Isolation of Targeted Hypothalamic Neurons for Studies of Hormonal, Metabolic, and Electrical Regulation
Summary
Here we present a protocol to grow specific hypothalamic cell subtypes in culture. The cells can be selected based on opportune/unique membrane markers and used in many applications, including immunofluorescence, electrophysiological, and biochemical assays.
Abstract
The hypothalamus regulates fundamental metabolic processes by controlling functions as varied as food intake, body temperature, and hormone release. As the functions of the hypothalamus are controlled by specific subsets of neuronal populations, the ability to isolate them provides a major tool for studying metabolic mechanisms. In this regard, the neuronal complexity of the hypothalamus poses exceptional challenges.
For these reasons, new techniques, such as Magnetic-Activated Cell Sorting (MACS), have been explored. This paper describes a new application of magnetic-activated cell sorting (MACS) using microbead technology to isolate a targeted neuronal population from prenatal mice brains. The technique is simple and guarantees a highly pure and viable primary hypothalamic neuron culture with high reproducibility. The hypothalamus is gently dissociated, neurons are selectively isolated and separated from glial cells, and finally, using a specific antibody for a cell surface marker, the population of interest is selected.
Once isolated, targeted neurons can be used to investigate their morphological, electrical, and endocrine characteristics and their responses in normal or pathological conditions. Furthermore, given the variegated roles of the hypothalamus in regulating feeding, metabolism, stress, sleep, and motivation, a closer look at targeted and region-specific neurons may provide insight into their tasks in this complex environment.
Introduction
The hypothalamus is a multipronged area of the brain that mediates endocrine, autonomic, visceral, and behavioral functions, including feeding, metabolism, sleep, body temperature, social behavior, and sex drive 1,2,3,4,5. Functional heterogeneity is achieved by a synergistic combination of biochemical and electrical mechanisms: hypothalamic neurons fire action potentials and secrete and release hormones and neuropeptides to modulate brain regions and organs of the body. Finally, hypothalamic neurons translate homeostatic messages from the body, responding with long-term and short-term feedback and feedforward regulations6.
The complex neuronal environment of the hypothalamus includes magnocellular endocrine neurons, releasing oxytocin and vasopressin; parvocellular neurons, primarily involved in the systemic hormonal regulation, releasing for instance, thyrotropin-release hormone (TRH), and corticotropin-release hormone (CRH) to the pituitary gland; large peptidergic projection neurons, releasing orexin and melanin-concentrating hormone (MCH); and parvocellular peptidergic neurons of the Arcuate Nucleus (ARC) releasing POMC (proopiomelanocortin) and AgRP (agouti-related protein), named ARCPOMC and ARCAgRP, respectively. Together with secretory cells, other excitatory and inhibitory neurons, including dopaminergic, glutaminergic, and GABAergic neurons 7, are involved in forming intrahypothalamic and extrahypothalamic circuits, thereby creating large-scale coordinated networks of considerable cellular heterogeneity8.
Hypothalamic diversity has been a challenge that researchers have been trying to overcome over the past 50 years. To study this heterogeneity in developing, mature, and aging hypothalami, investigators, on the one hand, have been employing single-cell RNA sequencing to explore neuronal organization, as well as molecular and transcriptomic signatures. This effort has provided an insightful look into the variegated roles of hypothalamic neurons and has addressed connections between cellular identity and its possible role in the physiological system8,9,10. On the other hand, neuronal functions have been investigated by optogenetic manipulations and fiber photometry behavioral approaches, affording a close look at the circuitry structure. In the past two decades, the Cre-recombinase technology has allowed researchers to ontogenetically stimulate or inhibit a targeted group of neurons while observing changes in behaviors and body responses6,11,12.
However, these approaches examine hypothalamic functions from a general perspective without diving deeper into the specific cellular mechanisms or the biological basis for their role within the complex hypothalamic environment. To address this, very few studies have focused on investigating molecular, biochemical, and electrical properties utilizing heterogeneous primary hypothalamic cultures. These studies sought to dissect specific neuronal processes in a complex environment and generated integrative models of physiological mechanisms13,14,15. Nonetheless, non-specific cultures pose significant challenges. For instance, the neurons' physiological connectivity and anatomical distribution are disrupted by plating neurons from different hypothalamic regions that normally would not interact, creating confounding effects. Additionally, each region has different roles and variegated neuronal populations, making it difficult to study simple biological processes.
To address these challenges, in the past decade, new approaches have been implemented to isolate neurons of interest, such as immunopanning, Fluorescent-Activated-Cell-Sorting (FACS), and Magnetic-Activated-Cell-Sorting (MACS). Immunopanning is a strategy employed to purify targeted cells using antibody-coated dishes for a series of non-neuronal (negative) and neuronal (positive) selections. While this technique could, in principle, generate high-yield purified cell cultures, in practice, is mostly used for astrocytes and oligodendrocytes since these cells can resist hours of manipulation16,17. FACS technology is a powerful tool to sort cells based on fluorescent markers and cellular characteristics using flow cytometry18,19,20. However, very few studies used this method to isolate cells for cell culture. The technique is expensive and requires highly skilled personnel to use and maintain; additionally, it is challenging to maintain viable and sterile cells at the end of the sorting procedure21. Overall, MACS appears to be a simple, non-expensive technique to obtain highly pure and viable cultures of hypothalamic primary neurons. The method utilizes magnetic beads linked to the cells via an antibody. This allows the cells to be isolated using the magnetic field of the column.
