A Thermoplasmonic Approach for Investigating Plasma Membrane Repair in Living Cells and Model Membranes
Summary
The thermoplasmonic puncture method integrates confocal microscopy, optical tweezers, and gold nanoparticles to study protein responses during plasma membrane repair in cells and giant unilamellar vesicles. The technique enables rapid and localized membrane puncture, allowing the identification of key proteins and their functional roles in the intricate plasma membrane repair machinery.
Abstract
The cell membrane is crucial for cell survival, and ensuring its integrity is essential as the cell experiences injuries throughout its entire life cycle. To prevent damage to the membrane, cells have developed efficient plasma membrane repair mechanisms. These repair mechanisms can be studied by combining confocal microscopy and nanoscale thermoplasmonics to identify and investigate the role of key proteins, such as annexins, involved in surface repair in living cells and membrane model systems.
The puncturing method employs a laser to induce highly localized heating upon nanoparticle irradiation. The use of near-infrared light minimizes phototoxicity in the biological sample, while the majority of the absorption takes place in the near-infrared resonant plasmonic nanoparticle. This thermoplasmonic method has been exploited for potential photothermal and biophysical research to enhance the understanding of intracellular mechanisms and cellular responses through vesicle and cell fusion studies. The approach has shown to be complementary to existing methods for membrane disruption, such as mechanically, chemically, or optically induced injuries, and provides a high level of control by inflicting extremely localized injuries. The extent of the injury is limited to the vicinity of the spherical nanoparticle, and no detrimental damage occurs along the beam path as opposed to pulsed lasers using different wavelengths. Despite certain limitations, such as the formation of nanobubbles, the thermoplasmonic method offers a unique tool for investigating cellular responses in plasma membrane repair in an almost native environment without compromising cell viability.
When integrated with confocal microscopy, the puncturing method can provide a mechanistic understanding of membrane dynamics in model membrane systems as well as quantitative information on protein responses to membrane damage, including protein recruitment and their biophysical function. Overall, the application of this method to reduced model systems can enhance our understanding of the intricate plasma membrane repair machinery in living cells.
Introduction
The cell membrane, serving both as a physical barrier and a signaling platform, is vital for cell survival1. Throughout its entire cell cycle, the plasma membrane (PM) is subjected to damage, such as mechanical2,3,4,5 and chemical6 stress-induced injuries. To maintain membrane integrity and ensure cell survival, the cell has developed robust plasma membrane repair (PMR) mechanisms. These mechanisms depend on various strategies, such as cytoskeleton reorganization, membrane fusion, and membrane replacement strategies7,8,9,10,11, all of which rely on the recruitment of specific proteins. Notably, members of the annexin protein family have been identified as key proteins associated with the processes of PMR1,9,12,13,14,15,16. Following PM injury, the cell experiences an influx of calcium ions (Ca2+), which poses an immediate threat to the cell's survival17. In response to Ca2+ influx, annexin proteins, which are predominantly located in the cytosol, bind to the inner leaflet of the damaged plasma membrane as a part of the PMR strategies18. Annexin A2 (ANXA2) was one of the first members of the annexin family to be associated with PMR in dysferlin-deficient muscular dystrophy and was suggested to mediate repair by fusing intracellular vesicles to the PM near the injury site5,19,20,21. Subsequently, several functions have been attributed to annexins22, and their role in PMR has garnered increased attention over the past 20 years. However, the exact role of annexins in PMR remains not fully understood15,18,21,22.
This article proposes a method for investigating protein-membrane interaction and membrane dynamics in a controlled and highly localized manner, utilizing a combination of confocal microscopy, optical tweezers, and gold nanoparticles (AuNPs). This method enables the quantitative study of protein, lipid, and small molecule interactions in response to membrane damage and Ca2+ influx. Despite the complexity and multiplicity of components involved in the process of membrane repair, simplified membrane systems that mimic the plasma membrane have been employed to gain a deeper mechanistic understanding of membrane dynamics and the response of annexin proteins to membrane disruption16. Giant unilamellar lipid vesicles (GUVs) were chosen as the model membrane system with a specified lipid composition. The GUVs were generated using the gel-assisted hydration method, specifically polyvinyl alcohol gel hydration, as described by Weinberger et al.23, which allowed for efficient encapsulation of annexins into GUVs.
The utilization of near-infrared (NIR) laser irradiation on metallic nanoparticles (NPs) induces significant heating of the NP, making it an effective method to establish a local heat source exploited in biomedical applications24. The method was initially used to directly measure the temperature surrounding a single AuNP in both 2D and 3D biomimetic assays. In these assays25,26, the plasmonic nanoparticles were irradiated on a supported lipid bilayer or optically trapped near GUVs undergoing a local thermal phase transition upon local heating, enabling quantification and control of the exact temperature profile around the particle. This reference temperature profile has been utilized when investigating or manipulating biological specimens. Further advancements in the method have facilitated the induction of nanoscopic pores in membranes27, allowing for vesicle and cell fusion28,29. Other studies have investigated the behavior of peripheral membrane proteins in GUVs29 and transmembrane proteins30 by creating novel hybrid vesicles, while cell-specific drug delivery has also been explored to control and study cellular responses or gene expression28,29,31,32,33. Recently, the method has been used to investigate protein responses to membrane damage32,34,35.
Several methods exist for disrupting the plasma membrane to explore cellular responses and membrane repair. These include microneedle punctures, microbead shaking, and cell scraping, all of which can disrupt the cell membrane mechanically14,36,37. Chemically induced damage can be achieved by adding detergents5,38 or bacterial toxins39,40 that destabilize the lipid bilayer and generate membrane pores across the plasma membrane. Furthermore, optically induced injuries by continuous wave and pulsed lasers have been used to study PMR components, such as annexin proteins5,14,21,41, in combination with plasmonic nanoparticles42,43,44,45. Despite the efficiency of high-power pulsed lasers, they can cause significant injuries and damage to the cell's interior along the beam path. Moreover, the detailed changes that occur in the biological matter upon pulsed laser irradiation and whether it creates a well-defined pore remain to be further investigated. An alternative method is presented in this article, employing thermoplasmonics to induce nanoscopic holes in the PM in a controlled manner34,35 without causing significant harm to the internal structures. This is accomplished by exposing plasmonic NPs to a highly focused NIR laser, resulting in an extremely localized temperature increase that can easily reach temperatures exceeding 200 °C, which can lead to small nanoscopic explosions25,46,47. This process can be controlled by adjusting the laser intensity as well as the size, shape, and composition of the NPs48. By employing this technique, researchers can explore the role of proteins in PM repair in living cells, which could help address some of the unanswered questions regarding the involvement of annexin proteins in membrane repair without compromising cell viability.
