONFs vary among different patients. Therefore, establishing an ONF disease model is necessary to verify the effectiveness of these treatment methods.
For several decades, researchers have generated the oronasal fistula (ONF) model in various animal species, including rats18,19, piglets20,21, minipigs22, and dogs23, as these species possess a substantial hard palate suitable for surgical manipulation. However, mice have a genetic sequence and whole genome similar to that of humans, making them an important model for researching and developing new drugs24,25,26. Furthermore, mice offer little variation from batch to batch, making them a favorable choice for establishing the ONF model12,13,27.
However, the detailed steps for creating ONF were not described, and the stability of the ONF size was not taken into consideration. Additionally, the verification of ONF formation relied solely on observation28, without ensuring direct communication between the oral and nasal cavities. It was not demonstrated through other means, such as the mouse's loss of body weight due to difficulties in eating caused by the ONF. Furthermore, normal variation in wound size was not considered, which is crucial for studies on drugs or materials that promote or inhibit wound healing. Therefore, there is a strong need to establish a stable and validated ONF model.
The objective of this study was to develop a practical ONF model that addresses the aforementioned issues, with the hope that this protocol will serve as the foundation for future research on the mechanisms of palatal wound healing and novel treatments for ONF.
Protocol
All animal procedures in this study were reviewed and approved by the Ethical Committee of the West China School of Stomatology, Sichuan University. Adult C57BL/6 mice (female) were used for the present study.
1. Surgical preparation
- Gather the necessary surgical instruments for the procedure: germinator, ophthalmologic cautery, microsurgical scissors, microsurgical tweezers, syringes, and needles (26 g x 0.63 inch) (Figure 1A,B) (see Table of Materials).
NOTE: Prior to the surgical procedure, autoclave the surgical instruments, including the ophthalmologic cautery, microsurgical tweezers, and microsurgical scissors, at 102.9 kPa (1.05 kg/cm2) and 121 °C for 20 min. - Collect the required surgical supplies: surgical drapes, latex gloves, sterile cotton, sterile sheets, sterile metal foil, foam board as the surgical platform, rubber bands (which can be obtained by tearing a medical latex glove), and tape (Figure 1C) (see Table of Materials).
NOTE: Use a separate set of supplies for each mouse, including syringes and sterile sheets for the surgical field. - Clean the surgical area and apparatus (light source, foam board, and temperature maintenance device, see Table of Materials) using alcohol wipes. Cover the knobs and handles of instruments that may be required during the procedure with sterile metal foil.
- Aseptically open the individual instruments and carefully position them in the surgical area. Activate the germinator (see Table of Materials) and the lights for use during the procedure. Place the ophthalmologic cautery into the germinator and heat it to 250 °C for 20 min.
2. Surgical procedure
- Perform fixation of the mouse and open the oral cavity following the steps below.
- Select a female C57BL/6J mouse weighing 20-25 g and aged 8-12 weeks. House the mouse for 7 days before conducting any procedures.
- Anesthetize the mouse by intraperitoneal injection of Zoletil50 (80 mg/kg) and Xylazine (5 mg/kg) (see Table of Materials). Apply ophthalmic eye ointment to the eye of the mouse. Wait until there is no toe-pinch response.
NOTE: The mouse is ready for the procedure when it is unable to turn over independently. - Secure the mouse to a foam board lined with sterile sheets. Use tape to tie the mouse to the surgical platform in a supine position (Figure 2A).
- Open the oral cavity of the mouse. Position two needles (26 g x 0.63 inch) in front of the orbital ear plane and two more behind it. Place a rubber band around the needles and cross the incisors to hold the mouth open. Use microsurgical tweezers to prop open the corners of the mouth (Figure 2B).
NOTE: Ensure that the hard palate is clearly exposed. Fix the tongue under the rubber band to prevent obstruction of the field of view and burning during subsequent experiments.
- Create the oronasal fistula (ONF) on the hard palate (Figure 3A-F).
- Retrieve the ophthalmologic cautery, which has been heated to 250 °C for 20 min. Place the cautery tip 1 mm away from the intersection of the midline of the palate and the line of the first premolar, creating a full-thickness mucosal injury to the hard palate in the midline.
NOTE: Avoid scalding the mouse's tongue. - After a few seconds, remove the ophthalmologic cautery when the mucosa around the cautery tip turns white.
