A Versatile Glass Jar System for Semihydroponic Root Exudate Profiling
Summary
We present a protocol for a glass-based, semihydroponic experimental system supporting the growth of a variety of phylogenetically distinct plants with or without microbes. The system is compatible with different growth media and permits nondestructive root exudate sampling for downstream analysis.
Abstract
Root exudates shape the plant-soil interface, are involved in nutrient cycling and modulate interactions with soil organisms. Root exudates are dynamic and shaped by biological, environmental, and experimental conditions. Due to their wide diversity and low concentrations, accurate exudate profiles are challenging to determine, even more so in natural environments where other organisms are present, turning over plant-derived compounds and producing additional compounds themselves. The semihydroponic glass jar experimental system introduced here allows control over biological, environmental, and experimental factors. It allows the growth of various phylogenetically distinct plant species for up to several months with or without microbes, in a variety of different growth media. The glass-based design offers a low metabolite background for high sensitivity and low environmental impact as it can be reused. Exudates can be sampled nondestructively, and conditions can be altered over the course of an experiment if desired. The setup is compatible with mass spectrometry analytics and other downstream analytical procedures. In summary, we present a versatile growth system suited for sensitive root exudate analysis in a variety of conditions.
Introduction
Within densely populated soils, the rhizosphere presents a carbon-rich niche. It is shaped by plant roots via the exudation of up to 20% of assimilated carbon and harbors microbial communities that are distinct from the resident soil microbiome1,2,3,4,5,6. As researchers are tapping into the beneficial functions of root-associated microbes and the potential for sustainable agriculture that goes with it7, this observation, often termed as the rhizosphere effect, has been the focus of growing scientific efforts. However, thus far, the chemical dialog between microorganisms and plants, which is proposed to be the driver of the rhizosphere effect, remains poorly understood and hence, the mechanistic understanding for the development of reliable microbial solutions in agriculture is limited8,9,10.
Deciphering root exudates in soil environments where metabolites are readily absorbed by soil particles and quickly turned over by microbial communities is not straightforward, especially for plant species with fine root systems such as the model plant Arabidopsis thaliana11. This is why, in most studies, root exudates are sampled from hydroponic systems. In these microcosms, aerial parts of plants are held in place by customized plant-holders or more low-key materials such as mesh, agar, and glass beads. The containers used range from Petri dishes over multi-well plates to various custom and commercial boxes with or without aeration filters12,13,14,15,16,17,18,19. Depending on the system, the plant-growth conditions will vary greatly and reflect natural conditions to a greater or lesser extent.
Here, we present a glass-based, semihydroponic system that is experimentally amenable and produces highly reproducible results. It is straightforward to assemble and use and is based on commonly available materials. The system is based on a glass jar filled with glass beads, taking advantage of the reusable nature and low-binding properties of glassware (Figure 1). The beads provide physical support for the growing plant and simulate mechanical impedance, contributing to more soil-like root architecture when compared to hydroponic setups19,20,21. If inoculated with microbes, the glass beads present surfaces for bacteria to attach to.
The glass jar can be closed to maintain sterility and the system is designed to allow for sufficient headspace and air circulation, avoiding an environment saturated in humidity. The jars are suitable for the prolonged growth of different plant species and can be scaled up and down using different-sized jars. Here, applications for six plant species are shown, covering C3 and C4 grasses, dicots, and legumes. Among them are the model species A. thaliana (dicot), Brachypodium distachyon (C3 monocot), Medicago truncatula (legume), as well as crop species such as Solanum lycopersicum (tomato, dicot), Triticum aestivum (wheat, C3 monocot), and Sorghum bicolor (sorghum, C4 monocot). The protocol presented includes the experimental setup of the system, seed sterilization and germination of six plant species, transplantation of seedlings to jars, different growth media, microbe inoculation, root exudate sampling, and exudate processing for analytics.
Protocol
1. Preparation of seedlings: Surface sterilization of seeds
NOTE: The surface sterilization of seeds and all following steps must be done in sterile conditions unless noted otherwise. Steps 1.1 to 1.4 are specific for surface sterilization of A. thaliana seeds. Other plant species have alternative variations in tube size (depending on the number of seeds and seed size), time in solutions, and sterilization solutions (Table 1).
- Add seeds to a microcentrifuge tube (maximum 20 mg of seeds) and cover the seeds with 70% ethanol.
CAUTION: Ethanol is flammable. Do not use near open flame. - Move the closed microcentrifuge tubes to a shaker for 15 min, and set the rotation such that the seeds are gently agitated.
- Carefully remove 70% ethanol with a pipette and replace it with 100% ethanol. Repeat step 1.2.
- Remove 100% ethanol and dry the seeds by leaving the microcentrifuge tube lids open in a sterile air flow.
- Once the seeds are dry, spread them out evenly onto 0.5x Murashige and Skoog (MS) medium with 0.7% phyto agar by flicking the tube or using sterile forceps. Close the agar plates and seal them with 1.25 cm micropore tape.
- Place the plates horizontally on a shelf in the growth chamber (16 h light/8 h dark, 22 °C day/18 °C night, 150-160 μmol m-2 s-1).
2. Preparation of seedlings: Transfer of germinated seeds to fresh plates
NOTE: The following steps are specific for A. thaliana and are not needed for other species.
- After germination, transfer 5 to 6 seedlings to new 0.7% phyto agar plates with 0.5x MS medium by placing the seedlings linearly, approximately 3 cm from the top (see the position of the rosette in Figure 2D).
- Seal agar plates with 1.25 cm micropore tape and grow vertically in the growth chamber (Figure 2D) until the seedlings are ready to be transferred into jars at 15-18 days after germination (Table 2).