The optical trapping of plasmonic nanoparticles has been well established by previous studies25,49,50,51,52; however, additional insights regarding the thermoplasmonic properties of the nanoparticles53,54,55 can be obtained in the supplementary materials (Supplementary File 1). The thermoplasmonic method can be used to create nanoscopic holes in the PM for the purpose of studying the cellular response and repair mechanisms. More precisely, the puncture can be achieved through the optical heating of AuNPs in close proximity to the membrane, as shown in Figures 1A and B. This localized puncture allows for Ca2+ influx, which was verified by a calcium sensor, thus activating the PMR machinery. For live cell experiments, AuNPs with a diameter of 200 nm were immobilized on the surface beneath the cell to monitor the role of ANXA2 in PMR via confocal microscopy. The NIR laser (Figure 1A,B), with a wavelength of 1064 nm, irradiates the AuNP, exploiting its plasmonic properties (Figure 1C), resulting in substantial local heating (Figure 1D) in the biological transparency window49 while causing minimal damage to the cell itself. The high-temperature region surrounding the AuNP rapidly decreases by 30-40% at a distance corresponding to the radius of the NP, as depicted in Figure 1E, allowing for an extremely confined injury in all three dimensions.
Supplementary File 1. Please click here to download this File.
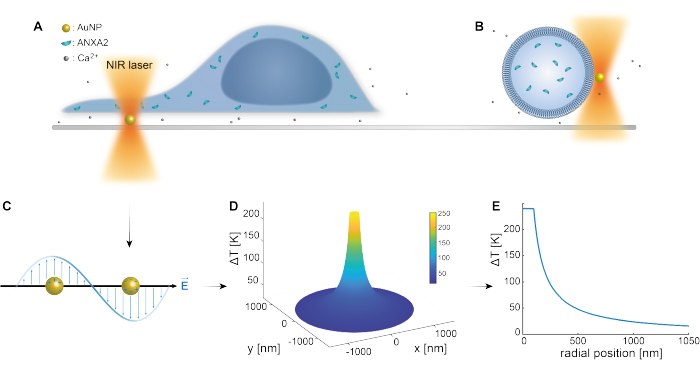
Figure 1: Schematic outline of the experimental method. (A) ANXA-transfected cells are situated on top of immobilized gold nanoparticles (AuNPs) on the surface, or (B) giant unilamellar vesicles (GUVs) with encapsulated ANXA are suspended in a medium containing AuNPs. (C) A single AuNP is irradiated by the NIR optical trap, where the interaction between the incoming electromagnetic field and the conduction electrons leads to the collective oscillation of electrons within the NP. (D) This process results in a highly confined yet significant increase in temperature. To estimate the temperature at the NP surface, Mie theory is employed, and a (E) temperature profile is calculated for an AuNP with a diameter of 200 nm and laser intensity I = 6.36 x 108 W/cm2. Please click here to view a larger version of this figure.
To minimize the thermal effect on the cell membrane, the AuNPs are only irradiated for ~1 second. This causes a transient and local burst of heating, which reduces the damage to proteins that typically require more time to unfold. Upon membrane puncture, annexin proteins are recruited in a fraction of a second, and within a few seconds, an annexin ring-like scaffold is formed around the site of injury (Figure 2). This approach has also been applied to explore the involvement of ANXA5 in both living cells and model membranes16 in an effort to shed light on the complete scheme of the repair processes. While the primary focus has been on the correlating recruitment of various annexin proteins, the biophysical aspects of the repair mechanism have yet to be elucidated.
To fully implement the proposed method, three key components are required: confocal microscopy, optical tweezers, and metal nanoparticles. Optical tweezers are used to trap AuNPs, and their construction can be achieved by following the procedure outlined by Neuman et al.49. However, if building an optical tweezer proves to be too challenging, a tightly focused NIR laser can be used to irradiate AuNPs immobilized beneath the cells. While spherical AuNPs were chosen for this protocol, a variety of plasmonic particles with tunable absorption spectra could also be utilized to achieve a highly localized temperature gradient within the NIR region48.
Fluorescence imaging is necessary for observing the role of the fluorescently labeled proteins, and therefore, total internal reflection microscopy (TIRF)56 could be considered as an alternative to confocal imaging. However, this technique only allows for surface imaging and would not be compatible with the model membrane vesicle experiments. Consequently, both the optical tweezers and confocal microscope are essential for the precise localization of the nanoparticle and detailed investigation of the local area surrounding the cell injury. To effectively irradiate the nanoparticle with a diffraction-limited laser focus, it is necessary to visualize the nanoparticle. This can be optimally achieved by reflection microscopy, which is a standard imaging feature of Leica confocal microscopes. However, if reflection or scattering imaging is not available, alternative methods, such as the less efficient fluorescent AuNP labeling, may be considered.
In summary, the highly controllable and localized thermoplasmonic method presented in this study has the potential to serve as an excellent platform for investigating the molecular components involved in cellular responses and PM repair mechanisms in living cells. In addition to studying the protein response upon PM damage, this approach can also be utilized for locally puncturing vesicles, thereby enabling an investigation of the protein response in both protein-protein and protein-membrane dynamics. Moreover, this method allows for a quantitative analysis of the interactions between proteins, lipids, and small molecules when membranes are disrupted. Collectively, these advances have the potential to shed light on some of the unresolved questions regarding the intricate and complex plasma membrane repair machinery.
Protocol
1. Cell membrane puncture preparation
- Cell seeding (Day 1)
- Culture human embryonic kidney (HEK293T) cells in culture medium at 37 °C in a 5% CO2 humidifier incubator until they reach 70% confluency.
- Detach the cells from the surface using 500 µL of trypsin, count 200,000 HEK293T cells, and seed them in a culture dish with a total volume of 3 mL of culture medium. Incubate the cells at 37 °C in a 5% CO2 humidifier incubator for 24 h.
NOTE: To prevent cell clustering in the center of the dish, spread the cells evenly and avoid swirling the dish, as this can decrease transfection efficiency.
- Cell transfection (Day 2)
NOTE: The transfected cells can be used up to 48 h after transfection.- Pipet the plasmid of interest and the transfection reagent for 5 s before use.
- In a sterile 2 mL tube, mix the following in the specific order: 500 μL of reduced-serum medium, 5 μL of transfection reagent (4 times more than the plasmid), and 1.25 μL of plasmid (1 μg/μL).
NOTE: To investigate calcium influx upon membrane rupture, follow the same procedure but use the membrane-bound calcium sensor probe GCaMP6-CAAX (1 μg/μL). - Gently but thoroughly pipet the transfection mixture and incubate it at room temperature (RT) for 30 min before adding it dropwise to the cells.
