Real-Time Detection of Ferulic Acid Effects on Rat Left Ventricle Using Pressure-Volume Conductivity Catheter
Summary
This protocol describes a method for measuring left ventricular pressure and volume using the pressure-volume conductance technique. This method enables continuous real-time monitoring of the effects of drugs on the heart.
Abstract
Decreased cardiac function can have a negative impact on other organs. The left ventricular pressure-volume relationship is considered to be a valid method for evaluating cardiac function. Real-time monitoring of cardiac function is important for drug evaluation. Under closed-chest conditions, the miniature transducer, which is an important component of the pressure-volume catheter, enters the left ventricle of the rat through the right carotid artery. The device visualizes the changes in cardiac function during the experiment in the form of a pressure-volume loop. The actual volume of the ventricle is calculated by altering the conductivity of the blood by injecting 50 µL of a 20% sodium chloride solution into the rat’s left jugular vein. The actual volume of the rat’s ventricular cavity is calculated by measuring the conductivity of the blood in a known volume using a pressure-volume conductance catheter. This protocol allows for continuous observation of the effects of drugs on the heart and will promote the rationale for the use of specialty ethnic drugs in cardiovascular disease.
Introduction
Cardiovascular disease has the highest mortality rate in the world1. Its causes include coronary artery stenosis (myocardial ischemia), coronary artery blockage (myocardial infarction), and ischemia-reperfusion injury2. As the heart is in a constant systolic and diastolic cycle, it is one of the most energy-demanding parts of the body. Therefore, when the coronary arteries have difficulty maintaining sufficient energy and oxygen, cardiac function inevitably decreases, which has a negative impact on other organs3,4. The heart is a powerhouse in the circulatory system, and cardiac function needs to be assessed rationally.
Assessment of cardiac function by ventricular pressure and volume relationships is considered to be a comprehensive method5. Real-time changes in ventricular pressure and volume during the complete cardiac cycle make up the pressure-volume loop. The ventricular pressure-volume loop allows quantitative analysis of cardiac function and reserve capacity in terms of different phases and energies of the ventricle. The normal ventricle has a small end-systolic volume with good beat work and efficiency5,6,7.
The pressure-volume conduction catheter technique is an invasive method for detecting the status of the left ventricle. It can be used to obtain a continuous real-time pressure-volume loop8. Pressure volumetric conductivity catheters are powerful tools, and sound handling procedures are essential for reproducible and reliable results, including in vivo analysis of myocardial parallel conductivity during saline calibration and in vitro measurement of blood conductivity in cuvette calibration3.
Ferulic acid (FA), a phenolic acid, is widely distributed in plant kingdoms such as Avena sativa and Ligusticum chuanxiong hort9,10. Ferulic acid has pharmacological effects of lowering blood pressure and arrhythmia. FA is a bioactive natural product with multiple functions. FA can resist oxidative damage, reduce inflammatory responses, inhibit platelet aggregation, and prevent coronary heart disease and atherosclerosis11. However, most studies on ferulic acid have focused on one aspect of the heart and rarely have the effects of ferulic acid been evaluated in the circulatory system12,13,14,15. Here we describe a closed-chest approach to isoflurane anesthesia combined with Ketamine (50 mg/kg) with a focus on the cardiac response to ferulic acid solution during jugular vein injection.
We will describe the complete procedure for using the tool under closed-chest conditions, including solution preparation, preparation of the transducer, pre-experimental rat preparation, catheter insertion into the right carotid artery and data analysis. The duration of the experiment is usually less than 4 h and it is determined by the different experimental protocols. In a single experiment, we can obtain detailed cardiac information such as left ventricular pressure, volume, and heart rate.
Protocol
The animal protocol was reviewed and approved by the Chengdu University of Traditional Chinese Medicine Experimental Animal Welfare Ethics Committee (Record No. 2023-04). Male Sprague Dawley (SD) rats (280 ± 20 g, 8-10 weeks old) were used for the present study. The rats were kept in an animal chamber and were free to drink and eat.
1. Solution preparation
- Prepare 0.9% NaCl solution to be used to keep the work area adequately moist.
- To prepare 20% hypertonic NaCl solution, dissolve 2 g of NaCl in 10 mL of double distilled water (ddH2O). In order to determine the parallel conductivity of the myocardium, it is necessary to alter the conductivity of the intraventricular liquid.