3. Preparation of hydroponic system: Jar setup
- Add 150 mL of clean 5 mm glass beads to each clean jar and close with the lid (Figure 1A).
NOTE: In this protocol, 5 mm glass beads are suitable for most species22, but the bead size can be adjusted if desired. - Place enough aluminum foil over the closed jars to cover the lid-to-jar junction and autoclave (121 °C for 20 min) (Figure 1B).
- Keep the prepared jars covered until the plants are ready to be transferred (Table 2).
4. Preparation of hydroponic system: Addition of seedlings
- When the seedlings are ready to be transferred into jars (Table 2), remove the aluminum foil and lids of the jars in the sterile bench.
- If the jars are to be inoculated with bacteria, perform the following steps before moving on to step 4.3; otherwise, skip step 4.2.
- Using a sterile inoculation loop, pick single colonies from pure bacterial cultures on agar plates and suspend them in 750 µL of sterile 10 mM MgCl2. Repeat until the solution is turbid.
- Optional: Wash the suspension 3x with 750 µL of sterile 10 mM MgCl2 by 5 min centrifugation at 1,000 × g and room temperature followed by the removal of the supernatant and resuspension of the pellet in 750 µL of 10 mM MgCl2.
- Optional: If inoculating several different bacterial species, mix the suspensions of single strains prepared in steps 4.2.1-4.2.2 in equal ratios before proceeding.
- Adjust the optical density at wavelength 600 nm (OD600) to 0.2-0.4 in 0.5x MS medium (pH 5.7 to 5.9, adjusted with KOH) or another growth medium of choice (see step 4.3). Measure the OD600 again to check if the solution was diluted correctly.
CAUTION: KOH is corrosive; wear gloves and lab coat. - Dilute the suspension further to OD600 0.002-0.004 in the same medium (1:100 dilution).
NOTE: Final cell densities will depend on the bacterial strain used, but in our experience, this inoculum corresponds to approximately 3-6 × 105 cells mL-1. - Add 35 mL of the final dilution per jar. Add 35 mL of sterile growth medium to control jars. Stir the beads to evenly coat with 0.5x MS media before plant transfer so that the roots do not dry. Proceed to step 4.4.
- Add 35 mL of 0.5x MS medium to each jar (pH 5.7 to 5.9, adjust with KOH). Stir the beads to evenly coat with 0.5x MS media before plant transfer so roots do not dry.
NOTE: The growth medium can be modified, for example, by the removal of nutrients, the addition of osmolytes to induce salt stress, or the use of different pH values or growth media. CAUTION: KOH is corrosive; wear gloves and lab coat. - Place 3-5 A. thaliana plants into a jar by placing the root systems between the glass beads.
NOTE: The number of plants per jar is different for other species (Table 2). - Move the beads around with sterile forceps or spoons to cover the roots and lift the leaves out of the media (Figure 1C).
NOTE: Carefully move the plants and beads to avoid breaking the roots and stressing the plants. - Add 2 strips of 1.25 cm micropore tape across the top of the jars and gently place the lid on top (Figure 1D-F).
NOTE: Do not push down on the lid to avoid obstructing air exchange. - Cover the gap between the lid and jar with 2.5 cm micropore tape to maintain sterility while allowing air exchange, and place the jars into a growth chamber (Figure 1G,H).
- Set up 4-8 jars per experimental condition, depending on the biological question and the variability expected.
- Make sure to include biological negative controls (e.g., jars without plants) to account for the metabolite background of the experimental system. Set up the same number of replicates for negative controls as for experimental treatments (e.g., quadruplicates).
- For extended growth periods, replace the growth medium weekly: remove the remainder of the growth medium with a 25 mL volumetric pipet and add in 35 mL of fresh medium.
5. Collection of root exudates
- When the plants reach the desired age, remove the 2.5 cm micropore tape and lid in the sterile bench.
NOTE: For A. thaliana in our growth conditions, 21 days after germination represents a mature vegetative stage before flowering onset. The stage of vegetative development is specific to the plant species, and the timing of the growth period changes with plant species; this timing can be modified depending on the research question. - For sterility testing, aliquot 20 µL of growth medium and pipet onto LB agar plates.
- For inoculated jars, aliquot 100 µL of growth medium to use for Colony-forming Unit (CFU) counts (e.g., in a dilution series23).
NOTE: In our experience, bacterial densities range within 105-108 live cells mL-1 of growth medium.
- For inoculated jars, aliquot 100 µL of growth medium to use for Colony-forming Unit (CFU) counts (e.g., in a dilution series23).
- Remove as much growth medium as possible with a 25 mL volumetric pipet, avoiding damage to the roots.
- Add 50 mL of collection medium (e.g., 20 mM ammonium acetate; pH 5.7-5.9 adjusted with HCl) by pipetting along the walls of the jar to avoid wetting the leaves. Close the jars with lids and incubate them in sterile conditions for the desired time of exudate collection. For time-sensitive experiments, 2 h is a good collection window.
CAUTION: HCl is corrosive; wear gloves and lab coat. For longer root exudate collection times, wrap the lids again with tape and move the jars to the growth chamber. - After 2 h, remove the desired amount of collection media into tubes (e.g., 50 mL for assays or analyses using concentrated exudates, or 2 mL for direct use). Store the collected root exudates at -80 °C until further processing or analysis.
- When working with inoculated jars, centrifuge the collected exudates for 10 min at 5,000 × g and 4 °C and only use supernatants for the analysis. Alternatively, filter-sterilize the exudates with a 0.22 μm or 0.45 μm filter.