Here we describe a method based on MACS technology, which is typically used with cortical neurons. This protocol allows to isolate, in principle, viable and highly pure hypothalamic neurons. In this study, we prepare primary cultures of neurons expressing the Leptin Receptor (LepR), such as ARCPOMC and ARCAgRP neurons, that are present only in the Arcuate Nucleus. These neurons respond to leptin, an anorexigenic hormone secreted by the adipose tissue, in biochemical and electrical ways. Therefore, the isolation of this group of neurons in culture allows for the study of their hormonal, metabolic, and electrical properties in vitro.
Protocol
NOTE: A general view of the experimental procedure is graphically illustrated in Figure 1A. All experiments with mice performed in this study were approved by our institution's Animal Care and Use Committee (IACUC). We used 3-month-old C57BL6/J mice, which were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-approved vivarium, under the care of a veterinarian. The mice lived in large cages, with a 12 h light-dark cycle, and were fed ad libitum.
1. Conception and pregnancy verification
- Place mice of any background and genotype of interest for breeding. Record the date and the weight of the female before conception.
- After 6 h, inspect the female for a plaque with a probe. If the plaque is present, separate the female from the male. If the plaque is not present, keep the female in the cage until the next day, then separate the mice.
- At days 7, 10, and 14 post conception, weigh the female to confirm pregnancy.
2. Media, 24-well plate, and material preparation
- On the day of cell isolation, place, ready-to-use, poly-D-lysine-coated glass coverslips (see Table of Materials) in a 24-well plate as follows:
- Under a biological hood, sterilize a single package containing 15 coverslips with 70% ethanol and let dry. Open the package and place the coverslips in a 60 mm plate. Shake the plate horizontally to separate the coverslips. Then, invert the plate to pick up single coverslips to be placed in the wells of a 24-well plate.
- Wash the coverslips one time with 1.0 mL of sterile Hank's Balanced Salt Solution (HBSS) for 5 min.
- In the meantime, prepare 20.0 mL of plating media as follows: to 18.31 mL of BME (Basal Medium Eagle, + Earle's Salts), supplemented with 1.0 mL of heat-inactivated fetal bovine serum (FBS), add 200 µL of sodium pyruvate (from a 100x stock), 200 µL of glutamine (from a 200 nM stock), and 100 µL of penicillin/streptomycin (from a 200x stock).
- Replace the HBSS in the wells with 1.0 mL of plating media and place the plate in the incubator at 37 °C.
- Using a Bunsen burner, fire-polish three Pasteur pipettes in decreasing diameters. Holding the pipet with one hand, insert the tip into the flame and quickly remove it. Repeat the process until the tip smoothens and the diameter is reduced to the desired diameter (assessed by eye).
3. Reagent preparation for Neural Tissue Dissociation, following the directions of the Neural Dissociation kit
- After warming the Digestion Buffer 1 at room temperature, prepare Enzyme Mix 1 by mixing 50 µL of Enzyme 1 with 1.91 mL of Buffer 1 and Enzyme Mix 2 by mixing 15 µL of Enzyme 2 with 30 µL of Digestion Buffer 2. The mixes are enough to be used for all embryos' brain tissue.
- Prepare 0.5% Bovine Serum Albumin (BSA) in HBSS, for example, 0.25 g in 50.0 mL of HBSS.
4. Embryo extraction
- Autoclave two straight fine forceps, one curved point forceps, and one pair of fine surgical scissors and sterilize them with 70% ethanol before use. Then, fill the Petri dishes with HBSS.
- Euthanize one E14-E16 pregnant dam in the CO2 chamber and perform cervical dislocation.
NOTE: The following steps must be performed under the hood under sterile conditions: - Sterilize the abdomen with 70% ethanol. Cut open the abdominal cavity from the pubic symphysis to the xiphoid process of the rib cage with surgical scissors and forceps.
- Extract the uterine horn and place it in a 100 mm plate filled with ice-cold HBSS and wash thoroughly.
- Extract and separate all the embryos from the uterus with fine forceps. Quickly decapitate the embryos with fine surgical scissors and/or forceps. Place the heads in the 60 mm Petri dish filled with HBSS.
5. Hypothalamus extraction, collection, and tissue dissociation
- Place one fine forceps in the eye cavity to hold the brain. Using the other fine forceps, remove the skin and the skull by peeling until the brain is visible. Distinguish the brain from other tissues based on its white appearance. Skin and skull are pink and rich in vasculature.
- Remove the brain from the skull by using the curved point forceps and scoop out the brain from the olfactory bulbs, flipping it upside down.
- Now the cortex is ventral, and the hypothalamus is visible dorsally on the superior surface (Figure 1B). With the curved forceps, remove the layer of meninges and blood vessels until the brain appears white and clear.
- With the curved forceps, separate the hypothalamic area from the rest of the brain.
- Cut the hypothalamus into 3-4 small pieces and with a pipet, transfer the pieces into a 15 mL tube.