- Before adding the transfection mixture, remove the medium from the culture dish, gently wash the cells with 2 mL of phosphate-buffered saline, and add 2000 μL of reduced-serum medium to the dish.
- Incubate the cells together with the transfection mixture at 37 °C in a 5% CO2 humidifier incubator for 2 h and 45 min before changing the medium to 3 mL of culture medium.
- Preparation of gold nanoparticle (AuNP) solution (Day 2)
- Vortex the 200 nm AuNP stock solution for 10 s at level 10 (see Table of Materials for further specification of apparatus), sonicate for 5 min (max amplitude), and vortex again for 10 s.
- Mix 150 μL of AuNPs with 850 μL of distilled water to a total volume of 1 mL.
NOTE: The diluted AuNP solution can be stored in the fridge and reused for up to 1 month.
- Preparation of the experimental dish (Day 2)
- Coat the microwell dish with 150 μL of 0.01%-0.1% cell attachment solution and incubate for 15 min at RT.
- Wash the glass surface twice with 500 μL of distilled water and allow it to air dry for ~10 min.
- Add 80 μL of AuNP solution dropwise to the dry surface.
NOTE: Vortex (10 s), sonicate (5 min), and vortex (10 s) the diluted AuNP solution before adding it to the coated glass surface to minimize AuNP aggregates. - Wait for ~10 min before introducing 1.5 mL of culture medium. Let the dish incubate at 37 °C overnight.
- Preparation of the experimental chamber (Day 3)
NOTE: The experimental chamber can be prepared on either day 3 or 4; however, ensure that the following preparations are done on the same day as the experiment.- Remove the medium from the cells in the culture dish and wash the cells with 2 mL of phosphate-buffered saline.
NOTE: This step is essential to remove any residual medium and debris that could interfere with the subsequent steps. - Add 500 μL of enzyme-based cell detachment solution to the well and incubate for 1-3 min until the cells have detached from the culture dish.
- Add 1.5 mL of fresh culture medium and pipette the cell solution to get a homogenous cell solution to minimize the likelihood of cell clusters.
- Carefully remove the medium from the AuNPs in the experimental microwell.
- Add the cell solution (2 mL) to the microwell and allow it to incubate for at least 5 h before performing the experiment.
NOTE: For optimal experimental conditions, avoid swirling the chamber, as this may cause cells to cluster in the middle of the chamber.
- Remove the medium from the cells in the culture dish and wash the cells with 2 mL of phosphate-buffered saline.
2. Cell membrane puncture experiment
- Optical settings of the experiment
- Conduct the experiments using a confocal scanning microscope combined with a 1064 nm trapping laser57.
- Carry out the optical trapping at the focal plane utilizing a 63x water-immersion objective with a numerical aperture (NA) of 1.2.
- Assume that the focus is the size of an Airy disc and that the focal beam width of the irradiation laser is ~540 nm in radius.
- Convert the laser power (P) to the corresponding laser intensity (I) by calculating the laser power per area (W/cm2).
- Utilize an acousto optical beam splitter (AOBS) to visualize multiple fluorescent signals detected using photomultiplier tubes and simultaneous detection of metallic NPs via their scattering signal.
NOTE: Not all confocal systems are equipped with AOBS, which allows reflection imaging of metallic NPs. Here, other forms of detection must be implemented, or a sequential scanning of the NIR laser in the area underneath the cell can be attempted. - During the experimental sessions, mount a glass-bottom open chamber containing cells, molecular fluorescent probes, and gold nanoparticles on the microscope.
- Move the trap relative to the cells by translating the sample mounted on a piezoelectric stage, enabling nanometer-precise lateral movements16.
NOTE: The optical trap is kept stationary.
- Experimental settings for the cell membrane puncture
- Place HEK293T cells, transfected with annexin plasmids coupled with a fluorescent protein, on top of isolated AuNPs that are immobilized on the glass surface (Figure 1A).
- Utilize an Argon 488 nm laser to visualize the GFP fluorescence signal and a HeNe 633 nm laser for observing AuNP reflection in the scanning confocal microscope.
- Employ the optical tweezer, which operates with a 1064 nm NIR laser, to irradiate a single AuNP for ~1 s, inducing a significant local increase in temperature that disrupts the plasma membrane.
- Apply irradiation between 200-295 mW to the focused particle, resulting in a substancial temperature increase.
NOTE: There is a substantial loss of power within the optics, with the laser power at the focal point reaching around 20% of the stated milliwatt, depending on the specific setup. The specific intensity (measured in W/cm2) depends on the exact alignment of the system, specifically the focal size. Additionally, the heat conduction of glass is higher than that of cell and water and consequently reducing the amount of heat released to the plasma membrane is slightly reduced48,58. - Achieve effective and localized PM injury and subsequent protein repair response by properly calibrating the localization of the NIR laser focus spot. This is achieved by capturing a single AuNP suspended in the same imaging medium and ensuring that the selected AuNP is in focus prior to irradiation.
NOTE: The nanoparticle is considered to be in focus when its scattering signal appears the sharpest, i.e., exhibiting clear edges and the absence of a halo around the particle, when observing it in confocal microscopy (Figure 2C (ii)).
- Cell and AuNP density conditions
- Choose single cells instead of cell clusters to prevent overlap of plasma membranes.
NOTE: The cells should be adhered to the surface, exhibiting a flattened morphology (Supplementary Figure 1), which allows puncturing of the cell periphery while preventing damage to the nuclear membrane (Supplementary Figure 2). - Incubate the cells according to the protocol or until they have settled and flattened to prevent AuNP cellular uptake. Avoid excessive incubation time and reduce the likelihood of AuNP endocytosis using PEGylated AuNPs.
- Ensure that the immobilized AuNPs are present as single particles, adequately spaced apart from each other to prevent aggregates. Aggregates can lead to a significant increase in the thermal gradient, resulting in elevated temperatures that may disrupt a substantial fraction of the cell.
- Replace the sample every 1-2 h to maintain cell health.
NOTE: Prolonged exposure can lead to a deterioration in cell health, compromising their ability to respond accurately to injury in terms of membrane repair. This decline in cell health is typically observed by a change in cell shape, as cells appear more spherical and more rigid, eventually culminating in cell death.
- Choose single cells instead of cell clusters to prevent overlap of plasma membranes.
Supplementary Information. Please click here to download this File.
3. Giant unilamellar vesicle (GUV) preparation
- Preparation of the lipid mixture
- Make the GUV lipid composition by combining 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1,2-dioleoyl-sn-glycero-3-phospho-L-serine (DOPS) lipids in a molar ratio of 4:1 (see Table 1). Aliquot the lipid stock into 1.5 mL glass vials according to the experimental needs and store them at -20 °C.
