A Synthetic Methodology for Preparing Impregnated and Grafted Amine-Based Silica Composites for Carbon Capture
Summary
This work aims to facilitate the development of standardized techniques for impregnating or grafting aminated compounds onto silica substrates, which are often broadly described in the literature. Specific amounts of solvent, substrate, amines, and the values of other important experimental parameters will be discussed in detail.
Abstract
Recently, there has been a significant effort towards reducing or mitigating CO2 emissions through the use of carbon capture materials for point source or direct air capture (DAC) methods. This work focuses on amine-functionalized CO2 adsorbents for DAC. These materials show promise for CO2 removal because they have low regeneration energy consumption and high adsorption capacity. The incorporation of amine species into a porous substrate combines the advantages of the amine species' affinity to CO2 with the large pore volumes and surface areas of the porous substrate. There are three methods commonly used to prepare amine-based CO2 sorbents, depending on the selection of the amine species, material support, and preparation method. These methods are impregnation, grafting, or chemical synthesis. Silica is a prevalent choice of substrate material because of its adjustable pore size, moisture tolerance, temperature stability, and ability to adsorb CO2 in low concentrations for DAC applications. Typical synthetic procedures and primary attributes of both impregnated and grafted amine-silica composites are described herein.
Introduction
The anthropogenic CO2 emissions over the past several decades have been widely implicated as the main factor driving the greenhouse gas effect and consequently, related climate change1,2,3,4. There are two general methods for CO2 capture, point source and direct air capture. For more than 50 years, wet-scrubbing CO2 capture technologies have been utilized for point source capture within the industry to mitigate CO2 emissions5,6. These technologies are based on liquid-phase amines that react with CO2 to form carbamates under dry conditions and hydrogen carbonates in the presence of water7,8, see Figure 1. The main reason that carbon capture and storage is utilized at large point (industrial) sources is to prevent the further release of large quantities of CO2, thus having a neutral effect on total CO2 concentration in the atmosphere. However, point-source carbon capture systems suffer from several drawbacks, such as equipment corrosion, solvent degradation, and high energy requirements for regeneration9. Direct air capture (DAC) goes beyond emission reduction and can facilitate the removal of CO2 from the atmosphere. The removal of this existing CO2 is necessary to limit continued climate change. DAC is an emerging methodology and must address the difficulties of removing low concentrations of CO2 in atmospheric conditions (400 to 420 ppm), operate in a variety of different environmental conditions, and address the need for cost-effective materials that can be reused many times1,2,3. Significant work is needed to identify materials that meet these requirements, which will accelerate the adoption of DAC and improve its economic feasibility. Most importantly, community consensus on critical parameters of measurement needs to be established, which is essential for benchmark materials to be developed.
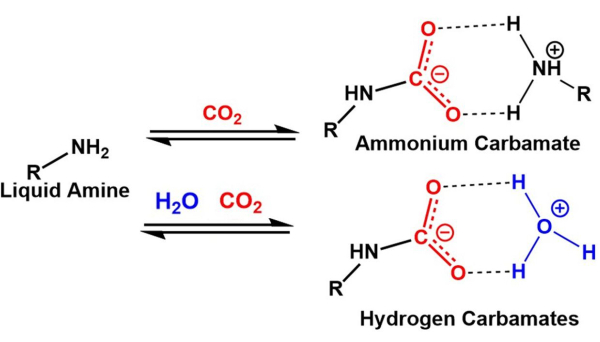
Figure 1: Schematic of the expected liquid amine adsorbent CO2 capture mechanism. The top reaction is in dry conditions, and the bottom reaction is in the presence of moisture. Please click here to view a larger version of this figure.
In an effort to remedy these drawbacks, considerable research and development of novel porous material technology have resulted in a wide array of promising materials that have the potential to be utilized as either capture materials or substrates for DAC. Some examples of such materials include mesoporous silica species10,11,12,13, zeolites14,15, activated carbon16,17, and metal-organic frameworks18. Many solid-supported amine adsorbents also show a higher tolerance to water, which is a vital consideration in CO2 removal through DAC approaches. For DAC applications, researchers must consider wet/dry environmental conditions, hot/cold temperatures, and an overall dilute atmospheric CO2 concentration. Amongst the various substrate materials, silica is commonly used because of its adjustable pore sizes, ability to surface functionalize, and large surface area1,2,3. Typical synthetic procedures and primary features of both impregnated and grafted amine-silica composites are described within this work (Figure 2). Direct synthesis, where the material is made in situ with both components, substrate and amine, is another commonly used methodology2.
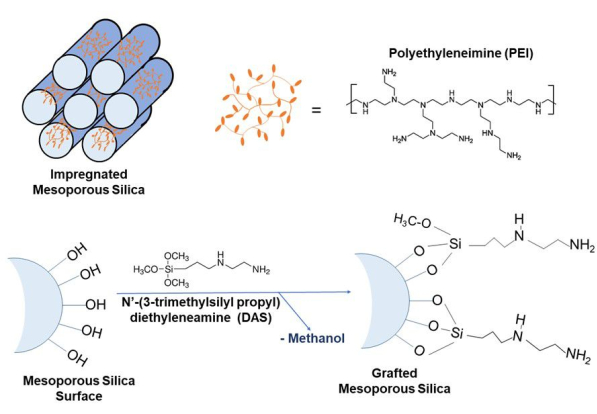
Figure 2: Schematic representations of impregnation. Mixing of PEI and silica substrate in methanol through diffusion (top) and grafted amine-silica composites through covalent tethering (bottom). Please click here to view a larger version of this figure.
Impregnation is a method in which an amine is physically adsorbed onto a surface, in this case, a porous silica medium, through van der Waals forces and hydrogen bonding between the amine and silica surface19, see Figure 2. Solvents such as ethanol and methanol are commonly used to promote the diffusion of the molecules into the porous structure of the substrate material. The solution can also be heated to increase the solubility of the high molar mass polyamines, thereby increasing the homogeneity of amine penetration within the pores. In the case of impregnated materials, the amount of amine introduced to a silica substrate is determined by the initial quantity of the amine and the surface area of the substrate. If the amount of amine introduced exceeds the available surface area of the silica substrate, the amine species will agglomerate on its surface. This agglomeration is readily apparent, as the impregnated material will appear to have a gel-like coating, often yellow, rather than the expected white and powdery appearance1. Among the many types of amine-base solid adsorbents, polyethyleneimine (PEI) and tetraethylene pentamine (TEPA) are the most widely used due to their high stability and high nitrogen content20. For physically impregnated systems, the theoretical loading quantity of amine can be calculated from the pre-weighted amounts of the substrate and the density of the amine. The obvious advantage of physical impregnation lies in the straightforward synthesis procedure to prepare it, as well as the potential for a large amine content due to the high porosity of the silica substrate. Conversely, the stability of the amine within the silica is limited because there is no covalent bonding between the amine and silica support. Therefore, after multiple cycles of CO2 uptake and regeneration through heat or steam, the amine can leach out of the pores. Despite these drawbacks, the implementation of such materials for DAC holds great promise for removing CO2 from the atmosphere.