- Repeat the steps for the other embryos while the tube is on ice.
- Fill the tube with 6.0 mL of HBSS and let the tissue settle, remove the supernatant, and add Enzyme Mix 1. Gently mix and agitate the tube to prevent the tissue.
- Incubate the tube in a 37 °C water bath for 15 min and agitate the tissue every 5 min to resuspend the tissue.
- After 15 min, add 30 μL of Enzyme mix 2. Dissociate the brain tissue by using the pipette Pasteur with the biggest diameter (<1 mm). Pipet up and down 10x without forming bubbles.
- Incubate for 10 min at 37 °C in the water bath. Gently agitate the tube to resuspend the tissue every 5 min.
- After 10 min, add the remaining 15 µL of Enzyme mix 2. Dissociate the tissue 10x with the other two fire-polished pipettes of decreasing diameter, up and down without forming bubbles.
- To the tube with the dissociated tissue, add immediately 10.0 mL of HBSS-0.5% BSA and centrifuge at 300 × g for 10 min at room temperature.
- Aspirate the supernatant and resuspend the cell pellet in 1.0 mL of HBS-0.5% BSA.
6. Cell counting
- Dilute the cell suspension 1:5 using HBSS-0.5% BSA.
- Place 10 µL of the diluted cell suspension in a Neubauer counting chamber.
- Under a brightfield microscope, count only the cells that are in the four chamber's corner squares. Calculate the average and multiply by 5 × 104.
NOTE: Make sure there are >106 cells to proceed to cell isolation; the optimal cell number is 107.
7. Negative selection
NOTE: Negative selection enables users to obtain a pure primary neuron culture by separating neuronal and non-neuronal cells. Use precooled solutions.
- Centrifuge the cell suspension at 300 × g for 3 min (centrifugation can be extended up to 10 min). Gently aspirate the supernatant and resuspend the pellet to a concentration of 107 cells in 80 µL of HBSS-0.5% BSA.
- Add 20 µL of Non-Neuronal Cell Biotin-Antibody Cocktail and incubate for 5 min at 4 °C.
- Wash the cells to remove free antibody with 2.0 mL of HBSS-0.5% BSA and centrifuge at 300 × g for 3 min.
- Gently aspirate the supernatant and resuspend the pellet in 80 µL of HBSS-0.5% BSA. Add 20 µL of anti-biotin Microbeads, mix thoroughly, and incubate for 10 min at 4 °C.
- Add 0.5 mL of HBSS-0.5% BSA for up to 107 cells and wait until the magnetic column is ready.
8. Magnetic separation, negative selection
NOTE: Magnetic separation is a crucial step that allows the separation of the non-neuronal cells from the neuronal cells. The sample containing neuronal and non-neuronal cells is passed through the magnetic field and the non-neuronal cells, which are bound to a biotin-antibody-magnetic bead complex, are trapped in the column (Figure 1C). The free neuronal cells are eluted through the column and are collected in a 15 mL tube.
- Prepare the stand (included in the kit) with the separator and the MS column as shown in Figure 1C.
- Open the column and set up the stand only when the cells are ready to be separated.
- Rinse the column with 0.5 mL of HBSS-0.5% BSA. Wait until the solution stops dripping.
- To collect neuronal cells, place a 15 mL tube under the column and pass 0.5 mL of the cell suspension through the column. Collect the eluate in the tube until it stops dripping. To capture residual neuronal cells, wash the column 3 x 0.5 mL of HBSS-0.5% BSA.
- To collect non-neuronal cells, remove the column from the magnet and place it inside a new 15 ml tube. Add 1.0 mL of HBSS–0.5% BSA to the column and use the plunger to collect the magnetically labeled non-neuronal cells.
- Centrifuge the neuronal and non-neuronal cells at 300 × g for 3 min. Gently aspirate the supernatant and resuspend the cells in 1.0 mL of HBSS-0.5% BSA. Count the cells as described previously in section 6.
- If needed, plate the non-neuronal cells in a 24-well plate; otherwise discard them.
9. Positive selection
NOTE: Once a pure neuronal cell suspension is obtained, positive selection is carried out to isolate the targeted cells. Cells can be isolated by using a specific biotin-conjugated antibody for a surface antigen. The antibody is recognized by anti-biotin magnetic beads. By flowing the cell suspension through the column, only the cells of interest are trapped in the magnetic field.
- Centrifuge the pure neuronal cell suspension at 300 × g for 3 min. Gently aspirate the supernatant and resuspend the pellet in 80 µL of HBSS-0.5% BSA. Add the specific antibody following the manufacturer's instructions and incubate at 4 °C for 10 min.
NOTE: If cells expressing the LepR are sought, we suggest a Mouse Leptin R Biotinylated Antibody (see Table of Materials) at a concentration of 0.50 µg/106 cells. - Wash off the excess antibody with 2.0 mL of HBSS-0.5% BSA and centrifuge at 300 × g for 3 min.
- Remove the supernatant, resuspend the pellet in 80 µL of HBSS-0.5% BSA, and add 20 µL of anti-biotin microbeads. Incubate at 4 °C for 10 min.
- For every 107 cells, add 0.5 mL of HBSS-0.5% BSA and wait until the magnetic column is ready.