- Place the ophthalmologic cautery in the germinator and continue heating it to 250 °C for 10 min. Repeat the previous step to enlarge the wound around the edges until it reaches a length of 2.0 mm and a width of 1.5 mm.
NOTE: Each extension should follow the edge of the last injury. Use a vernier caliper to measure the length and width of the injury. The injury should cover 10% of the palate. - Use microsurgical scissors to remove any excess denatured soft tissue around the wound. Use sterile cotton to stop bleeding and prevent inhalation asphyxiation of the mouse. Measure the wound to ensure it forms a full-thickness hard palate mucosal injury measuring 2.0 x 1.5 mm in the midline.
- Retrieve the ophthalmologic cautery, which has been heated to 250 °C for 20 min. Place the cautery tip 1 mm away from the intersection of the midline of the palate and the line of the first premolar, creating a full-thickness mucosal injury to the hard palate in the midline.
3. Post-operative care
- Administer Meloxicam to the mouse at the time of postoperative awakening, at a dose of 5 mg/kg/d for 3 days, subcutaneously29.
- Place the mouse on a temperature maintenance device until it fully regains consciousness.
NOTE: Ensure that the mouse is positioned in a way that facilitates breathing. Rotate the mice every 10-15 min to prevent blood pooling or collapse of lung lobes. Once the mouse has warmed up, return it to its cage. Provide sterile jelly and irradiated feed at the bottom of the cage for the mice to consume.
4. Verification of the oronasal fistula creation
NOTE: The success of the oronasal fistula (ONF) creation is assessed on the 7th day following the surgical procedure.
- Prepare the necessary surgical supplies: rubber band, tape, syringes, surgical drapes, latex gloves, sterile sheets, sterile metal foil, and foam board.
- Wear surgical drapes and sterile gloves to maintain aseptic conditions. Disinfect the foam board, light source, and temperature maintenance device with alcohol.
- Induce general anesthesia by intraperitoneal injection of Zoletil50 (80 mg/kg). Wait until there is no toe-pinch response. Use the same method as described in step 2.1.3 and 2.1.4 to immobilize the mouse and expose the hard palate.
- Perform anatomical structural verification by ensuring that the septum is still visible at the wound site, indicating successful ONF creation (Figure 4A,B).
- Perform functional verification: close the oral cavity of the mouse and inject sterile water into its oral cavity using a sterile syringe. The successful creation of ONF is confirmed when fluid flows from the mouse's nostrils.
- Place the mouse on the temperature maintenance device (37 °C) until it fully regains consciousness. Rotate the mice every 10-15 min to prevent blood pooling or collapse of lung lobes.
Representative Results
To assess the feasibility and stability of this experimental method, the same procedure was performed on ten mice, and observations were made regarding mortality, changes in wound size, body weight, and histologic analysis. The mice were euthanized on day 7.
The procedure exhibited a low mortality rate. The ophthalmologic cautery and germinator, depicted in Figure 1A–C, were the key instruments utilized in this experiment. The ONF model was created according to the provided protocol. Among the ten mice operated on, only one expired on the 7th day post-operation. The overall mortality rate throughout the experiment was approximately 10%.
The results revealed notable variability in the size of the ONF generated using the described method. On the day of surgery, all mice exhibited oval-shaped wounds measuring 2.0 mm in length and 1.5 mm in width. When assessing ONF formation on the 7th day after surgery, a significant variation in ONF size was observed (P = 0.0085) (Figure 5A,B).
The presence of ONF can result in complications such as food reflux and eating difficulties, potentially leading to changes in weight. Therefore, the body weight of the mice was also taken into account. The mice were weighed on the day of surgery (day 1) and on the 7th day (day 7) when ONF formation was examined. A significant reduction in weight was observed on day 7 compared to day 1 (P < 0.001) (Figure 6A,B). The loss of their body weight was 25.16%.
For histologic analysis, both the wound and normal tissue were harvested from the mice on day 7. Isolated palates were used as samples for histology sectioning. They were placed in tissue embedding boxes and fixed using 4% paraformaldehyde and 10% formic acid decalcification reagent. The tissues were then embedded in paraffin, sectioned into 7 µm slices along coronal planes, and stained with hematoxylin and eosin (H&E). Histological analysis of the ONF revealed loss of hard palate mucosa, denuded bone, and ONF formation (Figure 7). Histology of the lungs was performed, and no abnormalities were detected between normal and ONF mice.