Another option for the preparation of DAC materials is grafting. Grafting is a method through which amines are immobilized onto a porous silica substrate through a chemical reaction, as shown in Figure 2. This reaction proceeds by reacting an aminosilane with the surface's silanol functional group, resulting in a covalent bond. Therefore, the number of functional groups on the surface of the silica substrate impacts the grafted amine density21,22. Compared to amine-impregnated adsorbents, chemical grafting methods have had lower CO2 adsorption capacity mainly due to the low amine loading21. Conversely, chemically-grafted amines have increased thermal stability due to their covalently bound structure. This stability can be useful in the regeneration of the material as adsorbents (such as grafted silica) are heated and pressurized to remove the captured CO2 for reuse to save material and cost. In a typical synthesis procedure, the mesoporous silica substrate is dispersed in a solvent (e.g., anhydrous toluene), which is then followed by the addition of aminosilanes. The resulting sample is then washed to remove unreacted aminosilanes. Improvements in aminosilane density are reported to have been achieved through water addition, specifically with SBA-15, to expand pore size23. The procedure for grafting that will be described herein uses moisture-sensitive techniques. Therefore, additional water will not be used. Implementation of grafted aminosilane materials for DAC is promising due to their expected stability during CO2 adsorption and desorption processes. However, the major drawbacks of this methodology include the complex reactions/preparation of these materials, leading to increased cost, and their overall low CO2 adsorption capacity, meaning larger quantities are required.
Overall, results of many previous studies indicate that the structure of the substrate and amine-related modification has a significant impact on the adsorption performance with specific studies utilizing techniques such as transmission electron microscopy (TEM) and quasi-elastic neutron scattering (QENS) to fully characterize these materials24,25. In other words, the structural properties (e.g., porosity and surface area) of the substrate material determine the amine loading, so increasing these parameters can improve CO2 capacity24,25. Continued research into the optimization and design of substrate materials and preparation processes is critical to the development of high-performance adsorbents for DAC. The goal of this work is to provide guidance on impregnation and grafted amine synthesis in hopes of facilitating better transparency of synthetic techniques. Within the literature, specific details on the amounts of solvent, substrate, and amines are not always described, making it difficult to understand the correlation between experimental loading amounts and quantitative measurements of amine-silica composites. The exact loading amounts and a detailed description of the experimental procedures will be provided herein to better facilitate these types of comparisons.
Protocol
NOTE: Details related to the equipment, instrumentation, and chemicals used in this section can be found in the Table of Materials.
1. Impregnation of silica with polyethyleneimine of 800 g/mol molar mass (PEI 800)
- Preparation of reaction
- Use anhydrous methanol as the solvent in this reaction. It has a low boiling point; thus, its volatility facilitates its later removal at lower temperatures.
NOTE: Anhydrous solvent is important because water can prevent PEI 800 from entering the pores of the silica support. Another solvent commonly used is ethanol, which has a higher boiling point and requires longer drying times and higher drying temperatures. - Calculate the mass fraction (%) of amine using equation 1, where mamine = mass of amine, msilica = mass of silica used.
Equation 1: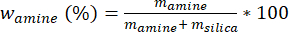
- Mass fraction of amine (wamine) in MCM-41 silica will be 59.9 % (750 mg of amine and 500 mg of silica). For every 1 g of amine, use 10 mL of anhydrous methanol. This is done so that the overall mixture is a dilute slurry. These calculated amounts will be classified as experimental (wamine_exp) and further classified for each synthetic methodology (e.g., wamine_exp_imp (impregnated) and wamine_exp_graft (grafted)).
- To ensure that all glassware is devoid of moisture, place them in an oven at 140 °C for at least 1 h prior to use.
- Use anhydrous methanol as the solvent in this reaction. It has a low boiling point; thus, its volatility facilitates its later removal at lower temperatures.
- Preparation of silica support
NOTE: MCM-41 silica is the solid substrate utilized in this process. Since MCM-41 is adsorbent silica, it is expected to adsorb water from the atmosphere or during manufacture.- Dry MCM-41 silica to ensure that no water has adsorbed into its pores. Place the desired amount of silica in a glass Petri dish, cover it with punctured aluminum foil, and then place it into a vacuum oven.
- First, apply the vacuum (typically less than 3 kPa, which changes based on each individual vacuum system), then set the oven to a temperature of about 110 °C to ensure water removal. Perform this step for a minimum of 2 h before proceeding with synthesis.
- Impregnation methodology
- Use a clean, dry laboratory spatula and transfer the desired amount (750 mg) of polyethyleneimine (PEI) into the reaction vessel (in this case, a 35 mL dry vial). Cap the reaction vessel when in transport.
- Transfer the reaction vessel into a chemical fume hood, clamp or secure it within the hood, and place it over a stir plate. Remove the lid of the reaction vessel.
- Place a clean, dry stir bar into the reaction vessel.
NOTE: Using a stir bar will ensure even mixing, can allow the solution to be stirred for longer durations, facilitate better dispersion, and can enable safe heating of the reaction without the need for manual mixing. - Using a pipette, add 7.5 mL of anhydrous methanol (for every 1 g of amine, use 10 mL of methanol) from a graduated cylinder. Turn on the stir plate. Allow the solution to mix for 15 min to ensure the PEI is fully dissolved and homogeneously dispersed within the solvent.
NOTE: After mixing, the solution will appear clear/transparent, denoting complete polymer dissolution. - Use a clean, dry laboratory spatula to transfer the desired amount (500 mg) of pre-dried silica (in this case, MCM-41) onto weighing paper. Transfer the silica into the reaction vessel inside the fume hood.
NOTE: This experimental loading amount of amine will match the actual measured amount by thermogravimetric analysis (TGA).