10. Magnetic separation, positive selection
- Prepare the stand with the separator and the MS column. Rinse the MS column with 0.5 mL of HBSS-0.5% BSA. Wait until the dripping stops.
- Place a 15 mL tube under the column, pass 0.5 mL of the cell suspension through the column, and collect the eluate comprising the non-specific neuronal cells. To clean the column of residual non-specific neuronal cells, wash with 3 x 0.5 mL of HBSS-0.5% BSA.
- Remove the column from the magnet, place it in a new 15 mL tube, and add 1.0 mL of HBSS-0.5% BSA. Use the plunger to flush out the targeted cells.
- Centrifuge both tubes at 300 × g for 3 min. Gently remove the supernatant and resuspend it in 0.5 mL of plating medium.
- Count the cells as previously described in section 6.
- Plate both targeted cells as the positive control and non-specific cells as the negative control at 120,000-200,000 cells/mm3 density in the 24-well plate previously prepared as described in section 2 and incubate at 37 °C in 5% CO2, 9% O2, and 95% humidity for 12 h.
11. Cell culture maintenance
- Prepare 20 mL of culture medium with 19.2 mL of Neuronal Culture Medium, 400 µL of B27 supplement (from a 50x stock), 200 µL of glutamine (from a 200 µM stock), and 100 µL of penicillin/streptomycin (from a 200x stock).
- Replace plating media from the 24 well-plate containing neuronal or non-neuronal cells.
- Wash with 2 x 1.0 mL of HBSS.
- Add 1.0 mL of culture media.
- Refresh the media every 2/3 days by replacing 0.5 mL of old media with 0.5 mL of fresh media.
NOTE: Cells can be maintained in culture and used for up to 21 days in vitro (DIV21).
12. Neuron immunofluorescence staining
- Twelve hours prior to the staining, prepare a solution consisting of 50/50 methanol and acetone and cool it at -20 °C overnight.
- Wash the neurons in the 24-well plate with 2 x 1.0 mL of 1x phosphate-buffered saline (PBS) for 5 min.
- Replace the PBS solution with 1.0 mL of the 50/50 solution and incubate in ice for 20 min.
- Wash with 1x PBS for 3 x 5 min.
- Block the neurons with 3% BSA in 1x PBS for 1 h at room temperature.
- Prepare the primary antibody solution in 3% BSA in 1x PBS, using the antibody concentration mentioned in the manufacturer's directions. The antibodies and concentrations used are listed in the Table of Materials.
- Replace the blocking solution with the primary antibody solution and incubate at 4 °C overnight.
- Wash the cells for 3 x 10 min with 1x PBS.
- Prepare the secondary antibody solution with 3% BSA in 1x PBS, using the concentrations of the antibodies following the manufacturer's instructions. The secondary antibodies used are listed in the Table of Materials.
- Incubate the cells with the secondary antibody solution at room temperature for 1 h.
- Wash the cells for 3 x 10 min with 1x PBS.
- Leave the neurons in 1x PBS during the mounting procedure. Place a small drop of mounting medium (with or without 4',6-diamidino-2-phenylindole for nuclear identification) on the microscope slide. Extract one glass coverslip containing neurons with forceps and tap the side of the coverslip on a paper tissue to dry the excess PBS. Flip the coverslip on the mounting medium, making sure the neurons are facing the microscope slides; gently press and remove the excess mounting media with tissue paper.
- The microscope slides are ready to be analyzed with a brightfield or a confocal microscope.
Representative Results
This paper describes a protocol for the isolation of targeted neurons of the hypothalamus (Figure 1). The scope of the method is to study specific neuronal characteristics in a controlled and isolated context. Thus, mouse embryos were extracted from the pregnant dams at E14-E16. The meninges were removed, and the hypothalamus was isolated from the rest of the brain. The tissue was gently dissociated with two mixtures of enzymes freshly prepared using the referenced dissociation kit. First, non-neuronal cells were separated from neuronal cells-glia, microglia, and neurons were collected in the same single-cell suspension. To this end, non-neuronal cells were labeled using a cocktail of antibodies recognizing non-neuronal surface epitopes. After incubation, the antibody-cell complex was conjugated with magnetic microbeads and subsequently passed through a magnetic column to trap the non-neuronal cells.
This step yielded two cell suspensions, one containing non-neuronal and the other containing neuronal cells. Both suspensions could be plated immediately. Alternatively, the neuronal cell suspension could be further manipulated to separate a neuronal sub-population (targeted suspension) from the rest using the same strategy. Based on the experiment of interest, the neuronal cells can be plated from 125,000 to 200,000 cells/mm3. The less-dense cultures can be used to analyze neurons at single-cell resolution: from axonal development, synaptic formation, and transmission to electrophysiology. The denser cultures can be used for biochemical analyses, including DNA and RNA extraction, western blot, Southern blot, Northern blot, real-time PCR, and RNA sequencing.