- If the experiment is part of a time series, remove all remaining collection medium from jars and add 35 mL of 0.5x MS medium. Replace the lid and 2.5 cm tape before returning the jars to the growth chamber.
- Measure the root and shoot fresh weight of all the plants in one jar to later normalize root exudates by plant weight.
NOTE: Plant tissues can be sampled in addition if desired.
6. Processing root exudates for mass spectrometry
- Depending on the analytical method, freeze-dry the root exudates to concentrate and remove the collection medium (-80 °C at 0.37 mbar until no liquid is present). Store at -80 °C until ready to analyze.
NOTE: The data presented here was generated with flow-injection TOF-MS analysis on an accurate-mass quadrupole time-of-flight instrument. - Prior to injection into the analytical instrument, reconstitute freeze-dried exudates or dilute unprocessed root exudates with collection medium according to the root fresh weight.
NOTE: The collection medium was chosen to be compatible with the mass spectrometer. However, depending on the analytical method, desalting steps might be necessary before injection.
Representative Results
The experimental system introduced here allows control of experimental and environmental factors altering root exudation profiles. We compared A. thaliana growth under different lighting conditions, plant age, and plant densities across two different laboratories (Figure 3). Plants looked healthy across laboratories (Figure 3A,B). Short-day conditions (10 h lights vs. 16 h light, Figure 3) resulted in higher root mass compared to long-day conditions (Figure 3C,D). Similarly, the total root mass of all plants grown in long-day conditions was smaller than in short-day (Figure 3C). Overall, the variability of growth within and between jars was low across laboratories.
The glass jar system is compatible with a variety of plant species and developmental stages. The endpoint for root exudate analysis is typically 21 days, as in our experimental conditions, many plant species are in a mature vegetative stage before transitioning to the reproductive stage (Figure 4A–E). Plants can easily be removed from the experimental system without visible damage to the root system. Thus, determining tissue weights or using tissues for downstream analysis is straightforward. The highest shoot weights were found for wheat, followed by sorghum and tomato. The highest root weights were found for sorghum, followed by wheat and M. truncatula. The root:shoot ratio differs between species. Overall, tissue weights varied between 100 mg and 800 mg.
A central aspect of experimental systems allowing root exudate collection is the controlled inoculation with microbes to study plant-microbe interactions in a defined environment. The experimental system presented can be kept sterile even with repeated manipulation (see 'control' condition in Figure 5), and bacteria can be added and maintained for extended periods. When 33-day-old A. thaliana plants were inoculated with a mixture of commensal bacteria at OD600 0.004, bacteria persisted for 12 days, which was the full duration of the experiment (Figure 5). Colony-forming units even increased 100-fold in the growth medium and also colonized the roots (Figure 5C). Phenotypes of inoculated plants were indistinguishable from those of sterile plants (Figure 5A,B).
The presented experimental system allows exudate collection in many experimental conditions. Here, we present exudation profiles of A. thaliana Col-0 collected either in ammonium acetate or in water from the same plants consecutively (Figure 6A). Exudates were stored at -80 °C until analysis, normalized by root weight, and analyzed by mass spectrometry. Samples of jars containing plants were filtered against experimental controls (jars without plants). Of 2,163 metabolites, 436 showed a signal above the background (20.16%) and were retained for analysis. Of these, 416 or 95% of compounds showed distinct abundance between the experimental conditions. However, 26 metabolites could not be attributed to any kind of compound and are thus not defined. Most metabolites (406 or 98%) were more abundant in water-collected exudates. The consecutive collection of exudates first in ammonium acetate and later in water might impact the exudation profile, as metabolic signals might dilute with time. However, the almost exclusively higher exudation signal in water collected as a second timepoint does not support this hypothesis: Exudate collection in pure water likely creates an osmotic shock for plants, which causes the increased metabolite abundances compared to a collection solvent equimolar to the growth solution (20 mM ammonium acetate is equimolar to 0.5x MS medium). Investigation of chemical classes of identified compounds of both growth conditions showed that the majority of the compounds are organic acids and derivatives (28.6%) followed by organic oxygen compounds (18%), organoheterocyclic compounds (14.2%), and lipids (13.2%). Only a small subset of metabolites belong to phenylpropanoids and polyketides (8.7%) and benzenoids (6%). Organic nitrogen compounds, nucleosides, organosulfur compounds, alkaloids, lignans, and related compounds represent between 2% and 0.5% of the classified compounds (Figure 6B). The distribution of metabolites depicted here corresponds to already published data using other types of root exudates collection systems24.
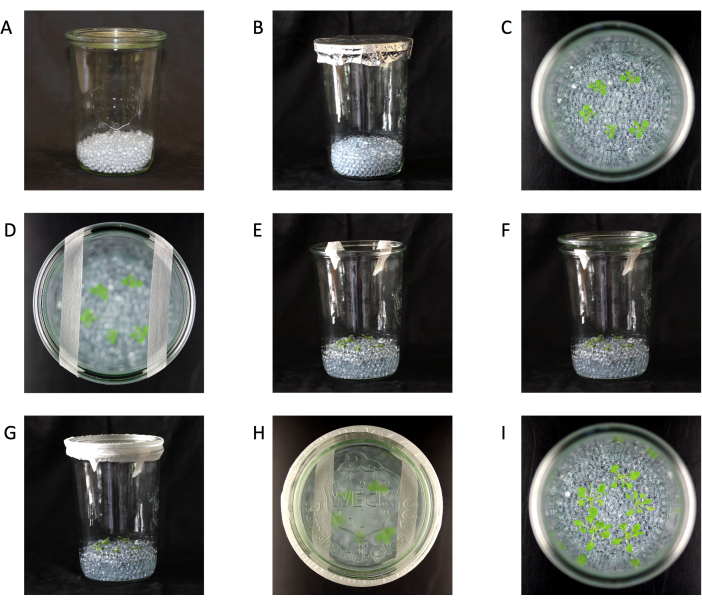
Figure 1: Glass jar setup. (A) Jar with glass beads. (B) Jar with glass beads ready to be autoclaved. (C) Seedlings in jars (top view). (D) Seedlings in a jar (top view) with 1.25 cm micropore tape. (E) Seedlings in jars (side view) with 1.25 cm micropore tape. (F) Seedlings in jars (side view) with 1.25 cm micropore tape and lid. (G) Complete jar setup with seedlings in jars (side view) with 1.25 cm micropore tape, lid, and 2.5 cm micropore tape. (H) Complete jar setup (top view). (I) Plants 21 days old and ready for harvest (top view). Jar size 147 mm height x 100 mm diameter. Please click here to view a larger version of this figure.