- Prepare 1% enzyme-active powdered detergent solution. Use this at the completion of the experiment, to immerse the pressure-volume electrical catheter in the solution for 1-2 h.
- Prepare FA solution by dissolving 10 mg of ferulic acid in 20 mL of ddH2O. Filter the solution through a 0.22 µm membrane. Inject the rat with 1 mL/kg ferulic acid solution.
2. Preparation of the sensor
- Immerse the pressure-volume sensor in 0.9% NaCl solution at 37 °C for about 30-60 min before the start of the experiment, which facilitates the stability of the experimental data.
- Connect the experimental apparatus. The system for measuring pressure-volume loops consists of a pressure-volume catheter, two control units, a recording unit, and computer running software. The pressure-volume loop module in the software will provide a reference experimental procedure.
- Press the Start button and the software will automatically record the monitoring data from the pressure-volume sensor.
- Use the Miro-Tip Pressure Volume (MPVS) software to calibrate the pressure and conductivity.
3. Pre-experimental rat preparation
- Administer ketamine (50 mg/kg) and fentanyl (0.25 mg/kg) to the rats via intramuscular injection5.
- Pinch the toes of the rats to verify anesthetic depth by an absence of reflexes. Emotion affects the physiology state of rats and pain causes alterations in cardiac function16. Use small animal shavers and depilatory creams to remove hair on surgical sites. Use iodophor and 75% alcohol to wipe the skin to maintain sterility.
- Immobilize fully anesthetized rats on an isothermal heating plate with the back in contact with the heating plate.
- Insert a temperature probe coated with petroleum jelly into the rectum of the rat. Maintain the rat body temperature at 37 °C ± 0.5 by adjusting the heating plate.
NOTE: It is necessary to keep the airway unobstructed during the experiment.
4. Catheter insertion into the right carotid artery
- Incise the skin on the right side of the median line of the neck of rats, longitudinally. Make a 4 cm incision and separate muscle and connective tissue by forceps. The carotid artery situated on the right side of the trachea is visible. The right carotid artery of the rat is dark red, strongly pulsating, and has a white vagus nerve parallel to it.
- Separate the carotid artery from other tissues and nerves using forceps. Place three 5-0 surgical lines below the clean carotid artery. Drip sterile 0.9% sodium chloride solution onto the surgical area to maintain the moistening of the carotid artery.
- Cut the skin above the left clavicle and peel off the tissue around the jugular vein. Then, place a 5-0 surgical thread under the left jugular vein.
- Use arterial clips to suspend blood flow proximally, using micro scissors to cut a section in the vessel where blood flow has stopped. It is normal for a small amount of blood to appear in the wound cross-section. If blood is exiting the vessel rapidly and intermittently, lift the proximal surgical line and apply the arterial clamp again.
- Insert the catheter from the cross-section along the carotid artery deep into the left ventricle. Ensure the lowest systolic pressure value after entering the left ventricle is close to 0 mmHg.
- To obtain a reasonable pressure-volume relationship, slightly adjust the pressure-volume catheter in the ventricular chamber. To prevent massive blood loss and the catheter from changing position due to heartbeat, ligate the proximal end of the surgical line.
NOTE: The body temperature, anesthesia level, pressure signal, and conductance signal of the rat should remain stable during this process. The respiratory tract of the rat should be kept open.
5. Drug injection and conductivity calibration
- Maintain the position of the pressure volume catheter in the ventricular chamber, after the data stabilizes, ligate the surgical line distal to the jugular vein ligated and slowly inject upto 1 mL/kg of ferulic acid solution. Observe for 5-10 min.
- Inject 50 µL of 20% NaCl solution from the left jugular vein to remove the parallel conductance generated by the myocardium. The volume range of the parallel conductance was approximately 130-280 µL5. Repeat this 3x at an interval of 2 min.
- After ventricular pressure and volume testing in rats, take blood from the rat's abdominal aorta using a blood collection needle. Place the collected blood in a sodium heparin collection tube and invert up and down 2x to prevent blood clotting. Euthanize the experimental rats by injecting 120 mg/kg of pentobarbital sodium through the left jugular vein.
- Perform conversion of measured conductance to actual blood volume using rat volume calibration tubes. Place blood mixed with sodium heparin, sequentially, into the orifices of the calibration tube, and the catheter detects the conductance values of the blood in the different orifices and records them in the pressure-volume monitoring module.