NOTE: For prolonged lipid preservation and to prevent the oxidation of unsaturated lipids, replace the air with argon in the aliquoted vials. - Before mixing the lipids, thoroughly clean a 50 μL and a 500 μL glass or metal syringe with chloroform five times to ensure they are free of contaminants for the chloroform-dissolved lipids.
CAUTION: Handle chloroform in a fume hood due to its toxicity.- Transfer the calculated volume of chloroform into a clean amber-glass vial, followed by the specified amount of each lipid (see Table 1).
NOTE: To avoid cross-contamination between lipid stocks, ensure that the syringes are cleaned with chloroform. - Finally, add the membrane dye and thoroughly mix the lipids by pipetting. Store the prepared lipid mixture at -20 °C for further use; the mixture remains viable for 2-3 weeks without any considerable lipid damage.
NOTE: The mixing should be done with a metallic or glass syringe. Always keep the lipids on ice when they are out of the freezer.
- Transfer the calculated volume of chloroform into a clean amber-glass vial, followed by the specified amount of each lipid (see Table 1).
- Make the GUV lipid composition by combining 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1,2-dioleoyl-sn-glycero-3-phospho-L-serine (DOPS) lipids in a molar ratio of 4:1 (see Table 1). Aliquot the lipid stock into 1.5 mL glass vials according to the experimental needs and store them at -20 °C.
- Preparation of polyvinyl alcohol (PVA) gel
- Prepare the GUVs using the gel-assisted hydration method described by Weinberger et al.23 with minor modifications.
- Prepare the PVA gel by dissolving 5 g of PVA in 100 mL of a buffer containing 50 mM sucrose, 25 mM NaCl, and 25 mM Tris (pH 7.4).
- Heat the PVA solution to 85 °C and stir until it becomes transparent. Allow it to cool down to RT and store it in a fridge for further use.
NOTE: PVA is not properly dissolved in the buffer, and therefore, heating up to 85 °C is necessary.
- Prepare the GUVs using the gel-assisted hydration method described by Weinberger et al.23 with minor modifications.
- Preparation of glass slides
- Clean glass slides with ethanol and let them air-dry. Then, treat the slides with an air plasma cleaner to remove any residual contamination from the glass surface.
- Preparation of PVA-coated glass slides
- Warm the PVA gel (5%) up to 60 °C for 30 min to increase its fluidity. Apply 90 μL of the warm PVA onto the glass slide, spread uniformly, and let it dry in a heating cabinet at 50 °C for 50 min.
- Once the PVA glass slide is ready, add 30 μL of the prepared lipid mixture using a glass or metal syringe and spread it into a thin film using the edge of the needle.
- Dry the lipid mixture by evaporating its chloroform content under gentle nitrogen pressure. Further, dry the glass slides under a vacuum for 1.5-2 h.
- Growing GUVs in a chamber
- Assemble the in-house chamber, similar in design to a previously published report59, using the prepared glass slide.
- In a separate 2 mL tube, dilute the recombinant protein of interest (in this case, ANXA5 or ANXA4) to a final concentration of 500 nM with a growing buffer (GB) consisting of 80 mM sucrose, 70 mM NaCl, and 25 mM Tris-HCl (pH 7.4).
- Add 400 μL of the diluted recombinant protein solution to the chamber. Incubate the chamber at RT for 1 h to allow GUVs to grow from the deposited lipid coat. Use the same buffer, excluding the protein, as a negative control.
NOTE: Wrap the chamber in polyolefin film to prevent buffer evaporation. - Collect the GUVs by transferring 400 μL of the chamber content to a 2 mL tube.
- Remove non-encapsulated proteins outside the GUVs by adding 1 mL of observation buffer (OB) containing 55 mM glucose, 70 mM NaCl, and 50 mM Tris-HCl (pH 7.4) to the collected solution. Then, centrifuge the solution at 600 x g for 10 min at 13 °C.
- After centrifugation, replace 1 mL of the supernatant with an equal volume of observation buffer. Disperse the GUVs through gentle pipetting and store them in the refrigerator until they are used in GUV experiments.
4. GUV puncture experiment
- Chamber preparation
- Use a 35-mm glass-bottom dish for the experiment. To prevent GUVs from sticking to the surface and bursting, coat the surface with β-casein (5 mg/mL) for 15-30 min.
- For the β-casein solution, dissolve 0.1 g of the protein in a 20-mL buffer of 20 mM Tris (PH 7.5) and 100 mM NaCl. Filter the protein solution, aliquot it into small vials, and freeze it for further use.
- Wash the chamber twice with observation buffer to remove any excess free β-caseins from the surface and let dry at RT.
- In a separate 2 mL tube, mix the collected GUVs with OB. Add CaCl2 to the mixture for the desired final concentration (in this case, 200 μM).
- Introduce 150 nm gold nanoshells (AuNSs) to the mixture in a ratio of 1:100. The final mixture comprises 250 μL of GUVs, 225 μL of OB, 20 μL of CaCl2 (5 mM), and 5 μL of the specified AuNSs.
- Use a 35-mm glass-bottom dish for the experiment. To prevent GUVs from sticking to the surface and bursting, coat the surface with β-casein (5 mg/mL) for 15-30 min.
- Experimental setup
- Transfer the mixture to the chamber and mount it on the microscope stage. Depending on the size of the chamber, add either the entire mixture or a portion of the mixture.
NOTE: Timing is critical since calcium ions can pass through the membrane and mediate the binding of the annexins to the internal leaflet of the membrane. - Employ the same optical setup used for the cell puncturing experiments.
- Use the optical tweezers to trap an individual AuNS in close proximity to or on the surface of a GUV by applying a laser power of ~ 125 mW.
- Subsequently, increase the laser power to ~ 300 mW. This produces a highly localized temperature increase, which disrupts and punctures the membrane at the target site.
NOTE: AuNSs are preferred for the GUV experiments due to their ability to generate a higher transient temperature increase while maintaining a smaller size compared to solid AuNPs.
- Transfer the mixture to the chamber and mount it on the microscope stage. Depending on the size of the chamber, add either the entire mixture or a portion of the mixture.
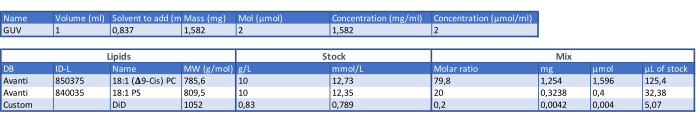
Table 1: Table for determining the GUV composition. Please click here to download this Table.
5. Measurements and data analysis of ANXA response in cell puncture experiments
- Use MATLAB to analyze the images and to calculate and plot the AuNP temperature profile (Figure 1D, E) based on Mie's equations60,61.
- Additionally, process all confocal images using the FIJI ImageJ distribution62,63.