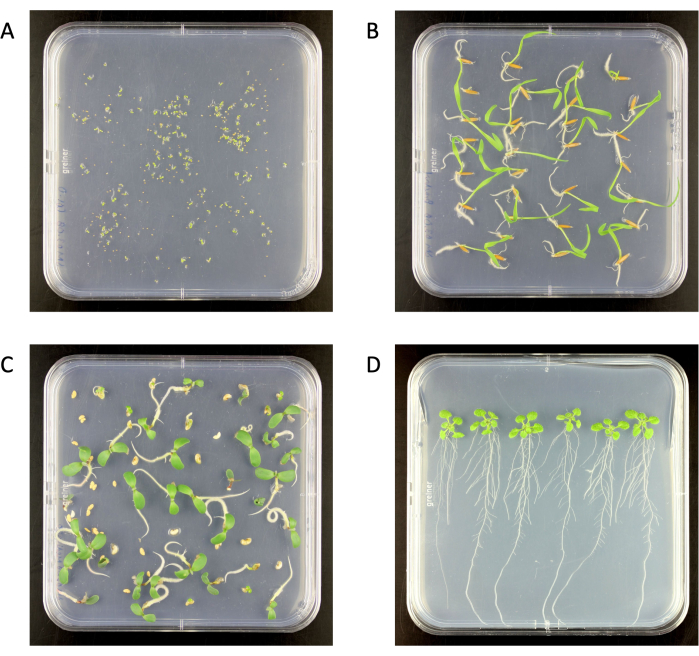
Figure 2: Surface-sterilized seedlings on 0.5x Murashige and Skoog medium agar plates in a growth chamber. (A) Arabidopsis thaliana (6 days old), (B) Brachypodium distachyon (6 days old), (C) Medicago truncatula (6 days old), (D) A. thaliana (18 days old). Agar plate size 120 mm x 120 mm. Please click here to view a larger version of this figure.
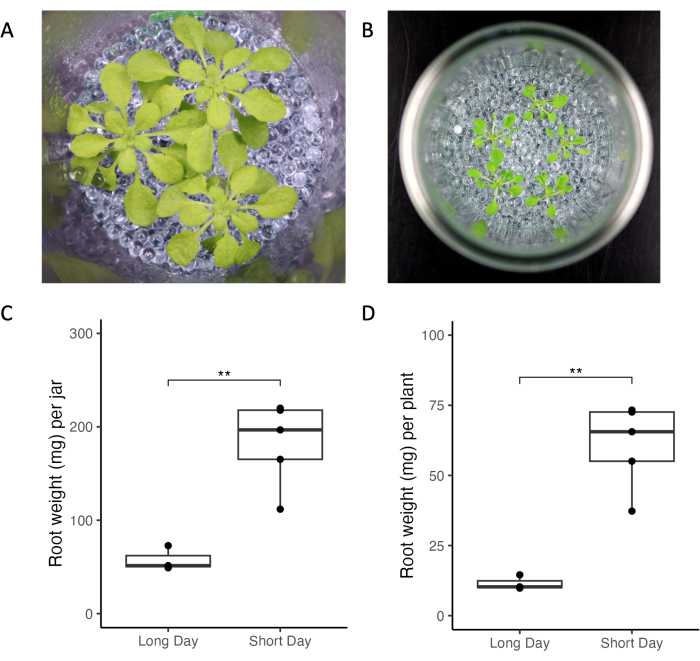
Figure 3: Arabidopsis thaliana Col-0 plants grown in jars under short- and long-day light conditions. (A) Jar with three 33-day-old plants grown in short-day conditions (10 h light/14 h dark, 220 µmol m-2 s-1 light intensity, 21 °C day/18 °C night) and (B) jar with five 21-day-old plants grown in long-day conditions (16 h light/8 h dark, 150-160 µmol m-2 s-1 light intensity, 22 °C day/18 °C night). Root weight of (C) all plants of one jar and (D) of single plants. ** represent significance values of t-test (p < 0.05). Please click here to view a larger version of this figure.