CAUTION: Breathing silica dust can damage lung tissue. It is recommended to wear an N95 respirator when working with silica substrates (consult local safety guidelines for appropriate choices for an individual laboratory) and work in a chemical exhaust hood. These silica materials often exhibit "static cling" properties and are easily dispersed within the fume hood. Perform this step quickly to avoid moisture adsorption onto the silica from the air. - Add additional methanol to rinse the silica into the vessel to ensure full exposure to PEI within the solution if needed. The mixture will appear as a slurry; see Figure 3.
- Place the vessel into a silicone oil bath, a heating block, or a heating mantle at 40 °C to 50 °C to ensure full solubility of PEI, homogeneous mixing, and to encourage amine loading into the porous silica.
NOTE: Elevated temperatures are not always utilized during impregnation procedures, and literature has shown that others have mixed at room temperature (RT)1,2,3. In this protocol, heating is utilized to facilitate homogeneous mixing. - Ensure that the stir bar is evenly mixing the solution. Allow the solution to stir under heat for about 1 h.
NOTE: Depending on reaction size and individual preference, the selection of reaction vessel may vary. Therefore, the way the reaction is heated (oil bath, heating block, or heating mantle) may vary to best accommodate the choice of the reaction vessel. - Remove the reaction vessel from the heat source and allow it to cool to RT while still stirring. When fully cooled, stop stirring and remove the stir bar.
- Put the vessel containing the sample under vacuum on a Schlenk line (typically <3 kPa, reduce the pressure slowly to avoid bumping).
- Let the reaction vessel remain on the Schlenk line until all solvent is visibly removed. Then, transfer the sample to a different storage container of choice, such as a glass Petri dish.
- Then place the sample in a vacuum oven, turn on the vacuum (typically <1.3 kPa), then set the oven to approximately 70 °C. Allow the sample to dry under vacuum for at least 18 h to ensure that a sufficient amount of methanol has been removed.
NOTE: Consider the level of solvent before placing the container of choice in a vacuum oven due to the risk of the sample and solution erratically leaving the vessel due to the vacuum. Typically, no more than 1 mL of solvent remains within the sample/ container before placing it in the vacuum oven. - After drying, the materials appear white and powdery. Store it in a moisture-free, air-free environment until needed for further use.
NOTE: This step can be in a vacuum desiccator or glove box that is prepared with an air and moisture-free environment. See Figure 4 for the expected end-product appearance.
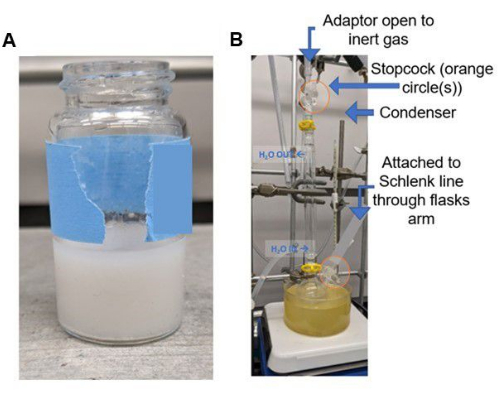
Figure 3: Representative images of reactions. (A) Photograph of PEI-silica slurry (in methanol) during PEI impregnation prior to transfer to a heating block and (B) apparatus for grafting of DAS after heating for 6 hours is completed. Please click here to view a larger version of this figure.
Figure 4: Representative appearance of end products after drying. (A) PEI impregnation at wamine_exp_imp = 59.9 %. (B) grafted DAS at wamine_exp_das = 90.0 %. Please click here to view a larger version of this figure.
2. Preparation of wet grafted silica with N'-(3-trimethylsilyl propyl) diethyleneamine (DAS)
- Preparation of solution
- Use anhydrous toluene in this reaction. It has a high boiling point (110 °C), thus allowing for high-temperature mixing. Aminosilane (N'-(3-trimethylsilyl propyl) diethyleneamine (DAS) is highly soluble in this medium.
NOTE: Performing this reaction in anhydrous conditions is important because water can interact with aminosilanes to change the nature of its bonding to the silica surface. The anhydrous toluene used comes with a septum-capped lid. Therefore, a gas-tight syringe will be used to transfer solvent into the reaction vessel. For every 1 g of DAS, 5 mL of toluene is used. Thus, for 5 mL of DAS (1.028 g/mL), 25 mL of solvent is used.
- Use anhydrous toluene in this reaction. It has a high boiling point (110 °C), thus allowing for high-temperature mixing. Aminosilane (N'-(3-trimethylsilyl propyl) diethyleneamine (DAS) is highly soluble in this medium.
- Preparation of silica support
- Dry the silica using the procedure described above in step 1.2.
- Preparation of siloxane
- The aminosilane is moisture sensitive, as the presence of water can cause polymerization to occur. Thus, handle the reaction as a moisture-free reaction. Store DAS within a septum-capped lid bottle, and use a gas-tight syringe for transfer.
CAUTION: There are many health risks and hazards associated with aminosilanes. Review the safety data sheet prior to beginning the experiment and observe all recommended safety precautions.
- The aminosilane is moisture sensitive, as the presence of water can cause polymerization to occur. Thus, handle the reaction as a moisture-free reaction. Store DAS within a septum-capped lid bottle, and use a gas-tight syringe for transfer.
- Grafted silica methodology
- It is important to note that, unlike the impregnation methodology, aminosilanes are expected to have low grafting nitrogen content on the silica substrate. Therefore, in this reaction, load wamine_exp_graft = 90.0 % of DAS experimentally to increase the probability of aminosilane locating silanol groups on the silica support and covalently bonding successfully.
- Dry all glassware in the oven for at least 2 h before use to ensure a moisture-free surface.
- Fill a round bottom Schlenk flask equipped with a magnetic stir bar with the desired amount (500 mg) of silica support (MCM-41).
- Insert a rubber septum into the reaction vessel and cycle the reaction vessel on a Schlenk line three times to remove air and moisture. Do this by opening the reaction vessel's stopcock to vacuum for about 30 s, closing the stopcock, switching to an inert gas (either N2 or Ar2) for about 30 s, then reopening the stopcock. After the reaction vessel has been cycled, maintain an inert gas environment for the following procedural steps.
- Insert a line of inert gas into the septum-capped lid (sure-seal) bottle, then use the gas-tight syringe and purge the syringe with inert gas before removing the desired amount of anhydrous toluene (in this case, 25 mL).