- Calculation of the ANXA ring radius
- Crop the region of interest containing the punctured area from the raw data prior to membrane injury analysis.
- Employ the in-house MATLAB workflow for processing each image by manually marking the center of the ANXA ring and its outer perimeter.
- Use these markings to set the processing limits for the region of interest.
NOTE: The area is subsequently processed radially within the set limits, and the fluorescence intensity at a specific distance to the center is the average over the full circle with the same center distance. This number is stored for each radius step. - Use the workflow to fit the averaged intensities over the entire scanning range to a one-dimensional Gaussian curve to identify the maxima (Rp) and full width at half maximum (FWHM) corresponding to Rext and Rint, respectively, as depicted in Figure 3A.
- Use these markings to set the processing limits for the region of interest.
- Finally, determine the radius of the ANXA ring by the distance from the ring's center to Rext given by the plot generated by the MATLAB workflow.
- Three-dimensional ANXA ring radius calculation
- Track the change in ANXA ring radius in the z-direction using the same MATLAB analysis workflow as in step 5.1. Apply the workflow to several confocal z-sections across the membrane wound, as illustrated in Figure 3C, E.
- Calculate the slope for each wound based on the change in radii over the z-sections, as depicted in Figure 3F.
- Time evolution of the ANXA ring radius
- Similarly, track the ANXA ring's evolution of the ANXA ring after thermoplasmonic membrane puncture using the MATLAB analysis workflow from step 5.1. Analyze consecutive time points to monitor changes over time, as demonstrated in Figure 3D, G, H.
Representative Results
In the present study, the thermoplasmonic method is used to investigate the annexin protein response to plasma membrane disruption; however, any protein that may be recruited upon membrane injury can be investigated using this assay, given that the respective protein is fluorescently labeled. Protein recruitment and function are monitored by confocal imaging in both human embryonic kidney (HEK293T) cells and giant unilamellar vesicles (GUVs). To elaborate, Figure 2 illustrates the experimental conditions in which a focused NIR laser beam at 1064 nm is used to irradiate a single AuNP (Figure 2A), resulting in membrane injury and an influx of Ca2+ into the cell, activating the PMR machinery. Subsequently, annexins are rapidly recruited to the injury site, where they bind to the negatively charged phospholipids at the wound perimeter, forming a ring-like structure within seconds (Figure 2B, i-iv). The model membrane experiments, using GUVs, demonstrated that membrane punctures were rapidly resealed in the absence of ANXA, as depicted in Figure 2C. However, in the presence of ANXA, a rapid accumulation of ANXA was observed at the injury site following membrane puncture (Figure 2D). Notably, ANXA continued to roll the exposed edges, ultimately leading to the bursting of the GUV. This rolling mechanism is believed to arise from the ability of ANXA to induce curvature and bend lipid membranes20.
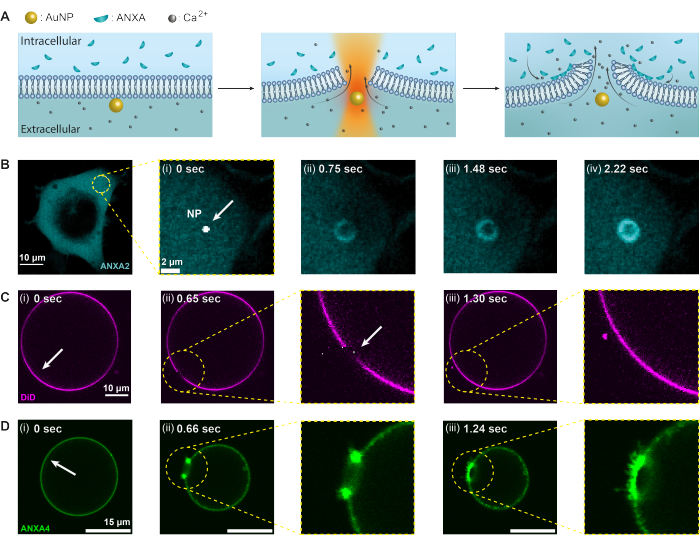
Figure 2: Annexins (ANXAs) response to thermoplasmonic-induced membrane disruption. Initially, (A) the plasma membrane acts as a barrier between the extracellular environment containing high levels of Ca2+ ions and the intracellular environment with encapsulated ANXAs. Upon irradiation with a near-infrared (NIR) laser, the AuNP generates substantial heat, causing the membrane to rupture and resulting in an influx of Ca2+ ions. Consequently, the plasma membrane repair (PMR) machinery is activated, which involves the recruitment of ANXAs to the site of injury, where they bind to negatively charged phospholipids. (B–D) Confocal microscopy images of a cell containing ANXA2 and a GUV containing ANXA4 demonstrate this process. (i) Prior to irradiation, the images show an intact cell, or GUV, with the irradiation sites denoted by the white arrows. (ii) Upon nanoparticle irradiation, (B) ANXAs are rapidly recruited to the injury site, forming a ring-like structure around the membrane wound (B [ii-iv]). Panel (C) shows a membrane-stained GUV without ANXA, which, upon puncture, reseals rapidly without observable membrane remodeling. On the other hand, panel (D) shows a GUV containing recombinant ANXA4, which is already bound to the membrane prior to (i) irradiation due to Ca2+ leakage across the membrane. (ii) Upon puncture, ANXA4 binds to the free edges, causing the GUV to collapse as the membrane rolls away from the edge. The scale bars measure 10 μm for figure (B), 2 μm for B (i), 10 μm for (C), and 15 μm for (D). This figure is reproduced from Moreno-Pescador et. al16 with permission from the Royal Society of Chemistry. Please click here to view a larger version of this figure.
The analysis of complete ANXA ring-like structures (Figure 2B and Supplementary Figure 1) provides useful insights into the wound size and morphology. The radius of the ANXA ring structure can be determined over time and space, as described in Section 5. Moreno et al.16 analyzed over 135 plasma membrane injuries in living cells, where the analysis of ANXA5 ring structures is depicted in Figure 3. The radius was determined by measuring the distance from the center of the ring to the external radius of the curve based on the full-width-half-maximum of the collapsed intensity profile (Figure 3A). The findings illustrated a heterogeneous distribution of ANXA5 ring sizes (Figure 3B), which remained constant over time (Figure 3D,G,H) and space (Figure 3C,E,F). These results suggest an accumulation of ANXA5 around the injury sites, indicative of an alternative ANXA5-mediated PMR strategy in living cells to the hypothesized funnel-like inward budding of the damaged membrane5.
