A Model for Experimental Exposure of Humans to Larval Ixodes scapularis Ticks
Summary
This article presents the methodology for exposing humans to larval Ixodes scapularis for clinical research. The technique is relatively simple, tolerable by the research volunteers, and can be modified according to experimental needs. Such research involving human subjects must be conducted under clinical study protocols approved by the appropriate regulatory authorities.
Abstract
Tickborne diseases are a significant public health problem in the United States and worldwide. Ticks are obligate blood-feeding arthropods; an ixodid tick must remain attached to the skin of the host and complete its multi-day feeding process to acquire its blood meal. Exposing animals to ticks is a common practice for studying host responses to tick bites and tickborne diseases. We developed the procedure, conducted the first human research study, and published the findings on exposing human volunteers to uninfected larval Ixodes scapularis ticks. This article describes the methodology used to construct the containment dressing, how to apply and secure the ticks to the host, how to maintain the dressing, and how to remove the ticks from the host. Exposing volunteers to tick bites is an experimental procedure and must be performed under a clinical research protocol approved by the appropriate regulatory authorities. This method allows for translational research to better understand the human response to tick bites and foster the development of diagnostics, prevention, and therapies for tickborne diseases.
Introduction
Hard ticks (Ixodidae: Acari) are obligate blood-feeding ectoparasites that occur worldwide and are capable of transmitting a broad range of pathogens, including bacteria, viruses, and parasites, of major medical and veterinary importance. Ixodid ticks must remain attached to the host for days to complete a blood meal, and they have the capacity to stay attached to the skin while avoiding recognition, preventing local blood coagulation, and facilitating long-term feeding1,2,3. Animal studies have demonstrated that non-permissive hosts acquire resistance to tick bites with repeated tick exposures, which can lead to a decreased ability to transmit a pathogen, while ticks can repeatedly parasitize permissive hosts. Acquired tick resistance is dependent on the nature of the host immune response4,5,6,7.
Tickborne diseases are an increasing threat in the United States (US), with the number of reported cases more than doubling between 2004 and 20168,9. Due to climate change, the geographic ranges of different ticks continue to expand10,11. The leading tickborne diseases in the US include Lyme disease, anaplasmosis, ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus disease8. Lyme disease, caused by infection with Borrelia burgdorferi sensu lato, is the most common tickborne disease in the US and Europe12. With approximately 476,000 individuals diagnosed with Lyme disease annually in the US, there is both a public health and economic burden to individuals and to society13,14,15.
Ixodes scapularis (the black-legged or deer tick) is the primary vector for Lyme disease, as well as anaplasmosis, babesiosis, Borrelia miyamotoi disease, and Powassan virus disease in the US. Other medically important tick species in the US include Amblyomma americanum (Lone star tick), Dermacentor variabilis (American dog tick), Ixodes pacificus (Western black-legged tick), Dermacentor andersoni (Rocky Mountain wood tick), Ixodes cookei (Groundhog tick), Dermacentor occidentalis (Pacific Coast tick), Rhipicephalus sanguineus (brown dog tick), and Amblyomma maculatum (Gulf Coast tick)16.
Developing a method to expose research volunteers to tick bites supports studies using the natural vector to search for evidence of infection, a procedure known as xenodiagnosis17,18,19,20,21, and to learn more about immunity induced by exposure to ticks, which can contribute to the discovery of an anti-tick vaccine5,6,7. The procedure described here was developed and used in the first human research study using laboratory-reared Ixodes scapularis larva for xenodiagnosis of B. burgdorferi infection after antibiotic therapy (NCT01143558), published in 201419. The system has been successfully used in a phase 2 study investigating whether a positive xenodiagnosis correlated with the persistence of symptoms after antibiotic treatment of Lyme disease (NCT02446626) and in a study exploring the host response to tick bites (NCT05036707).
This procedure protocol describes the process for creating the containment dressing, the tick placement procedure, and the tick removal procedure, as well as the site care needed to maintain the containment dressing. The details regarding the pathogen-free I. scapularis tick colony and tick exposure procedures used for the above-cited studies have been previously described19,22. This methodology offers a flexible research tool that can be adapted to studying different aspects of the human host response to tick bites, the effectiveness of tick prevention medications, as well as Lyme disease and other tickborne illnesses.