NOTE: See Figure 5 for an image of the sure-seal container with an inert gas inlet and a gas-tight syringe. The bend (blue arrow) is placed in the tube before transfer to prevent any drippage. This technique is used anytime gas-tight syringing of a liquid is needed. The amount of solvent is dictated by the amount of aminosilane added. For every 1 mL of aminosilane, use 5 mL of anhydrous toluene to ensure solubility. It is important to fill the syringe with 25 mL of toluene and then raise the needle above the solution level within the bottle. Then draw some inert gas from the headspace above the toluene before removing the syringe from the toluene container. - Ensure that the magnetic stir bar inside the reaction vessel is stirring smoothly before beginning this step. Transfer the anhydrous toluene contained in the gas-tight syringe by puncturing the septum on the reaction vessel and releasing the toluene into the vessel.
- Remove the needle with inert gas.
- Repeat the same steps (2.4.6 to 2.4.8) with the aminosilane (4.8 mL of DAS).
- Use an adaptor to attach a line from the Schlenk line to a water condenser using vacuum grease. Wrap the bottom of the condenser apparatus with polytetrafluoroethylene (PTFE) tape (this step ensures no contamination by grease). Then attach the condenser apparatus to the round bottom Schlenk flask to prepare the glassware set-up; see Figure 3.
- Attach 'cold' water lines to the water condenser and turn it on.
NOTE: The 'cold' water (below 23 °C) will travel into the bottom of the condenser and out the top into a sink. The tubes will be secured (with wires, zip ties, or steel hose clamps) to avoid leakage of water at the connection sites. - Lower the reaction vessel into a silicone oil bath or heating block, or place it in a heating mantle between 80 °C to 100 °C. This temperature is selected to help facilitate the grafting of aminosilane (DAS), homogeneous mixing, and encourage amine loading.
- Close the stopcock to the inert gas on the round bottom Schlenk flask and leave the stopcock on the condenser open; see Figure 3B.
NOTE: This step is performed to prevent toluene from rising into the tube located close to the apparatus (Schlenk flask side arm) while keeping the reaction under an inert atmosphere due to the inlet at the top of the condenser; see Figure 3 for this set-up. - Ensure the stir bar is evenly mixing the solution. Stir while heating for 6 h.
- Allow the reaction vessel to cool to RT. Use vacuum filtration to capture the solid grafted-amine silica on the filter paper and rinse with copious amounts of anhydrous toluene (3 times with 10 mL).
- To vacuum filter, equip an Erlenmeyer filer flask with arm to vacuum via a hose. Place a rubber bung at the opening, place Buchner funnel on top of the rubber bung, and lastly place a filter paper within the Buchner funnel. Wet the filter paper with anhydrous toluene.
- Turn on the vacuum and quickly dispense the solution onto the filter paper. It helps to rinse the reaction vessel with the anhydrous toluene before pouring it onto the filter paper during washes.
- The final material appears white on the filter paper. Remove the grafted silane material from the filter paper using a clean, dry laboratory spatula and place it in a vial.
- Cover the vial with punctured aluminum foil and place it in a vacuum oven. Turn on the vacuum. Set the oven to about 100 °C and allow to dry for approximately 18 h to remove excess toluene.
NOTE: The materials appear white and powdery after drying and are stored in a moisture-free, air-free environment. This can be in a vacuum desiccator or glove box that is prepared with an air and moisture-free environment. See Figure 4 for end-product appearance. - This procedure is repeated two more times (total 3 times, steps 2.4.1 – 2.4.16).
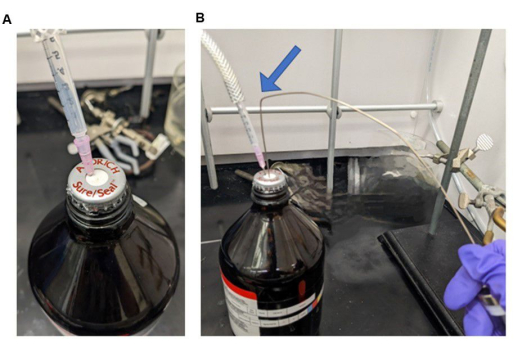
Figure 5: Photographs of a sure-seal container. (A) Container with a needle connected to an inert gas (N2 or Ar2) and (B) with inert gas connected and gas tight syringe attached, with 'bent' needle (blue arrow), that will be used to transfer without leakage. Please click here to view a larger version of this figure.
3. Analysis of silica-composite materials by TGA
NOTE: The standard uncertainty associated with this measurement is approximately ± 0.01 % in mass and ± 1 °C in temperature.
- Utilizing the instrument's application software for the TGA, tare an empty pan.
- Remove the tared pan from the sample loading area and add the specimen to the pan. Place the specimen in the center of the pan and use at least 2 mg to ensure adequate resolution of mass loss. Place the pan with the specimen back in the loading area.
- Using the instrument software, customize a procedural run that first equilibrates at approximately 50 °C for 5 min in a 100 % N2 environment with a gas flow rate of 60 mL/min. Then set a ramp of 2 °C/min to 5 °C/min to 1000 °C. Mark the end of the cycle. These measurements are denoted as wamine_TGA as they evaluate the real amine content within the material using TGA. This is further classified for each synthetic methodology (e.g., wamine_TGA_imp (impregnated method) and wamine_TGA_graft (grafted method)).
NOTE: Specific recommendations for flow rates may differ for individual TGAs. Consult the manufacturer's specifications before selecting the appropriate flow rate for an individual experiment. - Repeat steps 3.1-3.3 for any additional experimental runs.
- Apply step 3.1 for the CO2 adsorption experiment setup.
- Using the instrument software, customize a procedural run that first equilibrates at 100 °C for 5 min, then ramps at 20 °C/min to 40 °C. Then, apply an isothermal hold at 40 °C for 10 min before introducing a blended gas of 5 % CO2 in N2, 60 mL/min flow rate.
- Keep the sample at 40 °C under this gas mixture condition for 100 min. This procedure is performed to measure CO2 adsorption by weight gain. These measurements are denoted as wCO2 as they evaluate the CO2 adsorption within the material. This is further classified for each synthetic methodology (e.g., wCO2_imp (impregnated method) and wCO2_graft (grafted method)).
- For cycle studies, using the instrument software, customize a procedural run that first opens to 100 % N2 gas, isothermal hold for 5 min, before ramping at 20 °C/min to 105 °C, and isotherm hold for 5 min.