In this study, LepR was targeted to isolate neurons involved in the melanocortin system, such as the ARCPOMC and ARCAgRP neurons. Cells were plated at densities ranging from 120,000 cells/mm3, for LepR+ neurons, to 200,000 cells/mm3 for generic neuronal populations. After 48 h, LepR+ neurons began to form neurites (Figure 2). At DIV4, axonal extensions showed progress, while dendritic processes began to appear. At DIV6, the neurons were sufficiently developed and were therefore ready to be analyzed. Immunofluorescence experiments on LepR+ neurons showed 99% expression of LepR (green, Figure 3A). No glial cells or other non-neuronal cells were observed, confirming the purity of the primary neuronal culture. The neuronal nature of the cells was confirmed by microtubule-associated protein 2 (MAP2) staining, with the identification of axons and dendritic protrusions (Figure 3B). At DIV10, 30% of LepR+ cells expressed POMC (red). This is expected as the majority of LepR+ cells express either POMC or AgRP. Figure 3C,D illustrate co-localization between POMC and LepR signals. Note that the co-localization was prominent at and around the nucleus, as expected.
General cultures containing heterogeneous hypothalamic neuronal populations were used for control. Immunofluorescence demonstrated synaptic connectivity and functionality, as assessed by Synapsin-1 (green) and PSD 95 (red) co-staining (Figure 4A,B). The number of LepR+ neurons present in the general culture was ~5%, a percentage consistent with the notion that the majority of LepR-expressing neurons had been selected during the magnetic separation process (representative LepR+ cells are illustrated in Figure 4C,D). All data generated or analyzed during this study are available at https://doi.org/10.5061/dryad.cnp5hqc9c.
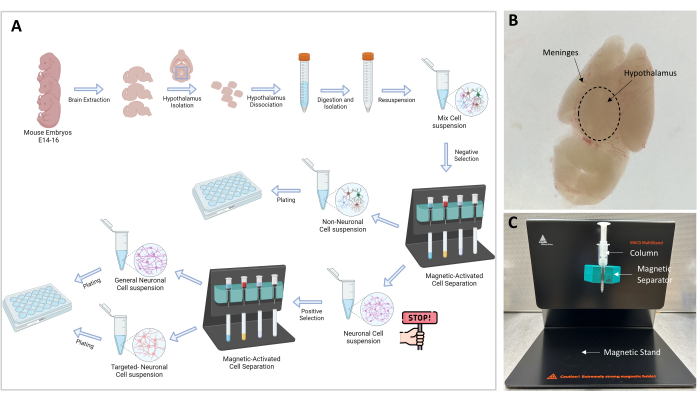
Figure 1: Experimental flow chart and setup. (A) Graphical representation of the experimental procedure. Go-no-go: ≥106 cells are necessary to proceed to cell isolation; the optimal cell number is 107. (B) Representative image of an E16 embryo brain. The hypothalamus and meninges are indicated. (C) MACS setup used for the separation and isolation of targeted cells. Magnetic stand, magnetic separator, and column are indicated. Please click here to view a larger version of this figure.
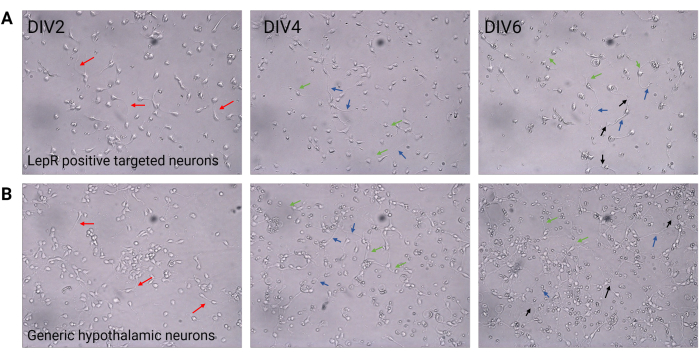
Figure 2: Neuronal culture between DIV2 and DIV6. (A) LepR+ cells obtained from the positive selection show reduced cell density and connectivity, but normal development of neurites (red arrows), axons (blue arrows), and dendrites (green arrows). Cells were plated at a density of 120,000 cells/mm3. Scale bar = 100 μm. (B) Generic hypothalamic neurons, plated at a density of 200,000 cells/mm3, display normal developmental and growth features and connectivity (black arrows). Scale bar = 100 μm. Abbreviations: DIV = days in vitro; LepR = leptin receptor. All data generated or analyzed to construct this figure are available at https://doi.org/10.5061/dryad.cnp5hqc9c. Please click here to view a larger version of this figure.