Protocol
Exposing volunteers to tick bites is an experimental method and must be conducted under a clinical research protocol approved by the relevant regulatory authorities. The clinical studies (NCT01143558, NCT02446626, and NCT05036707) were approved by the respective institutional review boards, conducted under investigational device exemptions granted by the US Food and Drug Administration, and carried out in accordance with Good Clinical Practice guidelines. Additionally, these studies were registered with ClinicalTrials.gov, and written informed consent was obtained from all participants.
1. Containment dressing preparation
- To create a measurement template, photocopy the 3" x 3" hydrocolloid containment dressing along the side of the measurement lines (Figure 1A and Table of Materials).
- Cut the photocopied template along the 1" line. Then, cut along the 2" line, creating a 2" circle with a 1" center (Figure 1B).
- Using this template, cut the non-adhesive foam dressing to create a 2" circle with a 1" center (Figure 2A,B, and Table of Materials).
- Using the same template, cut a 2" circle in the center of the 4" x 4" extra-thin hydrocolloid dressing (Figure 2C,D).
- Cut the hydrocolloid layer of the 3" x 3" containment dressing at the 1½" line.
- Pull back the paper liner of the hydrocolloid layer of the containment dressing to expose the adhesive. Place the cut foam dressing within the 2" circle of the adhesive (Figure 3A). This will ensure that the foam dressing extends beyond the opening of the containment dressing. Replace the paper liner onto the hydrocolloid layer of the dressing (Figure 3B).
NOTE: These measurements were used for studies involving up to 10 larval I. scapularis ticks. For studies involving up to 30 larval I. scapularis ticks, the template for the foam center was cut between the 1" and 1½" markings to allow for a larger area for placement. The measurements of the containment dressing can be adapted accordingly to the study design.
2. Tick placement
- Tick storage
- Store the larval I. scapularis ticks in a medical-grade refrigerator with the temperature set to 9-10 °C.
- Store the ticks in a vial covered with a mesh cap to allow adequate airflow. Package the vial of ticks in a sealable plastic bag with a lightly moistened sponge to maintain humidity and prevent desiccation.
- Placing the ticks
- Remove the ticks from the refrigerator to allow them to warm to room temperature. The ticks can become active in as little as 15 min outside the refrigerator.
- Position a white sheet around the placement area to easily visualize any inadvertently dropped tick.
- Dampen a 4"x 4" gauze pad with clean water or saline and use it to clean the skin of the placement site. The area should be patted dry or allowed to air dry.
NOTE: When selecting the body site for tick placement, consider areas that do not restrict the volunteer's daily activities and where the integrity of the dressing can be easily monitored by the participant. Do not place ticks in areas where they could be crushed due to direct pressure (e.g., the back). Evaluate the participant's body hair, and if necessary, carefully trim excessive body hair using scissors at the site of the adhesive, avoiding damage to the skin. Avoid using a razor for hair removal, as it may increase the risk of skin injury and infection. - Once the skin is dry, remove the adhesive liner from the hydrocolloid side of the prepared dressing and firmly affix it to the skin at the placement site, ensuring that the mesh layer remains open.
- Break the wooden swab (see Table of Materials) in half to expose jagged ends.
- Carefully open the vial of ticks.
- Using the jagged end of the wooden swab, transfer active ticks from the vial and place them onto the skin within the containment dressing (Figure 4A). Have an additional team member monitor the tick placement site to ensure the ticks remain inside the containment dressing. Use adhesive tape to trap ticks that escape from the vial during this process.
NOTE: Breaking a wooden swab and using the jagged end is effective for tick manipulation and transfer without causing damage to the ticks. A small brush with soft bristles in a light color also works for transferring ticks from the container to the skin site. - Once the specified number of ticks for the study is placed, remove the adhesive liner from the mesh layer of the containment dressing and close it over the opening.
- After ensuring that the adhesive is securely attached, apply the prepared 4" x 4" extra-thin hydrocolloid dressing over the containment dressing for additional security (Figure 4B).
NOTE: Additional information regarding the pathogen-free I. scapularis tick colony and the duration of the tick exposure procedures used in the cited studies can be found in the methods sections of the published studies19,22.
3. Site care
- Provide a water barrier cover and 2" hypoallergenic tape (see Table of Materials) to the research participant.
- Provide instructions for caring for the tick containment site.
- Periodically check the dressing and monitor the edges for secure adhesion to the skin. Apply additional tape to reinforce the dressing edges as needed.
- Do not scratch the dressing site, and refrain from opening or removing the dressing during the study period.