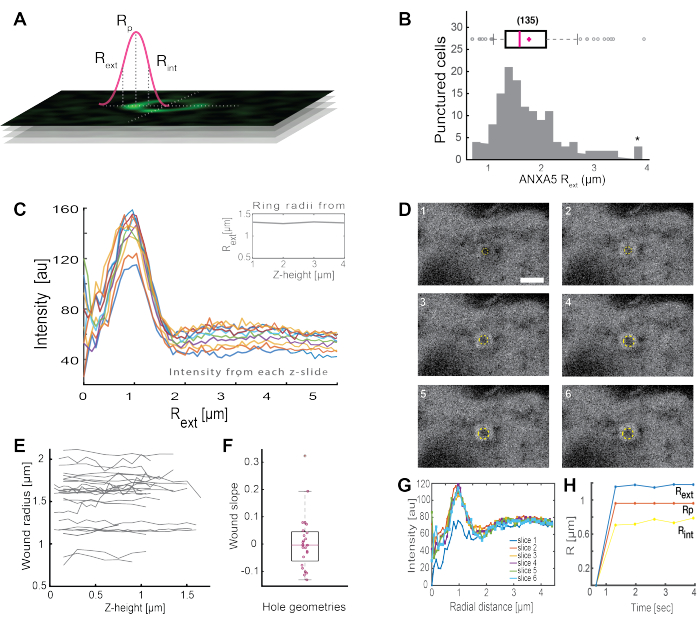
Figure 3: Analysis of ANXA5 ring structures surrounding an injury site in living cells. (A) The schematic representation illustrates the analytical approach, which is based on the full-width-half maximum of the ANXA intensity line profile. (B) The histogram illustrates 135 measured ANXA5 ring radii. (C) Fluorescence intensity line profiles across an ANXA5-GFP ring for each z-section of the z-stack. (D) The confocal images illustrate the time evolution of a wound, where ANXA5-GFP accumulates at the wound perimeter immediately after injury, followed by wound stabilization. The time frames labeled 1-6 were captured at 0.66 s intervals per frame. The scale bar is 2 μm. (E) The radii of the wounds were determined as a function of wound depth based on the method presented in panel A. (F) The slope of the wound was analyzed as ANXA5 ring radii as a function of z-height based on the data extracted from panel E. (G) The fluorescence intensity line profiles were measured across the ANXA5-GFP ring from panel D, where each time interval 1-6 is denoted as slice 1-6. Finally, (H) the time evolution of the ANXA5-GFP ring radii is presented as a function of time calculated from the data in panel G. This figure is reproduced from Moreno-Pescador et. al16 with permission from the Royal Society of Chemistry. Please click here to view a larger version of this figure.
In comparison to the ring-like structures formed by disrupting the PM alone (Supplementary Figure 1), injuries in close proximity to the nucleus (Supplementary Figure 2A) may influence the evolution and geometry of the wound. Occasionally, only a fraction of the ANXA rings were evident (Supplementary Figure 2B,C), which can also be analyzed using the in-house MATLAB analysis workflow (see Section 5), although additional data may be lost. Typically, ANXA ring formations observed in non-adherent cells (Supplementary Figure 2C) are located close to both the nucleus and the cell's periphery. As a consequence, a more elongated ring structure can be observed, which is suboptimal for the data analysis presented. Additionally, non-adherent cells appeared to be more susceptible to cell death following PM injury. Furthermore, when considering injuries resulting from the irradiation of AuNP aggregates, it is important to note that these injuries may be more severe and less controllable. This is due to the significant increase in plasmonic heating, which can damage a large fraction of the cell. As a result, such injuries have not been incorporated into the ANXA5 ring analysis.
Moreover, the preliminary findings indicate that the disruption of the plasma membrane using thermoplasmonics results in elevated intracellular Ca2+ levels. This was observed even at low-intensity irradiation of single AuNPs, suggesting PM permeabilization35, as depicted in Figure 4. The Ca2+ influx was observed in cells expressing a membrane-bound calcium probe, GCaMP6s-CAAX, which undergoes a conformational change upon Ca2+ influx, and thus, an increase in its intensity can be observed64. The calcium intensity was quantified for the entire footprint of the cell over time. To eliminate background noise, the background Ca2+ level was subtracted prior to membrane disruption and post-membrane repair. The maximum intensity was determined by normalizing the mean Ca2+ intensity within the cell, resulting in an intensity curve exhibiting a rapid initial Ca2+ intensity increase followed by a slower decrease, as depicted in Figure 4B.
The cell reached a maximal calcium intensity at ~ 6.6 s, which is consistent with the findings of Klenow et al.64, who suggested that the time of the calcium intensity peak (t = tc) corresponds to the time required for wound closure. Nevertheless, while further investigations are required to establish the underlying mechanism of membrane repair and wound healing, the preliminary findings showed that this Ca2+ process was observed exclusively in the injured cell and not in the uninjured cell used as a positive control. This confirms that the cell experienced Ca2+ influx upon thermoplasmonic membrane disruption, where the excess intracellular calcium is actively pumped out after successful PMR as the intracellular calcium level is no longer in competition with the Ca2+ influx, eventually achieving cell homeostatsis64.
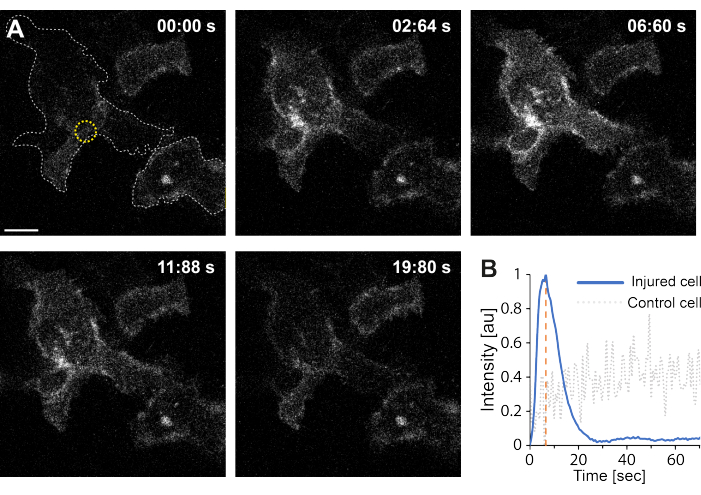
Figure 4: Calcium influx occurs as the plasma membrane of a HEK293T cell is ruptured by thermoplasmonics. A series of confocal images show two cells (the cell of interest and an uninjured cell used as a positive control) expressing the membrane-bound calcium probe, GCaMP6s-CAAX. The scale bar measures 10 μm. (A) Prior to irradiation, at 00:00 s, the footprint of the two cells is visualized by the gray dotted line, and the irradiation site is denoted by the yellow circle. Upon laser irradiation, a rapid influx of Ca2+ is observed, reaching maximum intensity at ~ 6.6 s, denoted by the orange dashed line, a timepoint that is presumed to correspond to the time of wound closure64. (B) The calcium intensity profile obtained from the GCaMP6s-CAAX probe in the injured cell (blue line) was compared to the Ca2+ intensity in a neighboring uninjured cell (gray dotted line), showing a clear Ca2+ influx exclusively upon PM disruption. Please click here to view a larger version of this figure.