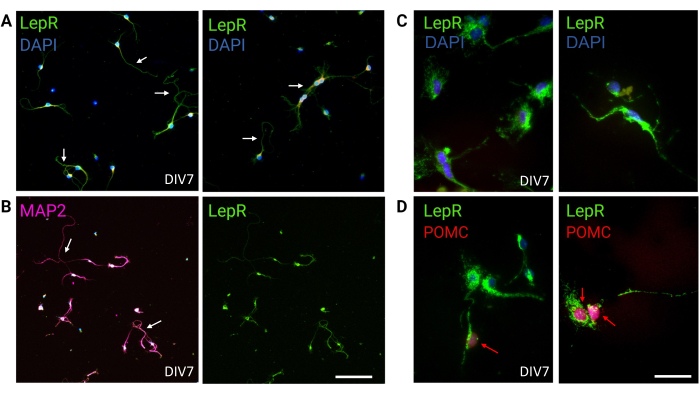
Figure 3: In vivo neurons are recapitulated by cultured LepR+ neurons. (A) Representative images of a culture of neurons expressing the LepR (green; DAPI is blue). Ninety-nine percent of the cells expressed the LepR. Neurons were plated at 120,000 cells/mm3 density. At DIV7, the neurons presented elongated axons, dendritic maturation, and neuronal connectivity (arrows). Scale bar = 40 μm. (B) Immunofluorescence with anti-MAP2 was used to confirm the neuronal nature of the cells. Neuronal-specific morphologies such as axons, dendrites, and protrusions are demonstrated (arrows). Scale bar = 40 μm. (C) Magnification of LepR+ cells. LepR in green and DAPI in blue. Scale bar = 10 μm. (D) Co-staining with LepR (green) and POMC (red). Roughly 30% of LepR+ neurons were co-immunoreactive to POMC, which was detected at the level of the nucleus (red arrows). Scale bar = 10 μm. Abbreviations: DAPI = 4',6-diamidino-2-phenylindole; MAP2 = microtubule-associated protein 2; POMC = proopiomelanocortin; LepR = leptin receptor. All data generated or analyzed to construct this figure are available at https://doi.org/10.5061/dryad.cnp5hqc9c. Please click here to view a larger version of this figure.
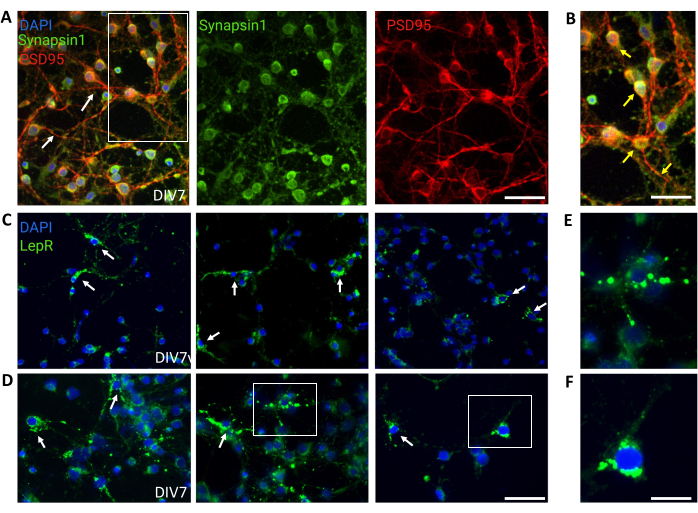
Figure 4: In vivo cells are recapitulated by cultured generic hypothalamic neurons. (A) Representative images of generic hypothalamic neuronal culture stained with Synapsin 1 (green), PSD95 (red), and DAPI (blue). The neurons exhibited well-developed connectivity and synaptic functionality (arrows). Scale bar = 40 μm. (B) Magnification of the box in (A), showing co-localization of Synapsin 1 (green) and PSD95 (red, arrows). Scale bar = 20 μm. (C,D) Representative images showing LepR+ cells in a general culture. LepR+ cells (green) accounted for ~5% of the total. Representative LepR+ cells are indicated by arrows. Scale bar = 40 μm. (E,F) Magnifications of the boxes in (C,D) showing green puncta leptin receptors localized at the soma. Scale bar = 20 μm. All data generated or analyzed to construct this figure are available at https://doi.org/10.5061/dryad.cnp5hqc9c. Please click here to view a larger version of this figure.
Discussion
Investigating the biochemical and electrical properties of hypothalamic neurons is key to understanding the molecular basis of metabolism, thermoregulation, mood management, feeding behavior, and more. However, the neuronal heterogeneity of the hypothalamus makes this effort challenging, and methods to isolate and study specific hypothalamic sub-populations are needed.
In vivo techniques employ CRE-recombinase, optogenetic, fiber photometry, and calcium imaging. These approaches allow primarily for the study of the electrical properties of hypothalamic neurons, and very few methods are currently available to investigate their non-electrical attributes. The MACS technology developed in this study could provide a technique amenable to isolating specific hypothalamic neuronal sub-populations in vitro, thereby affording targeted treatments and analyses. Neuronal cultures are simpler to manage compared to co-cultures of different neuronal populations. Additionally, pure cultures avoid confounding effects stemming from the presence of glia and microglia. Thus, neurons from the same hypothalamic region and type could be studied in response to specific metabolic and hormonal inputs.
In this protocol, we selected hypothalamic neurons expressing the LepR. Isolated LepR+ cells were cultured to investigate their cellular, morphological, and molecular characteristics which are difficult to study in vivo. The purity of the cultures was 99%, supporting the accuracy of the method. In addition, the LepR+ cells were healthy and viable at DIV7 until DIV21.
This technique, however, has some limitations. E18 or older pure neuron cultures are challenging to maintain. Therefore, the window of extraction is limited to E14-E16. This implies that cellular changes occurring after E16 are missed. For example, the expression of the leptin receptor in ARC neurons increases during the early postnatal period22. The procedure for the isolation must be performed as quickly as possible to reduce cellular stress and death and improve yield. The procedure can take up to 5 h; hence, it is essential to maintain sterile conditions and reduce manipulation to the minimum necessary. The positive selection can lead to low yield due to the low amounts of available tissue, limiting the number of experiments that can be performed with a single preparation. Elevated neuronal death was observed, probably due to low cell density and reduced neuronal connectivity and intraneuronal support.