- Avoid bathing or soaking in water. Also, avoid hot showers and limit the duration of showering. Before showering, protect the containment dressing with the provided water barrier cover and remove it after completing the shower. Gently pat the dressing dry, and inspect it to ensure it remains secure.
- Avoid activities that may lead to heavy perspiration and compromise the dressing's integrity (e.g., aerobic exercise, extended hikes).
4. Tick removal
- Collection vials preparation
- Label the tick collection vials with the desired identifiers (e.g., study code, date).
- If preservation of live ticks is necessary, puncture holes in the cap of the vials with a 20 G needle. Use a layer of nylon or polyester mesh along with the cap to seal the vial. Place a small piece of moist sponge in a sealable bag with the vial of ticks to maintain humidity.
- Removing the ticks
- Place white sheets around the bandage site and ensure a collection basin is within reach.
- Using alcohol wipes, carefully remove the bandage from the skin.
- Once fully removed, place the bandage in the collection basin and examine it for the presence of detached ticks.
- Assess the placement site for attached ticks. Ticks that have fed to repletion will detach (Figure 5A). For the remaining attached ticks, use fine-tip forceps for removal (Figure 5B).
- Clean the skin with alcohol wipes or soap and water.
Representative Results
The study demonstrated that the procedure is safe and well-tolerated, with the primary adverse event being mild itching at the site of the bites, observed in 58% of the procedures. There were no serious adverse events related to the procedure when using clean laboratory-reared larval I. scapularis ticks19. In the 43 procedures conducted, the mean percentage of recovery of attached ticks compared to placed ticks was 45% ± 27% (SD), with a median percentage of 40% (Figure 6).
The exact duration for larval I. scapularis ticks to attach to humans remains unclear, as the time from tick placement to tick attachment was not routinely observed. In one instance where a research volunteer was observed for 2½ h, none of the ticks had attached by the end of the period. It takes 4 to 5 days from placement for larval I. scapularis ticks to feed to repletion19.
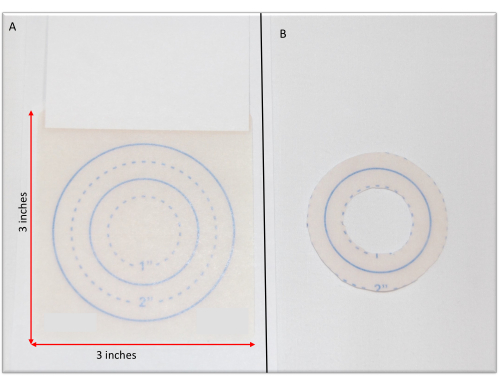
Figure 1: Containment dressing measurements. (A) A photocopy of the containment dressing. (B) The containment dressing measurement template created by cutting the photocopy at the desired markings. Please click here to view a larger version of this figure.
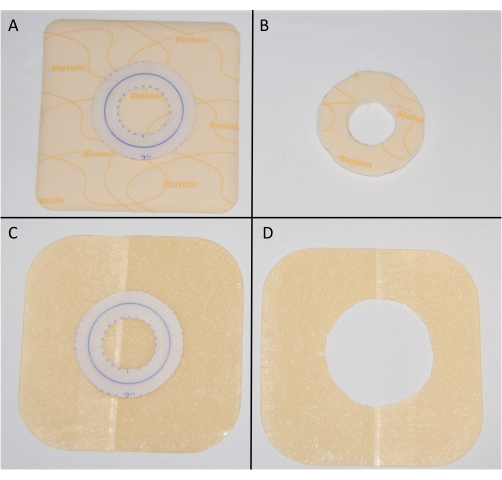
Figure 2: Creation of non-adhesive and hydrocolloid reinforcement dressings. (A) Using the containment dressing measurement template to cut the foam dressing. (B) A 2" circle with a 1" center foam dressing. (C) Using the containment dressing measurement template to cut the hydrocolloid dressing. (D) A 4" x 4" hydrocolloid dressing with a 2" center. Please click here to view a larger version of this figure.