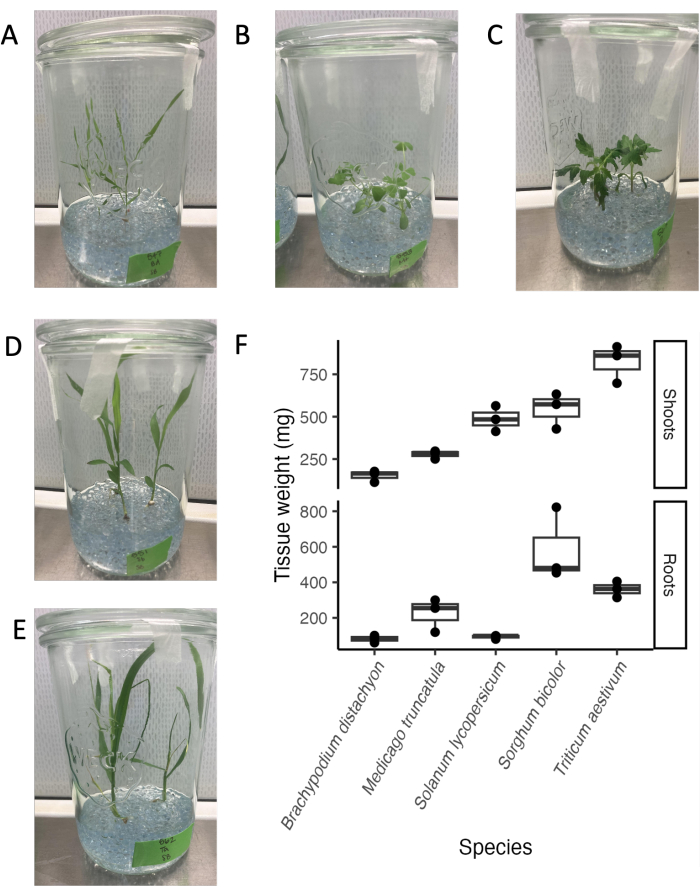
Figure 4: Five phylogenetically distinct species at day 21 in the sterile glass jar system setup. (A) Model monocot Brachypodium distachyon, (B) model legume Medicago truncatula, (C) dicot Solanum lycopersicum (tomato), (D) dicot Sorghum bicolor, (E) monocot Triticum aestivum (wheat). (F) Fresh weight of roots and shoots of the species grown in glass jars. Please click here to view a larger version of this figure.
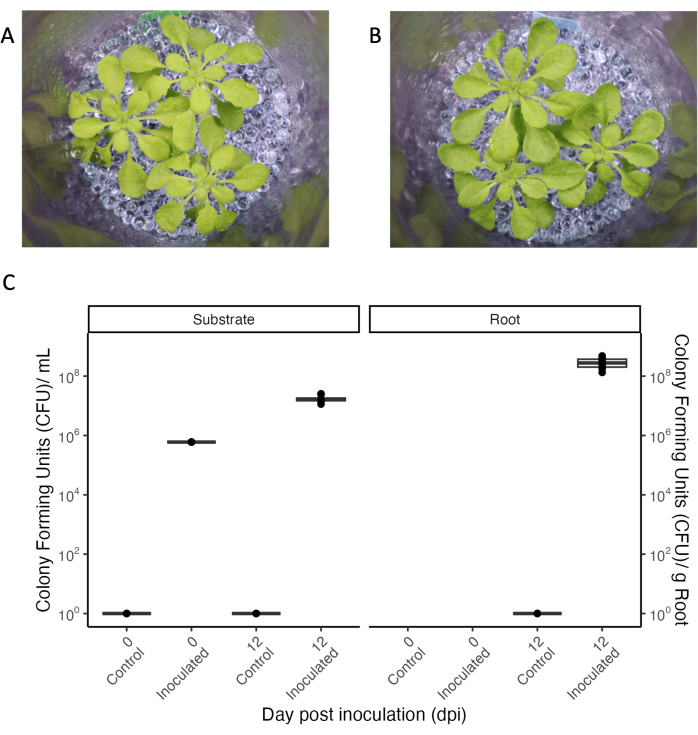
Figure 5: Arabidopsis thaliana Col-0 (33-day-old) grown in short-day conditions. (A) In a sterile setup or (B) inoculated for 12 days with a consortium of commensal bacteria. (C) Colony-forming units for sterile (control) and inoculated jars of the growth substrate (left) and the root (right). N = 4 jars for the sterile control condition, and n = 8 jars for the inoculated condition. Please click here to view a larger version of this figure.
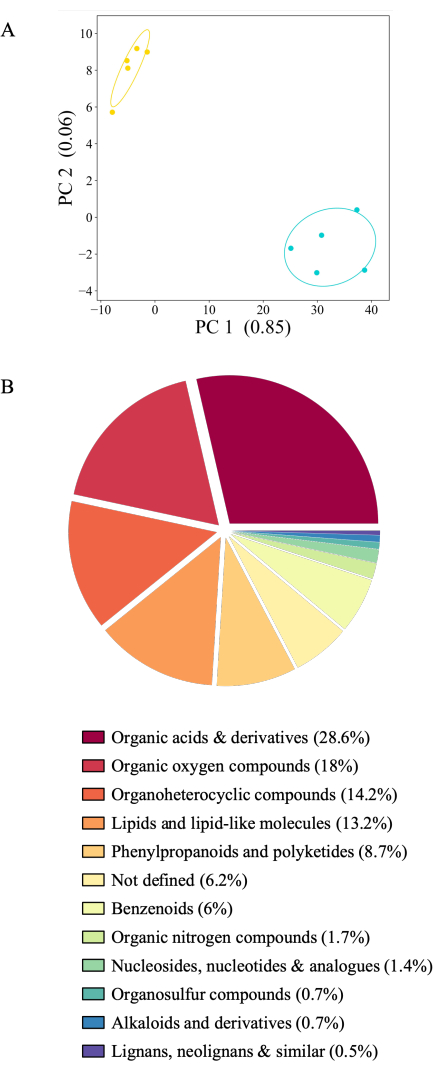
Figure 6: Distinct root exudate profiles for 21-day-old A. thaliana Col-0. Exudates were collected for 2 h in sterile 20 mM ammonium acetate (pH 5.7) followed by 2 h collection in sterile filtered deionized water. Metabolites were detected by mass spectrometry using direct injection. (A) Principal component analysis of 436 metabolites detected above background level (comparison of jars with plants against jars without plants). PC: principal component with the amount of variance explained. Blue: water-collected exudates, yellow: ammonium acetate-collected exudates. (B) Pie chart of 416 metabolites significantly different between experimental conditions (Tukey test) colored according to the superclass (https://cfb.fiehnlab.ucdavis.edu/). Please click here to view a larger version of this figure.