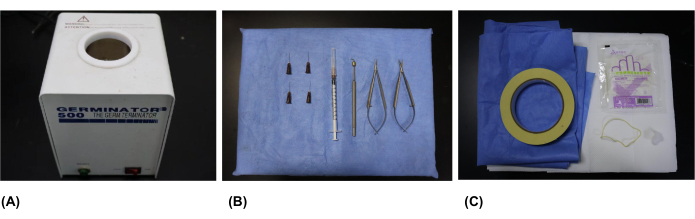
Figure 1: Surgical instruments and supplies. (A) The germinator used for heating the ophthalmologic cautery. (B) Surgical instruments: ophthalmologic cautery, microsurgical scissors, microsurgical tweezers, syringes, and needles (26 g x 0.63 inch). (C) Surgical supplies: surgical drapes, sterile gloves, sterile cotton, sterile sheets, sterile metal foil, foam board, rubber bands, and tape. Please click here to view a larger version of this figure.
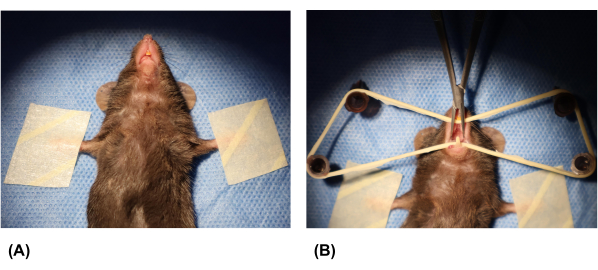
Figure 2: Fixation of the mouse and opening of the oral cavity. (A) The forelimbs of the mouse were taped to secure it. (B) Syringe needles were inserted into the foam board, and a rubber band was placed over the needles. The mouse's oral cavity was opened using a rubber band and microsurgical tweezers. Please click here to view a larger version of this figure.
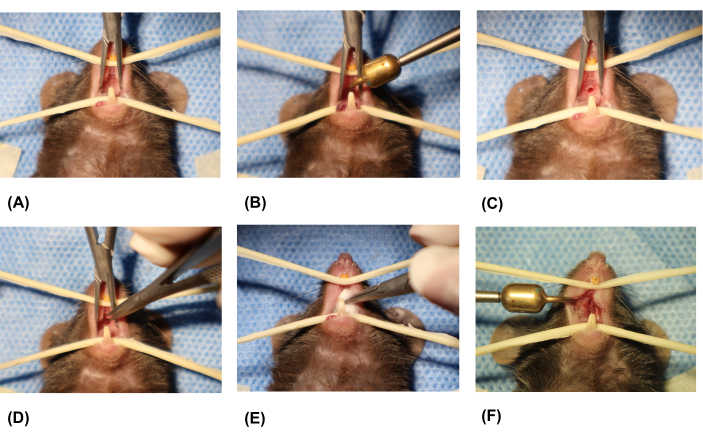
Figure 3: Creation of the oronasal fistula. (A) Exposing the oral cavity. (B) Placing the tip of the ophthalmologic cautery on the midline portion of the hard palate. (C) Removing the ophthalmologic cautery. (D) Removing excess soft tissue around the wound using microsurgical scissors. (E) Stopping bleeding using sterile cotton. (F) Final formed palatal wound. Please click here to view a larger version of this figure.
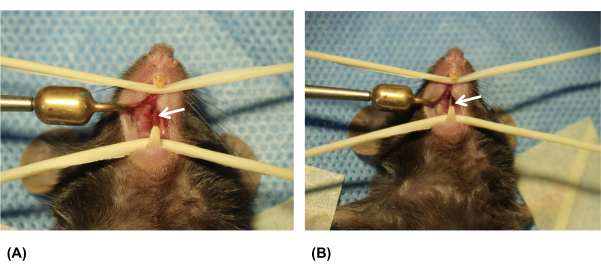
Figure 4: Examination of the palatal wound on the 7th day after surgery. (A) Palatal wound on day 1. (B) Palatal wound on day 7. White arrows indicate the oronasal fistula (ONF). Please click here to view a larger version of this figure.