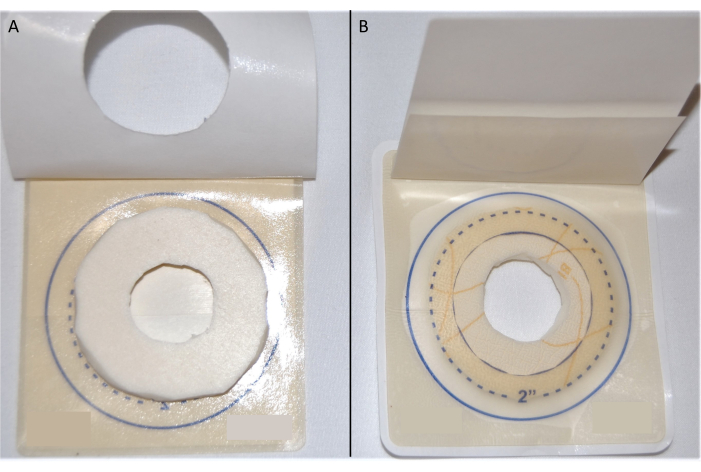
Figure 3: Containment dressing. (A) The foam dressing on the adhesive side of the hydrocolloid layer of the containment dressing. (B) Containment dressing used for tick feeding. Please click here to view a larger version of this figure.
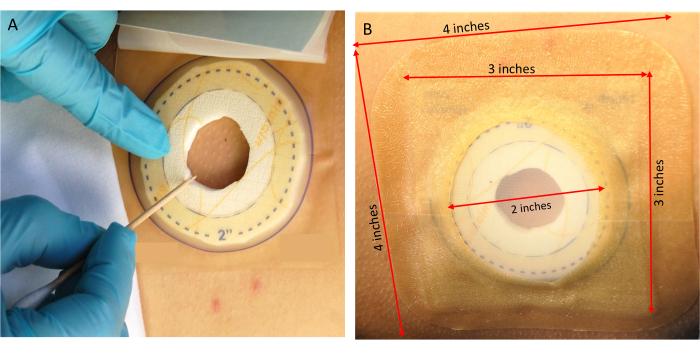
Figure 4: Tick placement. (A) Transferring ticks onto the research volunteer. (B) Securing the containment dressing. Please click here to view a larger version of this figure.
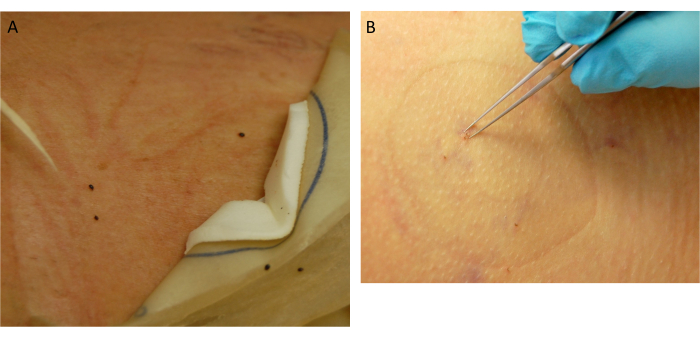
Figure 5: Tick collection. (A) Detached replete ticks. (B) Removal of attached ticks. Please click here to view a larger version of this figure.
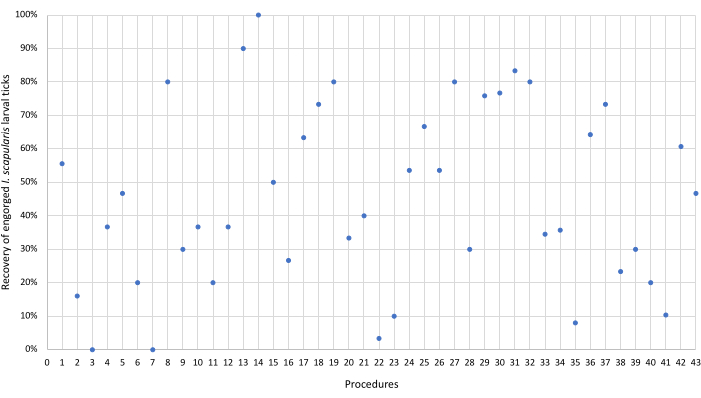
Figure 6: Recovery of engorged Ixodes scapularis larval ticks. Each dot represents the percentage of engorged I. scapularis larval ticks recovered using the described protocol. The average percentage of recovered ticks was 45 ± 27 (SD). Please click here to view a larger version of this figure.
Discussion
While animal studies involving exposures to ticks4,5,6,7,21 have been invaluable in increasing our understanding of host response to tickborne diseases and tick bites, these models have limitations in how well they predict the human host response. This model, which describes the methodology for exposing humans to tick bites in a controlled manner, can be easily adapted to answer different research questions and expands the capability to perform these needed studies in humans.