Moreover, the antibody targeting the antigen of interest must bind to the cell surface to guarantee a correct separation; usually, antibodies used for flow cytometry are suitable for the MACS technique. If the antibody has not been used before in cell separation methods, validation and titration experiments are needed to determine the ideal usage and concentration. The extraction of targeted cells requires a cell surface marker. Here we used a biotinylated antibody but in principle, antibodies conjugated with other molecules, such as FITC (fluorescein isothiocyanate) and PE (purified anti-phycoerythrin), could also be used. MACS technology could also be applied to neurons expressing a fluorophore, such as GFP or another Tag protein, potentially increasing specificity and yield. If a fluorophore is not employed, the alternative would be to confirm the expression of the molecule of interest by immunofluorescence before performing live cell experiments. Future studies will test the validity of these alternatives.
One important aspect that this study did not address concerns the "fidelity" of the sub-neuronal populations. We ascertained that the cultured LepR+ neurons expressed POMC, which is a signature of native ARCPOMC neurons. However, more tests will be necessary to conclude that the LepR+ neuronal cultures recapitulate their native in vivo counterparts. Overall, the MACS neuronal isolation protocol presented here may provide a valid and effective method to study in vitro hypothalamic mechanisms that would be otherwise difficult to investigate in vivo.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
Graphical figures were created with BioRender.com. This work was supported by an NIA grant (R01AG060919) and an NSF grant (2030348) to FS.
Materials
| Embryo extraction | |||
| 1 curved point forceps | Fine Science Tools | 11270-20 | Dumont |
| 1 fine surgical scissor | Fine Science Tools | 14058-11 | Dumont |
| 100 mm Petri dish | Corning | 430167 | |
| 2 straight fine forceps | Fine Science Tools | 11254-20 | Dumont |
| 60 mm Petri dish | Corning | 430196 | |
| 70% ethanol | Decon Laboratories, INC. | 2801 | Ethanol 190 Proof |
| Anti-Biotin MicroBeads 1mL | Miltenyi Biotec | 130-115-390 | |
| Anti-MAP2 antibody | Abcam | ab5392 | 1 : 800 |
| Bench pads | |||
| Bovine Serum Albumin | Sigma-Aldrich | A9418-50G | |
| Buffer Y | Miltenyi Biotec | 130-094-802 | |
| Buffer Z | Miltenyi Biotec | 130-094-802 | |
| Cell Culture | |||
| Anti-Biotin MicroBeads 1mL | Miltenyi Biotec | 130-115-390 | |
| Bovine Serum Albumin | Sigma-Aldrich | A9418-50G | |
| Buffer Y | Miltenyi Biotec | 130-094-802 | |
| Buffer Z | Miltenyi Biotec | 130-094-802 | |
| Enzyme A | Miltenyi Biotec | 130-094-802 | |
| Enzyme P | Miltenyi Biotec | 130-094-802 | |
| GG-12-1.5, 12 mm dia.#1.5 thick 100 pc cell culture tested German coverglasses | Neuvitro Corporation | GG-12-15 | |
| Gibco B-27 Supplement 10 mL | ThermoFisher | 17504-044 | |
| Gibco Basal Medium Eagle (BME) 500 mL | ThermoFisher | 21010046 | (+) Earle's Salts, (-) L-Glutamine |
| Gibco HBBS (1x) Hanks' Balanced Salt Solution 500 mL | ThermoFisher | 14025092 | Calcium, Magnesium, No phenol red |
| Gibco HI FBS 100 mL | ThermoFisher | 16140-063 | |
| Gibco L-Glutamine 200 mM (100x) | ThermoFisher | 25030-081 | |
| Gibco Penicilline/Streptomicine | ThermoFisher | 15140-122 | 10,000 U/mL |
| Gibco Sodium Pyruvate (100 mM) 100 mL | ThermoFisher | 11360070 | |
| MiniMACS Separator and Starting Kit | Miltenyi Biotec | 130-042-102 | |
| Mouse Leptin R Biotinylated Antibody | R&D Systems | ABAF497 | 0.25 μg/106 cells |
| MS Column | Miltenyi Biotec | 130-042-201 | |
| Neaubeaur-Improved Brightline 100 µm Chamber | Hausser Scientific | 3120 | |
| Neural Tissue Dissociation Kit – Postnatal Neurons | Miltenyi Biotec | 130-094-802 | |
| Neuronal Culture Medium 500 mL | ThermoFisher | 88283 | |
| Non-Neuronal Cell Biotin-Antibody Cocktail mouse 1 mL | Miltenyi Biotec | 130-115-389 | |
| Olympus SZ61 Zoom Stereomicroscope | Olympus Life Science | SZ61/SZ51 | |
| Pierce Primary Neuron Isolation Kit | ThermoFisher | 88280Y | |
| Staining | |||
| Anti-MAP2 antibody | Abcam | ab5392 | 1 : 800 |
| Donkey anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 | ThermoFisher | A32766 | 1 : 500 |
| Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 | ThermoFisher | A32790 | 1 : 500 |
| Dulbecco's Phosphate Buffered Saline (DPBS) | Sigma Aldrich | MFCD00131855 | |
| Goat anti-Chicken IgY (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 647 | ThemoFisher | A32933 | 1 : 500 |
| Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 | ThermoFisher | A11037 | 1 : 200 |
| Invitrogen Leptin Receptor Recombinant Rabbit Monoclonal Antibody (JA73-01) | ThermoFisher | MA5-32685 | 1 : 500 |
| Mouse Leptin R Biotinylated Antibody | R&D Systems | ABAF497 | 1 : 500 |
| POMC Rabbit mAb | Cell Signaling Technology | D3R1U | 1 : 500 |
| PSD95 (D74D3) XP Rabbit mAb | Cell Signaling Technology | D74D3#3409 | 1 : 500 |
| Streptavidin, Alexa Fluor 594 conjugate | ThermoFisher | S11227 | 1 : 500 |
| Synapsin 1 Monoclonal Antibody (7H10G6) | ThermoFisher | MA5-31919 | 1 : 500 |
| Vectashield Plus Antifade Mountina Medium with DAPI 10 mL | Vector Laboratories | H-2000 |
Referenzen
- Cone, R. D. Anatomy and regulation of the central melanocortin system. Nature Neuroscience. 8 (5), 571-578 (2005).