6. Data analysis
- By adding the measured conductivity value of a known volume of blood to the specified location, the software automatically plots the curve and extrapolates the conductivity of the blood. Use at least three sets of blood conductivity values to deduce the blood conductivity of the rat under test. The blood conductivity is individualized. For each rat under the test, perform this procedure individually.
- Hypertonic calibration: By adding the data obtained from three injections of hypertonic saline to a specified location, the software calculates parallel conductance averages and automatically calibrates the experimental data.
- Utilize regions with stable blood pressure and conductance values to analyze the left ventricular function of rats.
- Click on Analyze and the software will automatically calculate a variety of parameters based on the selected area, including EF (left ventricular ejection fractions) SW (stroke work), and CO (cardiac output), etc.
Representative Results
Each test (n = 3) was predicated on the entry of a pressure-volume conductivity catheter into the left ventricle. There are significant signal changes, such as a marked increase in the range of pressure, as the catheter enters the left ventricle from the carotid artery (Figure 1). Graphical analysis of the pressure-volume relationship is completed by plotting the volume (µL) on the Y-axis and the pressure (mmHg) on the X-axis. Rat left ventricular pressure was within 10-105 mmHg, and conductance values of the volume were within 65-115 µL.
The complete cardiac cycle is formed by the counterclockwise pressure-volume loop (Figure 2). Significant changes in heart function were observed in rats after ferulic acid was administered to them (Figure 3). Rat left ventricular pressure was within 0-85 mmHg and conductance values of the volume were within 30-100 µL.
As shown in Figure 4, changes occur in the left ventricular pressure-volume loop when hypertonic saline was injected into jugular vein of rats. Due to the direct measurement of pressure and conductivity signals within the ventricular cavity, injection of hypertonic saline through the left jugular vein can cause an increase in conductivity values. Interference from the myocardium can be eliminated by taking multiple measurements of the changes in conductivity values.
Pressure volumetric conductance was used for cuvette calibration (Figure 5). This is to convert the measured conductance values into volumetric.
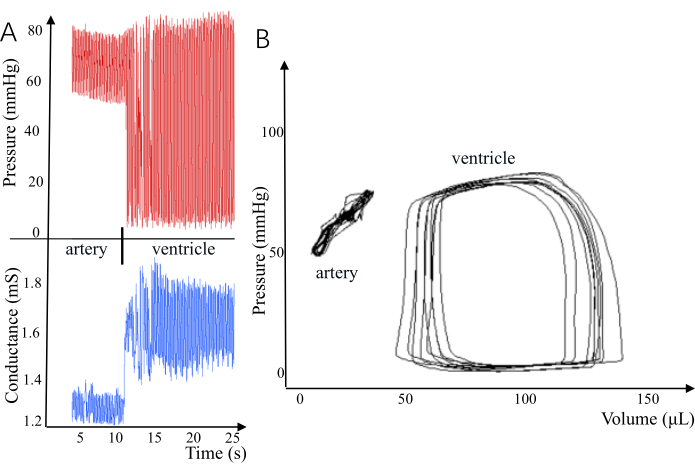
Figure 1: Different pressure-volume loops in the carotid artery and ventricle created by pressure-volume conductivity catheters. (A) There are significant differences in pressure and conductivity between arteries and ventricles. (B) The insertion of a miniature sensor into the ventricle can form a pressure-volume loop. Please click here to view a larger version of this figure.
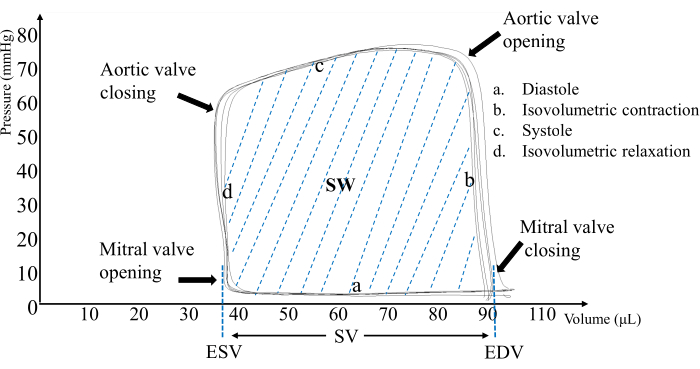
Figure 2: Pressure-volume loop. The pressure-volume loop includes four phases: diastole, isovolumetric contraction, systole, and isovolumetric relaxation. The area of the pressure-volume loop represents the work generated by one cardiac contraction. Subtracting the end-systolic volume (ESV) from the end-diastolic volume (EDV) yields the ventricular output. Please click here to view a larger version of this figure.