The initial study required the development of a new system that would allow the ticks to feed for days while also being comfortable and tolerable for the human subjects. After assessing different techniques, the containment dressing described here was ultimately the most successful. During the initial trials, the ticks wandered to the edge of the circular cut-out of the containment dressing and died on the exposed adhesive before attachment could take place. To account for this tick behavior, a non-adhesive foam ring was added under the hydrocolloid adhesive of the containment dressing. This provided a non-adhesive dark edge for the ticks to migrate to and attach to the skin. Unattached ticks desiccated and died in the dressing. If the ticks traveled beyond the foam layer, they were caught on the adhesive and died. Once the ticks attached to the skin, they generally remained embedded until they fed to repletion. Ticks that have fed to repletion will detach; hence, it is important to examine the removed dressing to collect these fed detached ticks. If the clinical protocol does not require the ticks to feed to repletion, use fine-tip forceps to remove the attached ticks.
The duration of tick feeding will depend upon the research question, the tick life stage, and the approved clinical protocol. For the studies using this colony of larval I. scapularis, it took 4 to 5 days for the ticks to feed to repletion19. Research volunteers are asked to refrain from activities such as exercise and swimming that can compromise the containment dressing. For showering, volunteers are provided with water barrier covers such as those used to cover central intravenous catheters and casts. In addition, they are instructed to avoid long hot showers. If care is taken to keep the containment dressing dry and to reinforce it with additional tape as needed, it will remain intact.
For the initial human research study in humans, larval I. scapularis were chosen for the procedure. The reasons for the choice included similarity to the study in mice23 as well as practical reasons. The use of larval ticks had fewer safety concerns, as this stage has not received a blood meal prior to usage in clinical protocols. In addition, their relative abundance (compared with other stages) made it easier to test and produce larger numbers. The testing of this colony of pathogen-free larval I. scapularis is described in Marques et al.19. New clinical studies are being performed using nymphal ticks, including a study evaluating the effectiveness of a medication to kill ticks (NCT05387083) and a tick challenge study (NCT05965635).
Exposing ticks to human research volunteers was a novel concept and has its limitations. The tick colony needs to be safely reared and tested for known pathogens that can be transmitted to humans before they can be used for clinical research. This process requires adequate expertise and resources.
Much effort is implemented to educate potential research volunteers on the safety and tolerability of the procedure as well as the process for maintaining the ticks under the containment dressing. Although this tick exposure methodology allows research volunteers to continue most of their daily activities, the lifestyle adjustments needed to maintain the containment dressing and the time commitment can be deterrents to participation.
Despite the limitations, the development of a model for exposing humans to larval I. scapularis ticks is an important research tool. The procedure has research utility to foster investigations on ticks, tick bites, the human response to ticks, and the prevention of tickborne diseases. The advantage of this methodology is its simplicity and adaptability. The methodology can be used to feed different life stages of ixodid ticks and is suitable for various types of experiments where exposing humans to ixodid ticks is required. Research procedures involving human subjects must be performed under clinical studies approved by the appropriate regulatory authorities.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases. We thank Linden T. Hu, Sam R. Telford III, Kenneth Dardick, Carla Williams, Erin Chung, and Christina Brandeburg for their participation in the development of the procedures.
Materials
| 20 G needle | Any brand | For puncturing the vial cap. | |
| 3" x 3" containment dressing | Monarch Labs Names | LeFlap | https://www.monarchlabs.com/ordering |
| 4" x 4" extra-thin hydrocolloid dressing | ConvaTec | DuoDerm | https://www.convatec.com/products/advanced-wound-care/brand-names/pc-wound-duoderm-granluflex/duoderm-extra-thin-dressing/ |
| 4" x 4" gauze | Monarch Labs Names | For cleaning skin | |
| Clean water or saline | For cleaning skin | ||
| Moisture barrier (e.g. 7" x 7") | AquaGuard | TIDI | For showering, ttps://www.tidiproducts.com/product-listing/aquaguard-shower-cover-sheets |
| Non-adhesive foam dressing | Coloplast | Biatain | https://www.coloplast.us/biatain-non-adhesive-en-us.aspx |
| Roll of 2" hypoallergenic tape | Monarch Labs Names | Durapore | For reinforcing containment dressing. |
| Roll of adhesive tape | For trapping ticks | ||
| Vials for collection (e.g. cryovials) | Ependorf | ECC200 |
Referenzen
- Yeh, M. T., et al. Determining the duration of Ixodes scapularis (Acari: Ixodidae) attachment to tick-bite victims. J Med Entomol. 32 (6), 853-858 (1995).