- Clarke, I. J. Hypothalamus as an endocrine organ. Comprehensive Physiology. 5 (1), 217-253 (2015).
- Mignot, E., Taheri, S., Nishino, S. Sleeping with the hypothalamus: emerging therapeutic targets for sleep disorders. Nature Neuroscience. 5 Suppl, 1071-1075 (2002).
- Baird, A. D., Wilson, S. J., Bladin, P. F., Saling, M. M., Reutens, D. C. Neurological control of human sexual behaviour: insights from lesion studies. Journal of Neurology, Neurosurgery, and Psychiatry. 78 (10), 1042-1049 (2007).
- Caria, A., Dall, O. G. Functional neuroimaging of human hypothalamus in socioemotional behavior: a systematic review. Brain Sciences. 12 (6), 707 (2022).
- Andermann, M. L., Lowell, B. B. Toward a wiring diagram understanding of appetite control. Neuron. 95 (4), 757-778 (2017).
- Romanov, R. A., Alpar, A., Hokfelt, T., Harkany, T. Unified classification of molecular, network, and endocrine features of hypothalamic neurons. Annual Review of Neuroscience. 42, 1-26 (2019).
- Hajdarovic, K. H., Yu, D., Webb, A. E. Understanding the aging hypothalamus, one cell at a time. Trends in Neurosciences. 45 (12), 942-954 (2022).
- Zhang, Y. H., et al. Cascade diversification directs generation of neuronal diversity in the hypothalamus. Cell Stem Cell. 28 (8), 1483-1499 (2021).
- Chen, R., Wu, X., Jiang, L., Zhang, Y. Single-cell RNA-seq reveals hypothalamic cell diversity. Cell Reports. 18 (13), 3227-3241 (2017).
- Ma, C., et al. Neural pathways from hypothalamic orexin neurons to the ventrolateral preoptic area mediate sleep impairments induced by conditioned fear. Frontiers in Neuroscience. 17, 1122803 (2023).
- Wang, F., et al. A parabrachial to hypothalamic pathway mediates defensive behavior. Elife. 12, e85450 (2023).
- Cowley, M. A., et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 411 (6836), 480-484 (2001).
- Parekh, R. U., et al. Hypothalamic kinin B1 receptor mediates orexin system hyperactivity in neurogenic hypertension. Scientific Reports. 11 (1), 21050 (2021).
- Schmidt, C. X., Tsang, A. H., Oster, H. Generation of mouse primary hypothalamic neuronal cultures for circadian bioluminescence assays. Bio-protocol. 11 (5), e3944 (2021).
- Foo, L. C. . Purification of rat and mouse astrocytes by immunopanning. 2013 (5), 421-432 (2013).
- Emery, B., Dugas, J. C. . Purification of oligodendrocyte lineage cells from mouse cortices by immunopanning. 2013 (9), 854-868 (2013).
- Zhao, H., et al. Changes of constituents and activity to apoptosis and cell cycle during fermentation of tea. International Journal of Molecular Sciences. 12 (3), 1862-1875 (2011).
- Zhang, Z. M., et al. Down-regulation of human leukocyte antigens class I on peripheral T lymphocytes and NK cells from subjects in region of high-incidence gastrointestinal tumor. Chinese Medical Journal. 124 (12), 1813-1817 (2011).
- Drake, S. S., Zaman, A., Simas, T., Fournier, A. E. Comparing RNA-sequencing datasets from astrocytes, oligodendrocytes, and microglia in multiple sclerosis identifies novel dysregulated genes relevant to inflammation and myelination. WIREs Mechanisms of Disease. 15 (2), e1594 (2023).
- Mattanovich, D., Borth, N. Applications of cell sorting in biotechnology. Microbial Cell Factories. 5, 12 (2006).
- Cottrell, E. C., et al. Developmental changes in hypothalamic leptin receptor: relationship with the postnatal leptin surge and energy balance neuropeptides in the postnatal rat. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 296 (3), R631-R639 (2009).