- Next, ramp down at 10 °C/min to 40 °C, and isotherm hold for 1 min before a blend of 5 % CO2 in N2 is released, and isotherm hold for 35 min. Repeat the procedural steps 10 times.
- Within the software, append this run as many times as desired to add extra cycle steps. Be sure not to change the pan number and to remove the weight stabilization step for the appended runs after the first run. This allows the user to place multiple 10-cycle runs together in a method.
4. Analysis of silica-composite materials by Fourier transform infrared spectroscopy (FTIR) using an attenuated total reflectance (ATR) accessory
NOTE: Standard uncertainties associated with this instrument are ± 1 % in peak intensity and ± 4 cm−1 in wavenumber, therefore, the uncertainty in intensity in the reported curve is ± 1.4 % using a linear propagation of uncertainties.
- Clean the window (diamond) on the FTIR-ATR accessory with a low-lint wipe and methanol.
- Collect a background spectrum using the basic measurement window of the software.
- Using a clean and dry spatula, place the sample over the FTIR-ATR window. Use the ATR compression probe to push the sample into contact with the window.
- Collect a sample spectrum by pressing the Collect Sample button in the basic measurement window, and load the associated background from the file obtained in step 4.2.
- Repeat steps 4.1 to 4.4 for all samples.
5. Analysis of silica-composite materials before and after impregnation and grafting of amines by scanning electron microscopy (SEM)
- Mount samples in powder form on aluminum stubs by carefully spreading them onto carbon conductive dual-sided tape. A stereo microscope aids in this procedure by increasing the visibility of the sample spread.
- Sputter coat each sample with a 5 nm gold-palladium (Au-Pd) conductive coating for optimal imaging conditions.
- Image the surface morphology of the substrate silica material before and after impregnation or grafting on a dual-beam, field emission SEM under high vacuum (i.e., less than 0.4 mPa, 3 x 10−6 torr).
NOTE: The chosen beam energy (1 keV) and probe current (6.3 pA and 25 pA) parameters were optimized for clear images with minimal charging, artifacts, and drift.
Representative Results
TGA is commonly used to quantify the amount of amine loaded or grafted to the silica surface for these materials. The obtained TGA curves show a loss of residual solvent and water between 60 °C to 100 °C, which is shown in the derivate weight (weight %/°C) curve as the first peak, and a loss of amine, which is shown in the derivate weight curve (weight %/°C) as the second peak. For PEI-impregnated silica, this loss of amine is expected to appear around 200 °C to 300 °C, which appears as the second peak in the derivate weight curve, and for DAS grafted silica, the loss of amine is expected to appear around 350 °C to 550 °C (Figure 6). The overall weight loss is indicative of the amount of amine loaded or grafted onto the silica substrate and is an important characterization parameter for judging the quality of the synthesis. For PEI-impregnated specimens, wamine by TGA (wamine_TGA_imp) = 59.2 % ± 0.3 % (n = 3) in contrast to the experimental (wamine_exp_graft)= 59.9 % (Figure 6B). For DAS-grafted specimens, wamine_TGA_graft = 22.3 % ± 0.1 % (n = 3) in contrast to wamine_exp_graft = 90.0 % when the synthesis is repeated three times (Figure 6A).
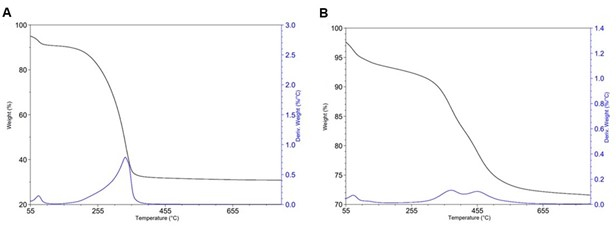
Figure 6: TGA. (A) PEI impregnated at wamine_exp_imp = 59.9 % observed, wamine_TGA_imp = 59.2 % ± 0.3 % (n = 3), and (B) Grafted materials at wamine_exp = 90.0 % observed, wamine_TGA = 22.3 % ± 0.2 % (n = 3). Please click here to view a larger version of this figure.
In Figure 7, overall CO2 adsorption was measured using TGA at 5 % CO2 in N2, a 60 mL/min flow rate, and held at 40 °C for 60 min. In Figure 7A, CO2 adsorption curves are shown for PEI-impregnated specimens with an average CO2 percent adsorption by weight (wCO2_imp) of 6.16 % ± 0.2 % (n = 3). In Figure 7B, CO2 adsorption curves are shown for DAS-grafted specimens with wCO2_graft = 2.03 % ± 0.04 % (n = 3). Within these TGA curves, the baseline is corrected to begin at 100 %.
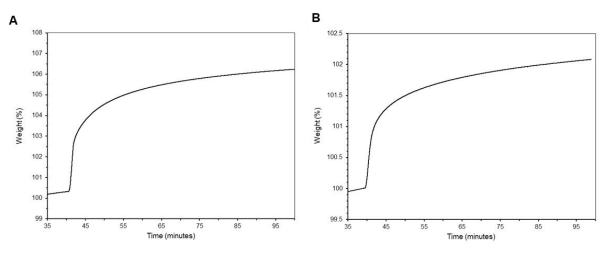
Figure 7: TGA curves of adsorption of CO2. (A) Impregnated PEI MCM-41 specimen wCO2_imp= 6.16 % ± 0.3 % (n = 3). (B) Grafted DAS MCM-41 specimen wCO2_graft = 2.03 % ± 0.04 % (n = 3). Please click here to view a larger version of this figure.
FTIR-ATR spectroscopy is a type of vibrational spectroscopy commonly used to understand the chemical structure of a material. Figure 8 shows FTIR-ATR spectra of neat PEI, DAS, and MCM-41 as compared to PEI-impregnated or DAS-grafted MCM-41 materials. Distinct peaks ranging from 2500 cm-1 to 3600 cm-1 are attributed to the amine-based N-H signals expected from the aminated materials. When comparing the spectra of the impregnated and grafted materials, a decrease in peak intensity is observed, which is attributed to a lower quantity of amine in the grafted material. Strong peaks corresponding to Si-O-Si range from 400 cm-1 to 1200 cm-1 can be seen within the spectra. The spectra presented corrected for attenuated total reflectance and then automatically baseline corrected through the instrument's software.