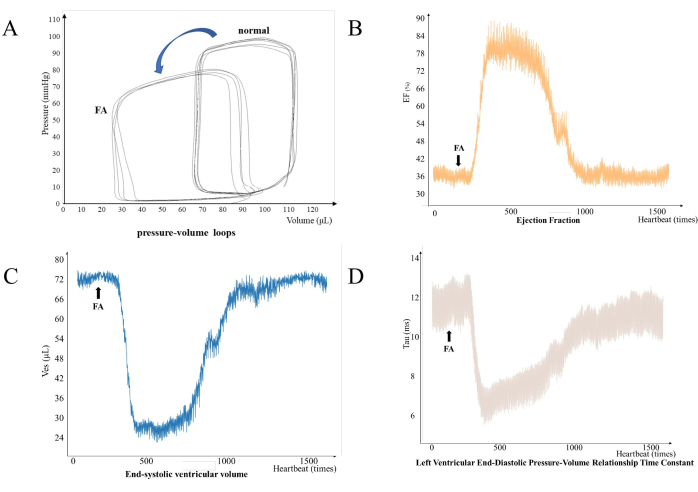
Figure 3: Left ventricular function is affected in rats after the injection of ferulic acid solution. (A) Left ventricular pressure-volume loop being affected. (B) Ejection fraction (EF) represents the percentage of stroke volume in relation to the end-diastolic volume of the ventricle: change in left ventricular ejection fraction with the count of heartbeats. (C) Left ventricular end-systolic volume varies with increasing number of heartbeats. (D) Left ventricular end-diastolic pressure-volume relationship time constant changes with an increase in heart rate. Please click here to view a larger version of this figure.
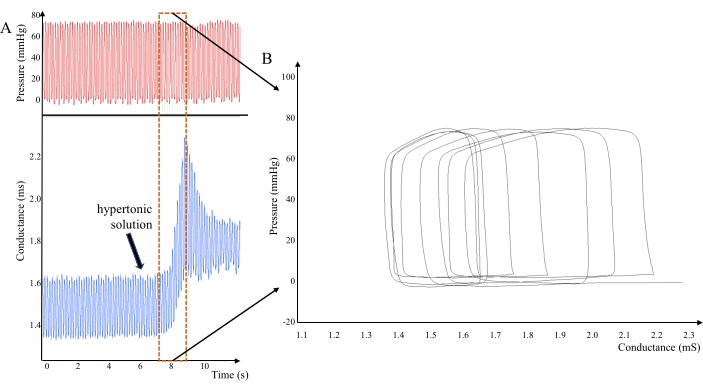
Figure 4: Pressure and conductance changes in the left ventricle of rats after 20% NaCl solution was injected into the vein. (A) Analyzed data where conductivity has been altered. (B) Pressure volume ring shifts to the right due to increased conductivity. Please click here to view a larger version of this figure.
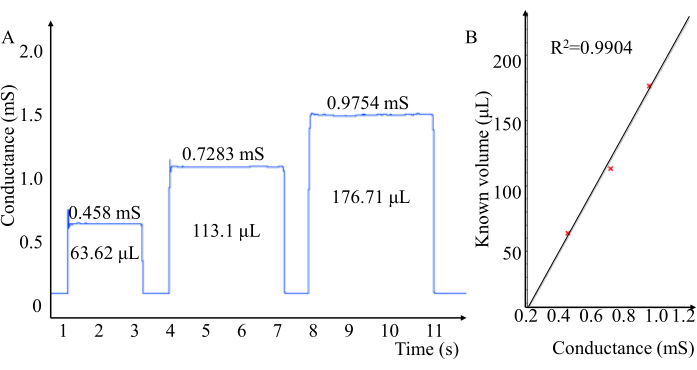
Figure 5: Pressure-volume conductance catheters are used to measure the conductance of a known volume of cuvette filled with rat blood. (A) Conductivity of different volumes. (B) Good correlation of conductivity measured by pressure-volume conductivity catheters. Please click here to view a larger version of this figure.
Discussion
It is essential to adopt a rational dosing strategy for different states of cardiac function. The pressure-volume conductance catheter technique is the most intuitive way to study left ventricular function5. This method enables the effects of drugs on cardiac function to be studied from a whole perspective. We describe the various stages of the experiment in detail. This will provide some facility for the study of heart function.