| Species | Seed preparation | Time in 70% ethanol (min) | Concentration of Sodium hypochlorite (NaClO) (v/v % bleach) | Time in bleach (min) | ||
| Arabidopsis thaliana | 15 | None; 100% ethanol | 15 | |||
| Brachypodium distachyon* | Dehusk | 0.5 | 6 | 5 | ||
| Medicago truncatula*a | 30 | 6 | 30 | |||
| Solanum lycopersicum* | 0.5 | 6 | 5 | |||
| Sorghum bicolor* | Dehusk | 0.5 | 6 | 30 | ||
| Triticum aestivum* | Dehusk | 0.5 | 12 | 20 | ||
Table 1: Surface seed sterilization methods for multiple species. *Washed 4-5x with filtered deionized water between ethanol and bleach and at the end; aincubation for 3-6 h after bleach, replacing with filtered deionized water every 30 min.
| Species | Day to jars | Number of plants |
| Brachypodium distachyon | 4 to 5 | 3 |
| Sorghum bicolor | 4 to 5 | 3 |
| Triticum aestivum | 4 to 5 | 2 |
| Medicago truncatula | 5 to 6 | 3 |
| Solanum lycopersicum | 7 to 8 | 3 |
| Arabidopsis thaliana | 17 | 3 to 5 |
Table 2: Age of seedlings in days for transfer to jars and number of plants per jar for various plant species.
Discussion
The experimental system presented here is based on glass jars and glass beads and thus provides a simple, low-maintenance, and versatile semihydroponic system to study root exudation in various contexts. It has been used in studies investigating the exudation profiles of different plant species25, the responses of exudation to different growth conditions25, as well as the influence of soil physiochemical properties on exudation22. The system is suitable for all plant species tested here for extended growth periods, ranging from weeks to months. The maintenance of sterile conditions is straightforward, as is the inoculation with bacteria, which persist over the analyzed 2-week growth period. Thus, the experimental system not only allows for a controlled collection of root exudates in sterile conditions, but it can also be used to study plant-microbe interactions. Furthermore, the plant growth media can be varied to study metabolic responses to different nutrient levels, and growth periods can be adjusted by adapting the light conditions or using different-sized jars.
Studying root exudates in hydroponic or semihydroponic conditions remains standard in the field mainly because of the enhanced resolution of low-concentration metabolites11. Many hydroponic approaches rely on Petri dishes, multi-well plates, or other small containers allowing sterility and high throughput but restricting experimentation to small plants or seedlings grown in high-humidity environments17,18,26,27. In the presented glass jar setup, sufficient head space is provided by the comparably large jars, allowing extended growth periods. Micropore tape stripes secure air exchange whilst maintaining sterility. Thus, even tall monocots such as barley and maize can be grown in the glass jar setup for multiple weeks. Small plants such as A. thaliana and clover can be studied for 4-5 weeks after germination, including vegetative and reproductive stages.
Alternative hydroponic setups are available for larger plants also, but these often require custom-made boxes and inlets made out of mesh, foam boards, and engraftment baskets for plant support15,28,29,30. In addition, these devices usually are not set up to be sterile, or they require challenging setup and maintenance procedures to keep them free from microbial and/or chemical contaminations. Setup and maintenance of sterility in the presented experimental system are straightforward. In addition, the use of glass for jars and beads reduces the presence of contaminants leaching from plastics and saves resources as it can easily be washed and reused.
Glass beads have been applied previously to mimic soil particles. They induce natural root development in root exudation sampling devices such as exudation traps31 or other semihydroponic systems19. The glass-jar setup takes advantage of this development and introduces the beads as a colonization surface for microbes. In soil, the microbiome around plant roots evolves in a semisolid environment, with compact particles and spaces filled with air or water. Even though the glass jar setup does not include active aeration of the growth medium due to which the lower liquid phase likely does not contain optimal oxygen levels, the combination of a larger bead volume with a smaller growth medium volume creates a humid yet aerated upper phase where microbes can grow under oxic conditions. Others have proposed to shake growth containers26,28 or use tubing coupled to air pumps19,29 to maintain air supply in hydroponic growth systems. However, those systems either are set up to not be sterile, or require specialized material and constant surveillance to maintain sterility. In addition, in the case of shaking, much care to avoid submerging of shoots in growth solutions and damage to root systems. Nevertheless, if desired, the experimental setup presented could be adapted with additional material for aeration.
A crucial aspect to consider in all plant-microbe interaction studies investigating metabolism is that microbes degrade plant-derived compounds and produce metabolites on their own. Without a specialized sterile experimental setup, it is not possible to distinguish between plant- and microbe-derived metabolites. To inhibit microbial activity and enrich plant-derived compounds, Oburger et al. proposed to chemically sterilize the root exudate sampling solution to inhibit bacterial degradation32. The effect of chemical inhibitors could be studied in the presented experimental system, comparing exudation profiles of sterile versus nonsterile plants treated with or without the inhibitor.