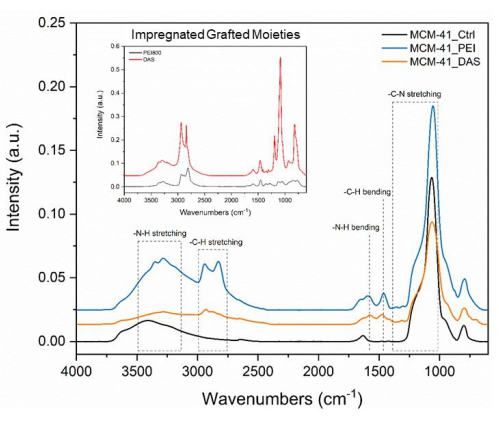
Figure 8: FTIR spectra. Representative FTIR spectra for neat PEI, MCM-41, DAS, DAS grafted specimen and PEI-impregnated material specimen. Please click here to view a larger version of this figure.
In Figure 9, SEM micrographs of unaltered MCM-41 are compared with MCM-41 impregnated with PEI at wamine_TGA_imp = 59.2 % and MCM-41 grafted with DAS at wamine_TGA_graft = 22.3 % for morphological and surface differences.
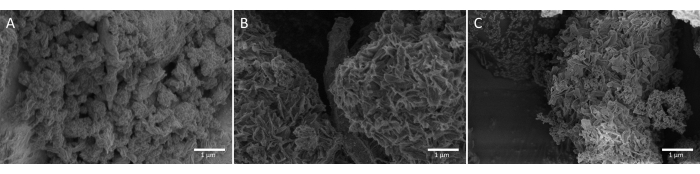
Figure 9: SEM images. (A) PEI impregnated MCM-41, (B) neat MCM-41, and (C) DAS grafted MCM-41. Please click here to view a larger version of this figure.
In Figure 10 and Figure 11, neat starting materials such as MCM-41, DAS, and PEI 800 are measured using TGA for baseline reference of weight loss and CO2 adsorption. In Figure 10A, neat MCM-41 TGA curves can be seen as a slow, gradual loss of weight but with no clear peaks in the derivate weight (weight %/°C). In Figure 10B, neat PEI 800 TGA curves with a total weight loss ranging from 200 °C to 370 °C can be seen.
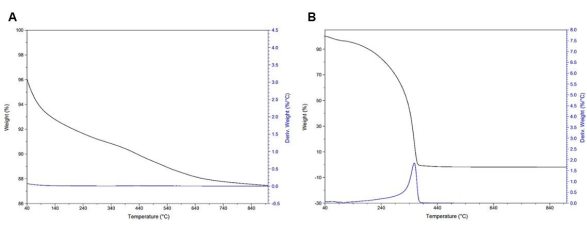
Figure 10: TGA curves. (A) Neat MCM-41 wMCM41 = 8.58 % ± 0.5 % (n =3 ). (B) PEI 800 molecular weight wPEI = 98.9 % ± 0.9 % (n = 3).. Please click here to view a larger version of this figure.
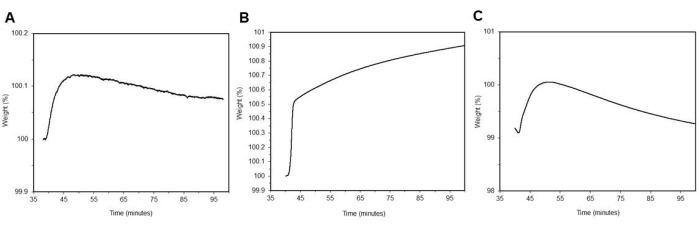
Figure 11: TGA curves. (A) Adsorption of CO2 of neat MCM-41 wCO2 = 0.223 % ± 0.2 % (n = 3). (B) Adsorption of CO2 of neat PEI 800 wCO2 = 0.879 % ± 0.3 % (n = 3). (C) Adsorption of CO2 of neat DAS wCO2 = 0.247 % ± 0.1 % (n = 3). Please click here to view a larger version of this figure.
In Figure 11A, CO2 adsorption curve are shown for neat MCM-41 with minimal CO2 adsorption wCO2 = 0.222 % ± 0.2 % (n = 3). In Figure 11B, neat PEI 800's CO2 adsorption curve is shown with minimal CO2 adsorption wCO2 = 0.879 % ± 0.3 % (n = 3). In Figure 11C, a CO2 adsorption curve is shown for neat DAS with minimal CO2 adsorption wCO2 = 0.247 % ± 0.1 % (n = 3). Note the adsorption for the neat materials is low because the CO2 adsorption is only occurring at the surface that is exposed to the blended gas environment. Notably, DAS is air and moisture sensitive and is exposed to air during the loading process into the TGA, which may affect its capacity to adsorb CO2.
In Figure 12, CO2 adsorption and desorption cycle curves are shown for impregnated MCM-41 (Figure 12A), and grafted MCM-41 (Figure 12B). Within this protocol, the sample is first activated by heating to 105 °C under 100 % N2 for 5 min, then ramped down to 40 °C and held for 1 min before a blend of 5 % CO2 in N2 is administered for 35 min. This procedure is then repeated (Figure 12C). The figure shows ten repeat cycles with decreasing adsorption capabilities of the material as time progresses. Data can be accessed through the Management of Institutional Data Assets (MIDAS) at https://doi.org/10.18434/mds2-3017.
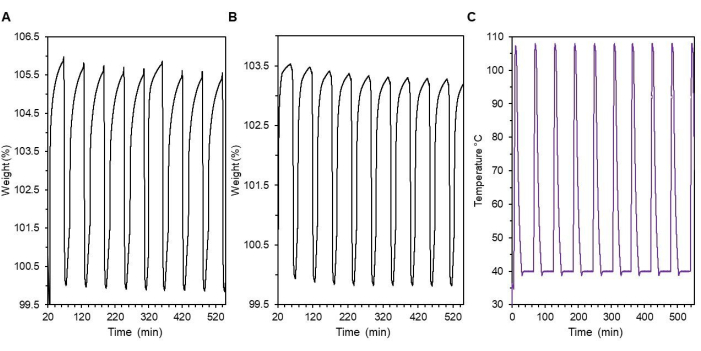
Figure 12: Cyclic TGA adsorption and desorption study. (A) PEI impregnated MCM-41, (B) DAS grafted MCM-41, and (C) temperature profile of the procedural runs for (A) and (B). Please click here to view a larger version of this figure.