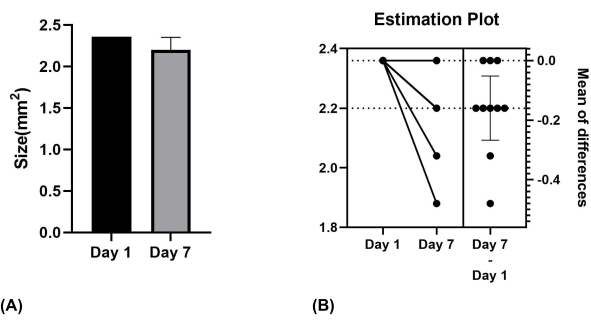
Figure 5: Size of the palatal wound on Day 1 and Day 7. (A) Mean values for mice on days 1 and 7. (B) Significant difference verified using paired samples t-test. Please click here to view a larger version of this figure.
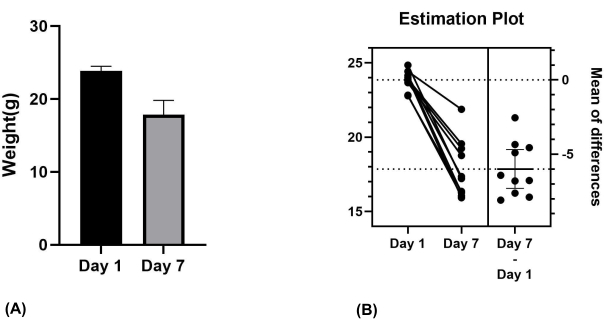
Figure 6: Weight of the mice on day 1 and day 7. (A) Mean values for mice on day 1 and day 7. (B) Significant difference verified using paired samples t-test. Please click here to view a larger version of this figure.
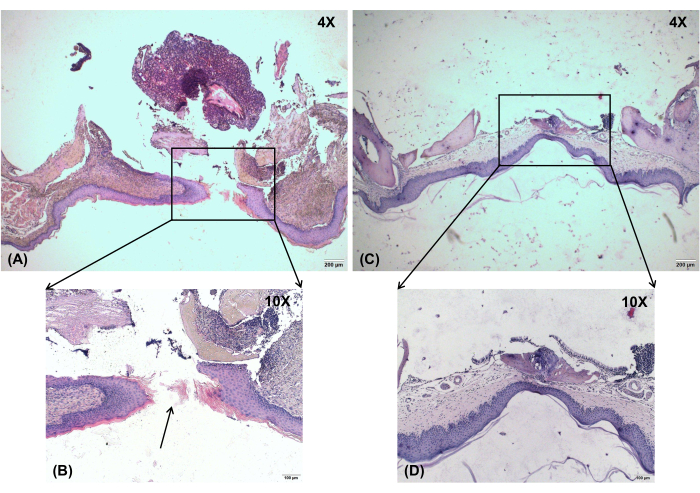
Figure 7: Histological observation. Histological analysis of the ONF shows loss of hard palate mucosa, denuded bone, and ONF formation. (A) Oronasal fistula on day 7, magnification: 4x. (B) Oronasal fistula on day 7, magnification: 10x. (C) No-injury control, magnification: 4x. (D) No-injury control, magnification: 10x. Black arrow shows the location of the ONF. Scale bars: A,C = 200 µm; B,D = 100 µm. Please click here to view a larger version of this figure.
Discussion
Researchers have explored various materials, drugs, and novel techniques for treating ONF10,11,12,13,14,15,16,17. With advancements in surgical procedures, the incidence and recurrence of ONF have been reduced. However, due to the unique characteristics of the disease, the number of ONF patients in the clinic is limited, necessitating a standardized model for studying potential treatments. While several methods for creating ONF models have been described18,19,20,21,22,23, they were often brief and lacked detailed discussion of the experimental method. The formation of ONF has been verified through microscopic and histological studies that describe the histopathological features12,13,27. This protocol aimed to establish a reproducible mouse model of ONF to facilitate research.
Achieving uniform ONF creation posed a challenge. To ensure reproducibility, it was crucial to uniformly damage the palates of the mice. Controlling the diameter of the ONF, minimizing wound healing, and effectively stopping bleeding were key steps in creating ONF. Microsurgical scissors were used to remove excess soft tissue around the wound after using the ophthalmologic cautery, thereby minimizing changes in wound diameter during the healing phase. However, using microsurgical scissors to remove excess tissue carried the risk of significant bleeding and even death of the mice, contributing to higher mortality rates observed in other experiments12,13,27. In this protocol, the combination of microsurgical scissors and a hemostatic ophthalmic cautery was employed to denature and remove excess tissue, while sterile cotton was used to control bleeding. This method significantly reduced bleeding or even achieved complete hemostasis due to the cauterizing effect of the heated ophthalmic cautery.