A main limitation of the presented glass jar setup is that the growth conditions remain very artificial compared to soil. Exudates from soil grown-plants are often either collected from percolation systems13, where solvent flowthroughs are gathered at the base of growth containers, or soil-hydroponic hybrid systems, where plants are initially grown in soil and then transferred to hydroponic conditions16,33. In contrast to the glass jar setup, these procedures usually are destructive, not allowing for multiple collections over time in changing growth environments. Furthermore, whilst in percolating systems, the soil background is sampled together with the exudates, in soil-hydroponic hybrid systems the problem of high soil metabolic background is circumvented with the transfer to hydroponic conditions for exudate collection. Although recovery times have been implemented to reduce metabolite leakage via wounded roots11, the plant transfer is very disruptive and wounds are likely to persist, and plant metabolism might change in response to transfer to hydroponic conditions. Moreover, in many instances, an osmotic shock is induced by transferring plants to water instead of a suitable growth solution16,33. In the presented protocol, the growth solution is exchanged with an equimolar solution to maintain osmotic balance, still allowing to capture exudation within a short, defined time window. The change of growth solution is common practice in many published studies and can easily be achieved in hydroponic setups without root wounding12,16,26,34. Due to its versatility, the experimental system presented can easily be adapted to mimic more natural conditions, for example, by using sterile or nonsterile soil extract as a growth solution with or without the presence of solid soil particles. The gradual change towards natural conditions allows for the study of the impact of the different physiochemical soil properties and microbial presence on plant metabolism and physiology. Before the scientific community has a good understanding of exudation in various environments, it is desirable to employ soil-based and hydroponic systems in parallel, as both setups have their advantages and limitations13.
In conclusion, the presented semihydroponic, glass-based experimental setup stands out because of its simplicity combined with high versatility of applications. It presents an accessible, low-cost way to collect and study exudation in sterile conditions, or in combination with microbes and plant-microbe interactions.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
We thank Prof. Dr. Nicola Zamboni and Prof. Dr. Uwe Sauer from ETH Zürich, Switzerland, for determining the root exudation profiles with direct injection and Prof. Dr. Klaus Schläppi from the University of Basel for the A. thaliana commensal bacteria. Further, we acknowledge the Swiss National Science Foundation (PR00P3_185831 to J.S., supporting S.M., A.S., E.M.S.) and the PSC-Syngenta Fellowship program (granted to Prof. Dr. Klaus Schläppi and J.S., supporting C.J.).
Materials
| Agar powder for bacteriology | VWR | 20767.298 | |
| Aluminum foil | FORA GmbH | ||
| Ammonium acetate | Sigma-Aldrich | 32301-1KG | ACS reagent, Eur >- 98% |
| Autoclave VX-150 | Systec | 1150 | |
| Balance | Sartorius | QUINTIX64-1S | |
| Centrifuge | Hermle Labortechnik GmbH | 305.00 V05 | |
| Cuvettes | Greiner Bio-One | 613101 | |
| Difco LB Broth, Lennox | BD | 240210 | |
| Ethanol | Reuss-Chemie AG | RC-A15-A-005L | |
| Filtered deionized water | Merck Millipore | Milli-Q IQ7000 | |
| Glass beads | Carl Roth | HH56.1 | 5 mm |
| Hydrochloric acid | Merk | 1.00317.1000 | |
| Inoculation loop | Karl Hammacher GmbH | HWO_070-21 | |
| Jars | Weck | 105741 | 850 mL |
| Lyophilizer | Christ | Alpha 2-4 LSCplus | |
| Magnesium chloride hexahydrate | Carl Roth | 2189.1 | |
| Matrix Orbital thermoshaker | IKA | 10006248 | |
| Microcentrifuge tube | Sarstedt AG & Co. KG | 72.695.500 | SafeSeal reaction tube, 2 mL, PP |
| Micropore tape | 3M | 1530-0 | 1.25 cm x 9.1 m |
| Micropore tape | 3M | 1530-1 | 2.5 cm x 9.1 m |
| Murashige & Skoog Medium (MS) | Duchefa Biochemie | M0221.0050 | |
| Growth chamber | Percival | SE41-TLCU4 | 16 hour light/8 dark. 22 °C day/18 night |
| Phyto agar | Duchefa Biochemie | P1003.1000 | |
| Potassium hydroxide | Sigma-Aldrich | 8.14353.0100 | |
| SmartSpec Plus Spectrophotometer | Bio-Rad | 170-2525 | |
| Sodium hypochlorite solution, 12% Cl | Carl Roth | 9062.4 | |
| Square petri dish | Greiner Bio-One | 688102 | 120x120x17 mm, with vents |
| Stericup Quick release | Millipore | S2GPU05RE | 0.22 µm PES, 500 mL |
| Sterile bench | FASTER S.r.l. | FlowFast H 18 |
Referenzen
- Sasse, J., Martinoia, E., Northen, T. Feed your friends: do plant exudates shape the root microbiome. Trends in Plant Science. 23 (1), 25-41 (2018).
- Lundberg, D. S., et al. Defining the core Arabidopsis thaliana root microbiome. Nature. 488 (7409), 86-90 (2012).
- Lopez, J. L., et al. Growth rate is a dominant factor predicting the rhizosphere effect. The ISME Journal. 17 (9), 1396-1405 (2023).
- Hu, L., et al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nature Communications. 9 (1), 2738 (2018).
- Edwards, J., et al. Structure, variation, and assembly of the root-associated microbiomes of rice. Proceedings of the National Academy of Sciences. 112 (8), 911-920 (2015).
- Bulgarelli, D., et al. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe. 17 (3), 392-403 (2015).