Discussion
The methods described herein are intended to provide a protocol for preparing impregnated and grafted amine silica-composite adsorbents. The procedures we have documented are based on review of techniques reported in the literature and those refined in our laboratory.1,2,3. Preparation of these materials is useful within the field of carbon dioxide removal research to develop or benchmark other materials that may be used to lower CO2 emissions either in the atmosphere (direct air capture) or in industrial processes (point source capture). Among solid-amine adsorbents, mesoporous silica is commonly used. Silica substrates tend to have simple syntheses or can be purchased commercially, with structural properties that make them a good choice for the impregnation or grafting of amine-based solid adsorbents21. Within this procedure, MCM-41 is used as the silica substrate due to its large surface area and narrow pore size distribution between 35 Å and 38 Å. However, another commonly studied silica support is SBA-15, which is also utilized in carbon capture research due to its large surface area, pore volume, and uniform mesopore size24. Systematic studies utilizing MCM-41 and PEI via impregnation have shown an increase in CO2 adsorption with an increase in PEI loading amount26. Prior results detailing the weight loss for impregnated MCM-41 silica composites are given by Xu and coworkers26, and for grafted silica composites by Sousa and coworkers27, who experimented with H2S and H2O effect on CO2 capture. Xu and coworkers26 found that their MCM-41 at 50 % (wt %) loading of PEI has an adsorption capacity of 112 mg CO2/g-adsorbent. Weight loss TGA of these impregnated materials is also presented within the manuscript. Comparatively, the MCM-41 59.9 % (wt %) impregnated PEI within this manuscript has a capacity of 61.6 mg CO2/g-adsorbent. While this appears to be much less than the reported amount, consider that the parameters of measurement are different – Xu and coworkers heated the sample to 75 °C, had a 99.8 % blend of CO2 in N2, and a 100 mL/min flow rate for 150 min. The increased temperature and nearly 100 % CO2 experimental conditions likely both aided in the adsorption of CO2. This highlights the challenges in comparing studies in which experimental procedures differ.
Even for a seemingly simple method such as impregnation, the selection of solvent, silica/solvent concentration, amine/solvent concentration, stirring/mixing method, temperature, and mixing time vary greatly in the literature. This paper is intended to describe one method of making these materials, but each individual researcher can make selections that are appropriate for their own research goals. Additionally, these methodologies are adaptable to other adsorbent substrates for carbon capture. It is our intent to help facilitate research into materials for carbon dioxide removal by providing a starting point for such work with this method.
Many studies have conveyed that the location of the amines within the structure of a silica composite can change for impregnated versus grafted amines. Impregnated materials loaded with PEI have higher degrees of functionalization (i.e., amine content) on the surface, which can prevent CO2 diffusion into the pores1,2,3,24. Pore blockage has been linked to colder reaction temperature conditions, higher amine loading, and steric hindrance, consequently decreasing the accessibility of amine sites and adsorption capabilities28.
Grafted materials have been shown to have amines located inside pore channels, and thus CO2 diffusion can readily occur through the whole structure, maximizing capture efficiency24,25. Comparing grafted amine silica-composites to one another poses a difficulty due to the variation in amine content both in the grafted material and within the aminosilane.
However, comparability between studies within the "same material(s)" still has significant limitations, such as variations in (1) synthesis, (2) materials, and (3) measurement. First, while many studies and review articles describe the preparation of impregnated or grafted silica materials, there tend to be varied protocols for synthesis with no clear guidelines as to the most critical steps in the synthesis. Second, there are variations in performance between silica substrates sourced from external suppliers and those prepared through synthetic routes utilized during these protocols. With variations in PEI molar masses, amine content in aminosilanes, and grafted or loaded percentages, there poses difficulty in comparing measured CO2 adsorption capacities between these varied materials. Third, CO2 adsorption capacity can be measured using many different types of commercial or specially engineered measurement tools, all of which have different associated measurement uncertainties. The overall percentage of CO2 within the gas source, the flow rate of gas, the selection of activation, adsorption, desorption temperatures, and the humidity within the gas source can vary between individual studies and individual instruments. All such experimental parameters are important to consider when comparing measured adsorption capacities for individual materials.
While this procedure focuses on silica substrates, the overall goal of eliminating carbon emissions and reducing atmospheric CO2 concentration is a complex, multifaceted problem that will require material innovations to tackle, consensus on measurement parameters to report, and a clear indication of critical procedural steps. Continued research to investigate novel solid-support adsorbents with CO2 adsorbent functionality is thus increasingly important to meet stated climate goals. This article highlights the need for a community consensus on relative parameters and demonstrates the need for benchmark materials.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
Charlotte M. Wentz would like to acknowledge funding through NIST Award # 70NANB8H165. Zois Tsinas would like to acknowledge funding through NIST Award # 70NANB22H140.
Materials
| Anhydrous methanol | Sigma-Aldrich | 322415 | Does not come with sure-seal |
| Anhydrous toluene | Sigma-Aldrich | 244511 | Comes with sure-seal |
| Ceramic Stirring Hot Plate | NA | NA | The size, watage, and thermal capabilities of the stirr plate will differ depending on individual lab facilities. |
| Fourier Transform Infrared Spectroscopy (FTIR) | Nicolet i550 series spectrometer | NA | Run on OMNIC standard software |
| Gastight syringe | NA | NA | As long as the gas tight syringe has a PTFE plunger and luer tip, is suited for air sensitive technique and can be used in this protocol. |
| Glass vial | NA | NA | As long as the vial is made if borosilicate glass and has a screw based cap the brand name, size, or general shape does not matter for the protocol. |
| MCM-41 silica | ACS Material | MSM41A01 | Cas no. 7631-86-9 |
| Metal needle | NA | NA | Syringe needles need to be stainless steel. It is recommended to determine length and outerdiameter of needle by what will be transferred using the gas tight syringe. For large quantities of liquid a larger outer diameter will improve transfer rates. |
| N’-(3-trimethylsilyl propyl) diethyleneamine (DAS) | Sigma-Aldrich | 104884 | Comes with sure-seal |
| Polyethyleneimine (PEI) | Sigma-Aldrich | 408719 | Does not come with sure-seal |
| Schlenk round bottom flask | ChemGlass AirFree | NA | As long as the flask is suited for high pressure and temperture but the brand name, size, or general shape does not matter for the protocol |
| Thermogravemetric Anlysis (TGA) | TA Advantage | NA | 550 series from Waters and TA Instruments |
Referenzen
- Zhu, X., et al. Recent advances in direct air capture by adsorption. Chemical Society Reviews. 51 (15), 6574-6651 (2022).