An alternative method for creating the ONF model in mice has been reported, involving the use of a biopsy punch13,27. While this method provided better control over the wound diameter due to the consistent size of the punch, it had a high failure rate and posed challenges in managing the required force, potentially leading to the death of the mice. Controlling the depth and strength of ONF creation with this method was difficult, and determining whether the nasal septum had been reached was challenging. Additionally, controlling bleeding was problematic, and mice were at risk of suffocation due to severe bleeding during the experiment.
However, there are limitations to this experimental method. Firstly, the size of the wound cannot be controlled by the same fistula size in each mouse compared to a biopsy punch with a fixed diameter size. And measuring tools should be used to maximize the size of each fistula. The size of the palatal wound is critical to the experiment, as delayed healing of the wound is central to ONF formation. Therefore, determining an appropriate size for the palatal wound is important. If the wound is too small, it may heal quickly, not meeting the time requirements for subsequent experiments. Conversely, if it is too large, mice may die from excessive blood loss during surgery or experience difficulty eating post-surgery, leading to starvation. Hence, exploring the optimal size of the palatal wound is warranted. Nonetheless, the size (2.0 mm x 1.5 mm) used in the current experiment was deemed appropriate. In this protocol, we only use female mice, but either female or male mice can be chosen according to the design of study.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Research and Development Program, West China Hospital of Stomatology, Sichuan University (RD-02-202107), Sichuan Province Science and Technology Support Program (2022NSFSC0743), and Sichuan Postdoctoral Science Foundation (TB2022005) grant to H. Huang.
Materials
| Germinator | Electron Microscopy Sciences | 66118-20 | Heating and disinfection equipment |
| Latex gloves | Allmed | or similar | |
| Lights | Olympus | A1813 | |
| Meloxicam | MedChemExpress | HY-B0261 | crushed; 5 mg/kg |
| Microsurgical instruments (scissors and tweezers) | Jiangsu Tonghui Medical Devices Co. | M-Y-0087 | Surgical instrument |
| Ophthalmologic cautery | Suqian Wenchong Medical Equipment Co. | 1.00234E+13 | Surgical instrument |
| Sterile cotton, | Yancheng Begu Technology Co. | or similar | |
| Sterile metal foil | Biosharp | or similar | |
| Sterile sheets | 3M | XH003801129 | or similar |
| Surgical drapes | Yancheng Begu Technology Co. | or similar | |
| Syringes | Yancheng Begu Technology Co. | S-015301 | or similar |
| Tape | Bkmamlab | or similar | |
| Temperature maintenance device | Harvard Apparatus | LE-13-2104 | |
| Zoletil50 | Virbac | 80 mg/kg |
Referenzen
- Alonso, V., et al. Three-layered repair with a collagen membrane and a mucosal rotational flap reinforced with fibrine for palatal fistula closure in children. International Journal of Pediatric Otorhinolaryngology. 127, 109679 (2019).
- Garg, R., Shah, S., Uppal, S., Mittal, R. K. A statistical analysis of incidence, etiology, and management of palatal fistula. National Journal of Maxillofacial Surgery. 10 (1), 43-46 (2019).
- Mahajan, R. K., Kaur, A., Singh, S. M., Kumar, P. A retrospective analysis of incidence and management of palatal fistula. Indian Journal of Plastic Surgery. 51 (3), 298-305 (2018).
- Huang, H., et al. Validation of the Chinese Velopharyngeal Insufficiency Effects on Life Outcomes Instrument. Laryngoscope. 129 (11), E395-E401 (2019).
- Sakran, K. A., et al. Evaluation of Postoperative Outcomes in Two Cleft Palate Repair Techniques without Relaxing Incisions. Plastic and Reconstructive Surgery. , (2023).
- Sakran, K. A., et al. Evaluation of late cleft palate repair by a modified technique without relaxing incisions. Journal of Stomatology, Oral and Maxillofacial Surgery. 124 (4), 101403 (2023).
- Sakran, K. A., et al. The Sommerlad-Furlow modified palatoplasty technique: postoperative complications and implicating factors. Laryngoscope. 133 (4), 822-829 (2023).
- Sakran, K. A., et al. Early cleft palate repair by a modified technique without relaxing incisions. The Cleft Palate-Craniofacial Journal. , (2022).