The pressure volume conductivity catheter technique is the most comprehensive and rigorous method. Information can be obtained on up to 30 indicators in a single experiment, including absolute values (pressure and volume) and relative values (EF), and even some information on drug metabolism.
The body temperature of rats was maintained at 37 °C ± 0.5 during the complete experimental procedure5. Blood loss from rats should be minimized during the experiment5. The blood volume of the rat should be noted during the experiment17.
Pressure-volume conduction catheter technology allows real-time detection of the status of the ventricles18. This technology can be very helpful in studying the effect of a single drug or combination of drugs on the heart. The conductance catheter will directly measure the pressure and conductance of the left ventricle. This is closely related to the body temperature and degree of anesthesia of the rat under test.In this experiment, after injection of the ferulic acid solution, the changes in left ventricular function were clearly demonstrated by the pressure-volume loop, including a decrease in end-systolic pressure and end-systolic volume (Figure 2A). The left ventricular ejection fraction of the rats was significantly increased, with a peak value of 89.87% (Figure 2B). The end-systolic pressure of the left ventricle in rats was significantly reduced, with a minimum value of 55.44 µL. This is consistent with the pharmacological effect of ferulic acid in reducing blood pressure as previously reported11.
Certain natural compounds found in food and medicinal plants can contribute to maintaining health. Ferulic acid is a phenolic compound widely present in plants, including Ligusticum chuanxiong and Angelica sinensis19, which are important active ingredients in various traditional Chinese medicines. Current research has reported that ferulic acid possesses multiple biological activities, including anti-inflammatory, anti-fibrotic, and anti-apoptotic effects11. It is necessary to study the impact of this readily available natural product from food on cardiac function in the circulatory system, although research has indicated its positive effects on cardiac morphological structure12,20.
The pressure-volume catheter can be placed in the ventricular chamber of the experimental animal to obtain ventricular pressure and conductance directly. Saline calibration and cuvette calibration are used to obtain true ventricular volumes. This experiment allows a continuous pressure-volume loop to be obtained, which will visually reflect changes in ventricular function. There are two methods of accessing the ventricular chamber with the pressure volume catheter, including the open-chest condition and the closed-chest condition. In the open-chest condition it is easier to control the position of the pressure volume catheter in the ventricular cavity. Measuring ventricular function under closed-chest conditions does not require assisted breathing of the animal, is less damaging to the animal and has a higher success rate. In addition, the pressure volume loop is observed under closed-chest conditions to determine whether the pressure volume catheter is in the ventricular cavity. If this catheter is compressed by the myocardium, the pressure volume loop will show an abnormal peak.
The heart is a vital organ that pumps blood throughout the body. A more rational approach to evaluating cardiac function is needed, including before and after loads as well as the state of the heart itself21. Pressure-volume loops are used to describe the changes in central ventricular chamber pressure and volume over the complete cardiac cycle in real time. This protocol describes a complete method for measuring left ventricular function with miniature sensors. Changing the microsensor model in the experimental protocol allows the measurement of cardiac function in different animals, such as pigs, mice etc4,8,22. The use of pressure-volume conductance catheters allows for the real-time observation of the effects of drugs on left ventricular pressure and volume, as well as the overall impact on the circulatory system of the experimental subject. This technique helps to minimize the potential negative effects of drugs on the heart.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Sichuan Provincial Major R&D Project (2022YFS043) and the Chengdu University of Traditional Chinese Medicine Youth Foundation Advancement Talent Special Project (QJJJ2022029).
Materials
| 1 mL syringe | Sartorius AG, Germany | – | |
| Animal temperature maintainer | Rayward Life Technology Co., Ltd | 69020 | |
| Dual Bio Amp | Millar, Inc., USA | DA-100 | |
| Enzyme-Active Powdered Detergent | Alconox Inc., USA | 1104 | |
| Ferulic acid | Macklin Biochemical Co., Ltd,Shanghai, China | F900027 | |
| Mikro-Tip Catheter Transducers, SPR-838NR | Millar, Inc., USA | SPR-838NR | |
| Millar Miro-Tip Pressure Volume (MPVS) Ultra | Millar, Inc., USA | SPR-869 | |
| Pet electric clippers | Jinyun County New Concept Home Supplies Co., Ltd. | - | |
| Power Lab 8 / 35 | Millar, Inc., USA | PL3508 | |
| Sodium Chloride, NaCl | Kelong Chemical Reagent, Chengdu, China | KX829463 | |
| Veet hair removal cream | Shanghai Songqi E-commerce Co., Ltd. | 3226470 |
Referenzen
- Zaman, R., Epelman, S. Resident cardiac macrophages: Heterogeneity and function in health and disease. Immunity. 55 (9), 1549-1563 (2022).