- Piesman, J., Mather, T. N., Sinsky, R. J., Spielman, A. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 25 (3), 557-558 (1987).
- Vora, A., et al. Ticks elicit variable fibrinogenolytic activities upon feeding on hosts with different immune backgrounds. Sci Rep. 7, 44593 (2017).
- Narasimhan, S., et al. Immunity against Ixodes scapularis salivary proteins expressed within 24 hours of attachment thwarts tick feeding and impairs Borrelia transmission. PLoS One. 2 (5), 451 (2007).
- Nazario, S., et al. Prevention of Borrelia burgdorferi transmission in guinea pigs by tick immunity. Am J Trop Med Hyg. 58 (6), 780-785 (1998).
- Krause, P. J., et al. Dermatologic changes induced by repeated Ixodes scapularis bites and implications for prevention of tick-borne infection. Vector Borne Zoonotic Dis. 9 (6), 603-610 (2009).
- Anderson, J. M., et al. Ticks, Ixodes scapularis, feed repeatedly on white-footed mice despite strong inflammatory response: an expanding paradigm for understanding tick-host interactions. Front Immunol. 8, 1784 (2017).
- Rosenberg, R., et al. Vital signs: trends in reported vectorborne disease cases – United States and Territories, 2004-2016. MMWR Morb Mortal Wkly Rep. 67 (17), 496-501 (2018).
- Paules, C. I., Marston, H. D., Bloom, M. E., Fauci, A. S. Tickborne diseases – confronting a growing threat. N Engl J Med. 379 (8), 701-703 (2018).
- Tardy, O., et al. Mechanistic movement models to predict geographic range expansions of ticks and tick-borne pathogens: Case studies with Ixodes scapularis and Amblyomma americanum in eastern North America. Ticks Tick Borne Dis. 14 (4), 102161 (2023).
- Molaei, G., Eisen, L. M., Price, K. J., Eisen, R. J. Range expansion of native and invasive ticks: a looming public health threat. J Infect Dis. 226 (3), 370-373 (2022).
- Marques, A. R., Strle, F., Wormser, G. P. Comparison of Lyme disease in the United States and Europe. Emerg Infect Dis. 27 (8), 2017-2024 (2021).
- Kugeler, K. J., Schwartz, A. M., Delorey, M. J., Mead, P. S., Hinckley, A. F. Estimating the frequency of Lyme disease diagnoses, United States, 2010-2018. Emerg Infect Dis. 27 (2), 616-619 (2021).
- Schwartz, A. M., Kugeler, K. J., Nelson, C. A., Marx, G. E., Hinckley, A. F. use of commercial claims data for evaluating trends in lyme disease diagnoses, United States, 2010-2018. Emerg Infect Dis. 27 (2), 499-507 (2021).
- Hook, S. A., et al. Economic burden of reported Lyme disease in high-incidence areas, United States. Emerg Infect Dis. 28 (6), 1170-1179 (2022).
- Eisen, L. Tick species infesting humans in the United States. Ticks Tick Borne Dis. 13 (6), 102025 (2022).
- Embers, M. E., et al. Variable manifestations, diverse seroreactivity and post-treatment persistence in non-human primates exposed to Borrelia burgdorferi by tick feeding. PLoS One. 12 (12), 0189071 (2017).
- Hodzic, E., Imai, D., Feng, S., Barthold, S. W. Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PLoS One. 9 (1), 86907 (2014).
- Marques, A., et al. Xenodiagnosis to detect Borrelia burgdorferi infection: a first-in-human study. Clin Infect Dis. 58 (7), 937-945 (2014).
- Hodzic, E., Imai, D. M., Escobar, E. Generality of post-antimicrobial treatment persistence of Borrelia burgdorferi strains N40 and B31 in genetically susceptible and resistant mouse strains. Infect Immun. 87 (10), e00442 (2019).
- Bockenstedt, L. K., Mao, J., Hodzic, E., Barthold, S. W., Fish, D. Detection of attenuated, noninfectious spirochetes in Borrelia burgdorferi-infected mice after antibiotic treatment. J Infect Dis. 186 (10), 1430-1437 (2002).
- Turk, S., Williams, C., Marques, A., Pal, U., Buyuktanir, O. . in Borrelia burgdorferi: Methods in Molecular Biology. , 337-346 (2018).
- Hodzic, E., Feng, S., Holden, K., Freet, K. J., Barthold, S. W. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob Agents Chemother. 52 (5), 1728-1736 (2008).