- Zhao, P., Zhang, G., Yan, H., Zhao, Y. The latest development on amine functionalized solid adsorbents for post-combustion CO2 Capture: Analysis review. Chinese Journal of Chemical Engineering. 35 (8), 17-43 (2021).
- Chen, D., Zhang, S., Row, K. H., Ahn, W. -. S. Amine-silica composites for CO2 capture: A short review. Journal of Energy Chemistry. 26 (5), 868-880 (2017).
- Nie, L., Mu, Y., Jin, J., Chen, J., Mi, J. Recent developments and considerations issues in solid adsorbents for CO2 capture from flue gas. Chinese Journal of Chemical Engineering. 26 (11), 2303-2317 (2018).
- Nithyashree, N., Manohara, G. V., Maroto-Valer, M. M., Garcia, S. Advanced high-temperature CO2 sorbents with improved long-term cycling stability. American Chemical Society Applied Material Interfaces. 12 (30), 33765-33774 (2020).
- Song, C., et al. Alternative pathways for efficient CO2 capture by hybrid processes-A review. Renewable and Sustainable Energy Review. 82, 215-231 (2018).
- Rochelle, G. T. Amine scrubbing for CO2 capture. Science. 325 (5948), 1625-1654 (2009).
- Vaidye, P. D., Kenig, E. Y. CO2-alkanolamine reaction kinetics: A review of recent studies. Chemical Engineering & Technology. 30 (11), 1467-1474 (2007).
- Veawab, A., Tontiwachwuthikul, P., Chakma, A. Corrosion behavior of carbon steel in the CO2 adsorption process using aqueous amine solutions. Industrial & Engineering Chemical Research. 38 (10), 3917-3924 (1999).
- Chen, S., Bhattacharjee, S. Trimodal nanoporous silica as a support for amine-based CO2 adsorbents: Improvement in adsorption capacity and kinetics. Applied Surface Science. 396, 1515-1519 (2017).
- Jiao, J., Cao, J., Xia, Y., Zhao, L. Improvement of adsorbent materials for CO2 capture by amine functionalized mesoporous silica with worm-hole framework structure. Chemical Engineering Journal. 306, 9-16 (2016).
- Guo, X., Ding, L., Kanamori, K., Nakanishi, K., Yang, H. Functionalization of hierarchically porous silica monoliths with polyethyleneimine (PEI) for CO2 adsorption. Microporous and Mesoporous Materials. 245, 51-57 (2017).
- Fatima, S. S., Borhan, A., Ayoub, M., Ghani, N. A. Development and progress of functionalized silica-based adsorbents for CO2 capture. Journal of Molecular Liquids. 338, 116913 (2021).
- Cheng, J., Liu, M., Hu, L., Li, Y., Wang, Y., Zhou, J. Polyethyleneimine entwine thermally-treated Zn/Co zeolitic imidazolate frameworks to enhance CO2 adsorption. Chemical Engineering Journal. 364, 530-540 (2019).
- Zagho, M. M., Hassan, M. K., Khraisheh, M., Al-Maadeed, M. A. A., Nazarenko, S. A review on recent advances in CO2 separation using zeolite and zeolite-like materials as adsorbents and fillers in mixed matrix membranes (MMMs). Chemical Engineering Journal Advances. 6, 100091 (2021).
- Wang, J., Wang, M., Zhao, B., Qiao, W., Long, D., Ling, L. Mesoporous carbon-supported solid amine sorbents for low-temperature carbon dioxide capture. Industrial & Engineering Chemistry Research. 52 (15), 5437-5444 (2013).
- Ünveren, E. E., Monkul, B. O., Sarioğlan, S., Karademir, N., Alper, E. Solid amine sorbents for CO2 capture by chemical adsorption: A review. Petroleum. 3 (1), 37-50 (2017).
- Demir, H., Aksu, G. O., Gulbalkan, H. C., Keskin, S. MOF membranes for CO2 capture: Past, present and future. Carbon Capture Science & Technology. 2, 100026 (2022).
- Xu, X., Song, C., Andresen, J. M., Miller, B. G., Scaroni, A. W. Novel polyethylenimine-modified mesoporous molecular sieve of MCM-41 type as high-capacity adsorbent for CO2 capture. Energy & Fuels. 16 (6), 1463-1469 (2002).
- Gelles, T., Lawson, S., Rownaghi, A., Rezaei, F. Recent advances in development of amine functionalized adsorbents for CO2 capture. Adsorption. 26 (94), 5-50 (2020).
- Rao, N., Wang, M., Shang, Z., Hou, Y., Fan, G., Li, J. CO2 adsorption by amine-functionalized MCM-41: A comparison between impregnation and grafting modification methods. Energy Fuels. 32 (1), 670-677 (2018).
- Anyanwu, J. T., Wang, Y., Yang, R. T. Amine-grafted silica gels for CO2 capture including direct air capture. Industrial & Engineering Chemistry Research. 59 (15), 7072-7079 (2020).
- Anyanwu, J. -. T., Wang, Y., Yang, R. T. CO2 capture (including direct air capture) and natural gas desulfurization of amine-grafted hierarchical bimodal silica. Chemical Engineering Journal. 427 (14), 131561 (2022).
- Sanz, R., Calleja, G., Arencibia, A., Sanz-Pérez, E. S. Amino functionalized mesostructured SBA-15 silica for CO2 capture: Exploring the relation between the adsorption capacity and the distribution of amino groups by TEM. Microporous and Mesoporous Materials. 158, 309-317 (2012).
- Moon, H. J., et al. Understanding the impacts of support-polymer interactions on the dynamics of poly(ethyleneimine) confined in mesoporous SBA-15. Journal of the American Chemical Society. 144 (26), 11664-11675 (2022).
- Xu, X., Song, C., Andresen, J. M., Miller, B. G., Scaroni, A. W. Preparation and characterization of novel CO2 "molecular basket" absorbents based on polymer-modified mesoporous molecular sieve MCM-41. Microporous and Mesoporous Materials. 62 (1-2), 29-45 (2003).
- Sousa, J. A. R., et al. H2S and H2O combined effect on CO2 capture by amino functionalized hollow microsphere silicas. Industrial & Engineering Chemistry Research. 60 (28), 10139-10154 (2021).
- Rim, G., et al. Sub-ambient temperature direct air capture CO2 using amine-impregnated MIL-101(Cr) enables ambient temperature CO2. JACS Au. 2 (2), 380-393 (2022).