- Schefold, J. C., Filippatos, G., Hasenfuss, G., Anker, S. D., von Haehling, S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. 12 (10), 610-623 (2016).
- Medert, R., Bacmeister, L., Segin, S., Freichel, M., Camacho Londoño, J. E. Cardiac response to β-adrenergic stimulation determined by pressure-volume loop analysis. J Vis Exp. (171), e62057 (2021).
- Hieda, M., Goto, Y. Cardiac mechanoenergetics in patients with acute myocardial infarction: From pressure-volume loop diagram related to cardiac oxygen consumption. Heart Fail Clin. 16 (3), 255-269 (2020).
- Pacher, P., Nagayama, T., Mukhopadhyay, P., Bátkai, S., Kass, D. A. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 3 (9), 1422-1434 (2008).
- Ziegler, T., Laugwitz, K. L., Kupatt, C. Left ventricular pressure volume loop measurements using conductance catheters to assess myocardial function in mice. Methods Mol Biol. 2158, 33-41 (2021).
- Rosch, S., et al. Characteristics of heart failure with preserved ejection fraction across the range of left ventricular ejection fraction. Circulation. 146 (7), 506-518 (2022).
- Meyers, T. A., Townsend, D. Early right ventricular fibrosis and reduction in biventricular cardiac reserve in the dystrophin-deficient mdx heart. Am J Physiol Heart Circ Physiol. 308 (4), H303-H315 (2015).
- Alaerts, G., et al. Exploratory analysis of chromatographic fingerprints to distinguish rhizoma Chuanxiong and rhizoma Ligustici. J Chromatogr A. 1217 (49), 7706-7716 (2010).
- Serreli, G., et al. Ferulic acid derivatives and Avenanthramides modulate endothelial function through maintenance of nitric oxide balance in HUVEC cells. Nutrients. 13 (6), 2026 (2021).
- Li, D., et al. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 284, 119921 (2021).
- Monceaux, K., et al. Ferulic acid, Pterostilbene, and Tyrosol protect the heart from ER-stress-induced injury by activating SIRT1-dependent deacetylation of eIF2α. Int J Mol Sci. 23 (12), 6628 (2022).
- Liu, Z., et al. N-terminal truncated peroxisome proliferator-activated receptor-γ coactivator-1α alleviates phenylephrine-induced mitochondrial dysfunction and decreases lipid droplet accumulation in neonatal rat cardiomyocytes. Mol Med Rep. 18 (2), 2142-2152 (2018).
- Sun, Y., et al. Shuangxinfang prevents S100A9-induced macrophage/microglial inflammation to improve cardiac function and depression-like behavior in rats after acute myocardial infarction. Front Pharmacol. 13, 832590 (2022).
- Panneerselvam, L., et al. Ferulic acid attenuates arsenic-induced cardiotoxicity in rats. Biotechnol Appl Biochem. 67 (2), 186-195 (2020).
- Hsueh, B., et al. Cardiogenic control of affective behavioural state. Nature. 615 (7951), 292-299 (2023).
- Townsend, D. Measuring pressure volume loops in the mouse. J Vis Exp. (111), e53810 (2016).
- Bastos, M. B., et al. Invasive left ventricle pressure-volume analysis: overview and practical clinical implications. Eur Heart J. 41 (12), 1286-1297 (2020).
- Wang, L. Y., et al. Effects of ferulic acid on antioxidant activity in Angelicae Sinensis Radix, Chuanxiong Rhizoma, and their combination. Chin J Nat Med. 13 (6), 401-408 (2015).
- Liu, Z., et al. Ferulic acid increases intestinal Lactobacillus and improves cardiac function in TAC mice. Biomed Pharmacother. 120, 109482 (2019).
- Baan, J., et al. Continuous measurement of left ventricular volume in animals and humans by conductance catheter. Circulation. 70 (5), 812-823 (1984).
- Dam Lyhne, M., et al. Effects of mechanical ventilation versus apnea on bi-ventricular pressure-volume loop recording. Physiol Res. 71 (1), 103-111 (2022).