Discussion
The study highlights the thermoplasmonic approach as a promising technique for exploring protein responses in living cells and model membranes following membrane disruption. This method not only provides extensive information on protein recruitment but also on the biophysical function of proteins involved in protein-membrane dynamics. Consequently, it facilitates the identification of molecular components responsible for surface repair and advances the understanding of the complex yet vital machinery of plasma membrane repair. Although various methods exist for inducing membrane disruption, such as mechanical, chemical, and optical techniques, these methods suffer from limitations, such as being non-specific to cells, generating multiple injuries to the cell membrane, or causing significant damage to the membrane and ablating internal cellular material along the laser path when using high-power pulsed lasers. While the integration of confocal microscopy and optical tweezers offers the most comprehensive information, alternative imaging modalities could also be used. For instance, as the imaging of the plasmonic nanoparticle is achieved using reflection microscopy, a built-in imaging mode in Leica confocal microscopes, additional imaging techniques, such as darkfield microscopy65,66, other scattering methods like iSCAT67,68, or fluorescent labeling of the nanoparticle, could be employed for AuNP visualization, although this might limit the applicability of the method.
The presented method is additionally capable of inducing nanoscopic holes in model membranes, enabling investigation of the synergy effects between different annexins. This is achieved by encapsulating differently labeled recombinant annexins, e.g., RFP and GFP, respectively, followed by thermoplasmonic puncture. This model system provides insight into how annexins interact with membranes in the vicinity of free edges, as demonstrated in Figure 2D. However, unlike in cells, the holes inflicted on GUVs continue to expand, followed by destabilization of the vesicle. Imaging the hole evolution using confocal microscopy can be challenging due to the rapid expansion of the hole diameter, but can be accomplished by capturing several z-stacks over time. An alternative method would be to use a spinning disk confocal for faster imaging. Furthermore, the thermoplasmonic approach typically yields a limited number of optimal results per hour when applied to single cells or GUV experiments, usually two to three, at sample temperatures between 20 °C and 30 °C. To obtain the most accurate observation of protein-membrane dynamics, it is recommended to keep the cells in a HEPES-containing buffer and replace the sample every hour. Alternatively, the experimental window could be extended by performing the experiments in a cell incubating chamber, i.e., at a constant temperature of 37 °C with 5% CO2. Furthermore, combining this approach with other imaging techniques, such as stochastic optical reconstruction microscopy (STORM), could provide a deeper understanding of the biophysical function and interaction of key proteins involved in membrane repair on a single-molecule level. This could provide detailed information on the site of injury, including the wound geometry and location of annexin proteins, as well as identify other key players involved in repairing the membrane surface.
In order to achieve maximum effectiveness and precision in inducing membrane injury, it is imperative to verify the location of the laser focus prior to each experiment and ensure that the axial position of the laser focus coincides with the confocal focus. This alignment optimizes the intensity during AuNP imaging, which leads to a maximum local temperature increase and consequent membrane injury at lower laser power. This process is manually executed and therefore susceptible to variability in membrane rupture efficiency as the focus is manually translated to a position that coincides with the particle's location. In microscopes that lack a reflection mode, as in some commercial systems, co-localization of the laser focus and particle can be challenging. In such cases, alternative imaging modes (e.g., bright field) can be employed, and a slow raster scan can be performed around the expected particle position. It should be noted that low laser power is likely to induce membrane permeabilization only, while high laser power can generate temperatures around the NP that exceed the boiling point of water, even if the glass surface has a cooling effect. It is estimated that the formation of nanobubbles surrounding the NPs occurs between 200 °C and 300 °C25,48, where the explosive heat may result in either particle displacement from the laser focus or particle fragmentation. Additionally, the formation of nano or microbubbles during heating poses a challenge to this method. As air interfaces dewet membranes and can cause protein destabilization, which is undesirable, it is imperative to limit the heating when investigating membrane repair. Notably, gold nanoshells do not tolerate high temperatures and will degrade under these conditions, as demonstrated by high-resolution microscopy58.
This article provides a detailed protocol for using thermoplasmonics to perform highly localized punctures in membranes, which is applicable to both cells and model membranes. To further reduce the extent of heating, smaller nanoparticles resonant with NIR light can be utilized, enabling intracellular punctures in endosomes, the endoplasmic reticulum, and the nuclear envelope. Such nanoparticles, including rods and nanomatryoshkas48, can be used to investigate nuclear envelope repair by targeting endocytosed gold nanoparticles that are readily taken up at the cell surface and trafficked toward the nucleus69. Overall, this technique enables the identification and examination of key molecular components involved in PMR, elucidating their biophysical function and role while preserving the viability of the cells.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We wish to thank Jesper Nylandsted for providing us with recombinant annexin proteins and plasmids encoding for annexins. This work was financially supported by the Danish Council for Independent Research, Natural Sciences (DFF-4181-00196), by the Novo Nordisk Foundation Interdisciplinary Synergy Program 2018 (NNF18OC0034936), the Scientific Committee Danish Cancer Society (R90-A5847-14-S2), the Lundbeck Foundation (R218-2016-534), and by the Lundbeck Foundation Center of Excellence (Biomembranes in Nanomedicine).