- Li, J., Wang, J., Liu, H., Macdonald, C. A., Singh, B. K. Application of microbial inoculants significantly enhances crop productivity: A meta-analysis of studies from 2010 to 2020. Journal of Sustainable Agriculture and Environment. 1 (3), 216-225 (2022).
- Escudero-Martinez, C., Bulgarelli, D. Engineering the crop microbiota through host genetics. Annual Review of Phytopathology. 61, 257-277 (2023).
- Poppeliers, S. W., Sanchez-Gil, J. J., de Jonge, R. Microbes to support plant health: understanding bioinoculant success in complex conditions. Current Opinion in Microbiology. 73, 102286 (2023).
- O’Callaghan, M., Ballard, R. A., Wright, D. Soil microbial inoculants for sustainable agriculture: Limitations and opportunities. Soil Use and Management. 38 (3), 1340-1369 (2022).
- Oburger, E., Jones, D. L. Sampling root exudates – Mission impossible. Rhizosphere. 6, 116-133 (2018).
- Yuan, J., et al. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome. 6 (1), 156 (2018).
- Vismans, G., et al. Coumarin biosynthesis genes are required after foliar pathogen infection for the creation of a microbial soil-borne legacy that primes plants for SA-dependent defenses. Scientific Reports. 12 (1), 22473 (2022).
- Strehmel, N., Böttcher, C., Schmidt, S., Scheel, D. Profiling of secondary metabolites in root exudates of Arabidopsis thaliana. Phytochemistry. 108, 35-46 (2014).
- Song, Y., Pieterse, C. M. J., Bakker, P., Berendsen, R. L. Collection of sterile root exudates from foliar pathogen-inoculated plants. Methods in Molecular Biology. 2232, 305-317 (2021).
- Oburger, E., et al. A quick and simple spectrophotometric method to determine total carbon concentrations in root exudate samples of grass species. Plant Soil. 478 (1-2), 273-281 (2022).
- Koprivova, A., et al. Root-specific camalexin biosynthesis controls the plant growth-promoting effects of multiple bacterial strains. Proceedings of the National Academy of Sciences of the United States of America. 116 (31), 15735-15744 (2019).
- Gao, J., et al. Ecosystem fabrication (EcoFAB) protocols for the construction of laboratory ecosystems designed to study plant-microbe interactions. Journal of Visualized Experiments. (134), e57170 (2018).
- Lopez-Guerrero, M. G., et al. A glass bead semi-hydroponic system for intact maize root exudate analysis and phenotyping. Plant Methods. 18 (1), 25 (2022).
- Boeuf-Tremblay, V., Plantureux, S., Guckert, A. Influence of mechanical impedance on root exudation of maize seedlings at two development stages. Plant and Soil. 172, 279-287 (1995).
- Groleau-Renaud, V., Plantureux, S., Guckert, A. Influence of plant morphology on root exudation of maize subjected to mechanical impedance in hydroponic conditions. Plant and Soil. 201, 231-239 (1998).
- Sasse, J., et al. Root morphology and exudate availability are shaped by particle size and chemistry in Brachypodium distachyon. Plant Direct. 4 (7), 00207 (2020).
- Katagiri, F., Thilmony, R., He, S. Y. The Arabidopsis thaliana-Pseudomonas syringae interaction. Arabidopsis Book. 1, 0039 (2002).
- Monchgesang, S., et al. Natural variation of root exudates in Arabidopsis thaliana-linking metabolomic and genomic data. Scientific Reports. 6, 29033 (2016).
- McLaughlin, S., Zhalnina, K., Kosina, S., Northen, T. R., Sasse, J. The core metabolome and root exudation dynamics of three phylogenetically distinct plant species. Nature Communications. 14 (1), 1649 (2023).
- Badri, D. V., Chaparro, J. M., Zhang, R., Shen, Q., Vivanco, J. M. Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. Journal of Biological Chemistry. 288 (7), 4502-4512 (2013).
- Massalha, H., Korenblum, E., Malitsky, S., Shapiro, O. H., Aharoni, A. Live imaging of root-bacteria interactions in a microfluidics setup. Proceedings of the National Academy of Sciences of the United States of America. 114 (17), 4549-4554 (2017).
- Nathoo, N., Bernards, M. A., MacDonald, J., Yuan, Z. C. A hydroponic co-cultivation system for simultaneous and systematic analysis of plant/microbe molecular interactions and signaling. Journal of Visualized Experiments. (125), e55955 (2017).
- Nguyen, N. T., McInturf, S. A., Mendoza-Cozatl, D. G. Hydroponics: a versatile system to study nutrient allocation and plant responses to nutrient availability and exposure to toxic elements. Journal of Visualized Experiments. (113), e54317 (2016).
- Yi, Y., Li, Z., Kuipers, O. P. Plant-microbe interaction: transcriptional response of Bacillus Mycoides to potato root exudates. Journal of Visualized Experiments. (137), e57606 (2018).
- Phillips, R. P., Erlitz, Y., Bier, R., Bernhardt, E. S. New approach for capturing soluble root exudates in forest soils. Functional Ecology. 22 (6), 990-999 (2008).
- Oburger, E., et al. Root exudation of phytosiderophores from soil-grown wheat. New Phytologist. 203 (4), 1161-1174 (2014).
- Neal, A. L., Ahmad, S., Gordon-Weeks, R., Ton, J. Benzoxazinoids in root exudates of maize attract Pseudomonas putida to the rhizosphere. PLoS One. 7 (4), e35498 (2012).
- Miao, Y., et al. Exogenous salicylic acid alleviates salt stress by improving leaf photosynthesis and root system architecture in cucumber seedlings. Scientia Horticulturae. 272, 109577 (2020).