- Chen, J., Yang, R., Shi, B., Xu, Y., Huang, H. Obturator manufacturing for oronasal fistula after cleft palate repair: a review from handicraft to the application of digital techniques. Journal of Functional Biomaterials. 13 (4), 251 (2022).
- Yussif, N., Wagih, R., Selim, K. Propylene mesh versus acrylic resin stent for palatal wound protection following free gingival graft harvesting: a short-term pilot randomized clinical trial. BMC Oral Health. 21 (1), 208 (2021).
- Miron, R. J., et al. Platelet-rich fibrin and soft tissue wound healing: a systematic review. Tissue Engineering Part B: Reviews. 23 (1), 83-99 (2017).
- Ballestas, S. A., et al. Improving hard palate wound healing using immune modulatory autotherapies. Acta Biomaterialia. 91, 209-219 (2019).
- Ferreira, C. L., et al. Electrical stimulation enhances early palatal wound healing in mice. Archives of Oral Biology. 122, 105028 (2021).
- Lindley, L. E., Stojadinovic, O., Pastar, I., Tomic-Canic, M. Biology and Biomarkers for Wound Healing. Plastic and Reconstructive Surgery. 138 (3 Suppl), 18s-28s (2016).
- Xu, Y., et al. Rapid Additive Manufacturing of a Superlight Obturator for Large Oronasal Fistula in Pediatric Patient. Laryngoscope. 133 (6), 1507-1512 (2022).
- Leenstra, T. S., Kuijpers-Jagtman, A. M., Maltha, J. C. The healing process of palatal tissues after palatal surgery with and without implantation of membranes: an experimental study in dogs. Journal of Materials Science: Materials in Medicine. 9 (5), 249-255 (1998).
- In de Braekt, M. M., van Alphen, F. A., Kuijpers-Jagtman, A. M., Maltha, J. C. Wound healing and wound contraction after palatal surgery and implantation of poly-(L-lactic) acid membranes in beagle dogs. Journal of Oral and Maxillofacial Surgery. 50 (4), 359-365 (1992).
- Suragimath, G., Krishnaprasad, K. R., Moogla, S., Sridhara, S. U., Raju, S. Effect of carbonated drink on excisional palatal wound healing: A study on Wistar rats. Indian Journal of Dental Research. 21 (3), 330-333 (2010).
- Zhu, T., Park, H. C., Son, K. M., Yang, H. -. C. Effects of dimethyloxalylglycine on wound healing of palatal mucosa in a rat model. BMC Oral Health. 15 (1), 60 (2015).
- Kirschner, R. E., et al. Repair of oronasal fistulae with acellular dermal matrices. Plastic and Reconstructive Surgery. 118 (6), 1431-1440 (2006).
- Rohleder, N. H., et al. Repair of oronasal fistulae by interposition of multilayered amniotic membrane allograft. Plastic and Reconstructive Surgery. 132 (1), 172-181 (2013).
- Kesting, M. R., et al. Repair of oronasal fistulas with human amniotic membrane in minipigs. British Journal of Oral and Maxillofacial Surgery. 48 (2), 131-135 (2010).
- Ayvazyan, A., et al. Collagen-gelatin scaffold impregnated with bFGF accelerates palatal wound healing of palatal mucosa in dogs. Journal of Surgical Research. 171 (2), e247-e257 (2011).
- Takao, K., Miyakawa, T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences of the United States of America. 112 (4), 1167-1172 (2015).
- Rongvaux, A., et al. Development and function of human innate immune cells in a humanized mouse model. Nature Biotechnology. 32 (4), 364-372 (2014).
- Shan, L., Flavell, R. A., Herndler-Brandstetter, D. Development of humanized mouse models for studying human NK cells in health and disease. Methods in Molecular Biology. 2463, 53-66 (2022).
- Keswani, S. G., et al. Role of salivary vascular endothelial growth factor (VEGF) in palatal mucosal wound healing. Wound Repair and Regeneration. 21 (4), 554-562 (2013).
- Amanso, A. M., et al. Local delivery of FTY720 induces neutrophil activation through chemokine signaling in an oronasal fistula model. Regenerative Engineering and Translational Medicine. 7 (2), 160-174 (2021).
- Antiorio, A. T. F. B., et al. Administration of meloxicam to improve the welfare of mice in research: a systematic review (2000 – 2020). Veterinary Research Communications. 46 (1), 1-8 (2022).