Materials
| 1064 nm trapping laser | Spectra Physics | N/A | Spectra Physics J201-BL-106C, Nd: YVO4 NIR laser |
| 160 nm Gold Nanoshells | NanoComposix | NCXGSIR150 | |
| 200 nm Gold Nanoparticles | BBI Solutions | EM.GC200/7 | |
| 35 mm glass surface MatTex microwell | MATTEK | P35G-1.5-14-C | |
| Amber-glass vials | Supelco Sigma Aldrich | 243438 | |
| Annexin A2 plasmids | N/A | N/A | Received from our collaborator at the Danish Cancer Research Center |
| Annexin A4 recombinant-protein | N/A | N/A | N-terminal GFP tagged ANXA4 received from our collaborator at the Danish Cancer Research Center |
| Annexin A5 recombinant-protein | N/A | N/A | N-terminal GFP tagged ANXA5 received from our collaborator at the Danish Cancer Research Center |
| beta-casein | Sigma Life Science | C6905-1G | |
| CaCl2 | Suprlco (sigma Aldrich) | 10035-04-8 | |
| Centrifuge 5702 | Eppendorf | 5702 | |
| Chloroform | VWR Chemicals | 67-66-3 | |
| Culture dish (Nunclon Delta Surface) | Thermo scientific | 150460 | |
| DID cell-labelling Solution | Invitrogen | 7757 | |
| Distilled water | Gibco | 15230-089 | |
| DOPC | Avanti Polar Lipids | 850375C | Dissolved in chloroform |
| DOPS | Avanti Polar Lipids | 840035C | Dissolved in chloroform |
| Dulbecco's Modified Eage's Medium | Thermo Fisher Scientific | 11995065 | |
| FIJI ImageJ distribution | ImageJ2 | N/A | |
| GCaMP6s-CAAX | N/A | Received from our collaborator at the Danish Cancer Research Center | |
| Gibco Fetal Bovine Serum | Fisher Scientific | 11573397 | 10% of the culture medium |
| Glucose | PROLABO | 24 374.297 | |
| Hamilton syringes | Hamilton Company | N/A | 50 and 500 microliters |
| Harrick Plasma Cleaner PDG-002 | Harrick Plasma | N/A | |
| HEK293T cells | N/A | Received from our collaborator at the Danish Cancer Research Center | |
| Leica Acousto-Optical Beam Splitter (AOBS) | Leica | N/A | |
| Leica PL APO 63x water immersion objective, NA = 1.2 | Leica | N/A | |
| Leica SP5 confocal scanning microscope | Leica | N/A | |
| Lipofectamine | Fisher Scientific | 15338030 | |
| MatLab | The Mathworks, Inc., Natick, Massachusetts, United States | N/A | |
| NaCl | VWR Chemicals | 7647-14-5 | |
| Opti-MEM Reduced-Serum Medium | Thermo Fisher Scientific | 11058021 | |
| Parafilm | Bemis | PM-992 | |
| Penicillin-Streptomycin | Thermo Fisher Scientific | 15140122 | 1% of the culture medium |
| Phosphate Buffered Saline (PBS) | Thermo Fisher Scientific | 10010023 | |
| Piezoelectric stage (PI 731.20) | Physik Instrumente (Germany) | N/A | |
| Poly-L-Lysine | Sigma-Aldrich | P8920-100ML | 0.01-0.1% for coating |
| Polyvinyl alcohol | Sigma-Aldrich | 363065-25G | |
| round glass slide 25 mm Ø | VWR | 631-1584 | |
| Sonicator Brandson 2800 | Brandson | N/A | |
| sucrose | Sigma Life Science | 57-50-1 | |
| T25 tissue culture flask | Falcon | 353108 | Blue Vented cap |
| Tris-HCl | Invitrogen | 15567-027 | |
| TrypLE | Thermo Fisher Scientific | A1285901 | |
| Trypsin-EDTA | Fisher Scientific | 11590626 | |
| VWR Mixer mini vortex 230V EU | VWR | 12620-84 | ECN: 444-2790, SN: 150713022 |
References
- Bendix, P. M., et al. Interdisciplinary synergy to reveal mechanisms of annexin-mediated plasma membrane shaping and repair. Cells. 9 (4), 1029 (2020).
- Gajic, O., Lee, J., Doerr, C. H., Berrios, J. C., Myers, J. L., Hubmayr, R. D. Ventilator-induced Cell Wounding and Repair in the Intact Lung. American Journal of Respiratory and Critical Care Medicine. 167, 1057-1063 (2003).
- McNeil, P. L., Khakee, R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. The American Journal of Pathology. 140 (5), 1097-1109 (1992).
- Yu, Q. C., McNeil, P. L. Transient disruptions of aortic endothelial cell plasma membranes. The American Journal of Pathology. 141 (6), 1349-1360 (1992).
- Boye, T. L., et al. Annexin A4 and A6 induce membrane curvature and constriction during cell membrane repair. Nature Communications. 8, 1623 (2017).
- Bischofberger, M., Gonzalez, M. R., van der Goot, F. G. Membrane injury by pore-forming proteins. Current Opinion in Cell Biology. 21, 589-595 (2009).
- Tang, S. K. Y., Marshall, W. F. Self-repairing cells. Science (New York, N.Y.). 356, 1022-1025 (2017).
- Abreu-Blanco, M. T., Verboon, J. M., Parkhurst, S. M. Single cell wound repair: Dealing with life’s little traumas. Bioarchitecture. 1, 114-121 (2011).
- Sønder, S. L., et al. Annexin A7 is required for ESCRT III-mediated plasma membrane repair. Scientific Reports. 9, 6726 (2019).
- Andrews, N. W., Almeida, P. E., Corrotte, M. Damage control: cellular mechanisms of plasma membrane repair. Trends in Cell Biology. 24 (12), 734-742 (2014).
- Idone, V., Tam, C., Goss, J. W., Toomre, D., Pypaert, M., Andrews, N. W. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. The Journal of Cell Biology. 180 (5), 905-914 (2008).
- Lauritzen, S. P., Boye, T. L., Nylandsted, J. Annexins are instrumental for efficient plasma membrane repair in cancer cells. Seminars in Cell & Developmental Biology. 45, 32-38 (2015).
- Häger, S. C., Nylandsted, J. Annexins: players of single cell wound healing and regeneration. Communicative & Integrative Biology. 12 (1), 162-165 (2019).
- Jaiswal, J. K., et al. S100A11 is required for efficient plasma membrane repair and survival of invasive cancer cells. Nature Communications. 5, 3795 (2014).
- Draeger, A., Monastyrskaya, K., Babiychuk, E. B. Plasma membrane repair and cellular damage control: The annexin survival kit. Biochemical Pharmacology. 81 (6), 703-712 (2011).
- Moreno-Pescador, G. S., et al. Thermoplasmonic nano-rupture of cells reveals annexin V function in plasma membrane repair. Nanoscale. 14 (21), 7778-7787 (2022).
- Zhivotovsky, B., Orrenius, S. Calcium and cell death mechanisms: A perspective from the cell death community. Cell Calcium. 50 (3), 211-221 (2011).
- Gerke, V., Moss, S. E. Annexins: From structure to function. Physiological Reviews. 82 (2), 331-371 (2002).
- Idone, V., Tam, C., Andrews, N. W. Two-way traffic on the road to plasma membrane repair. Trends in Cell Biology. 18 (11), 552-559 (2008).
- Boye, T. L., et al. Annexins induce curvature on free-edge membranes displaying distinct morphologies. Scientific Reports. 8, 10309 (2018).
- Bouter, A., et al. Annexin-A5 assembled into two-dimensional arrays promotes cell membrane repair. Nature Communications. 2, 270 (2011).
- Boye, T. L., Nylandsted, J. Annexins in plasma membrane repair. Biological Chemistry. 397 (10), 961-969 (2016).
- Weinberger, A., et al. Gel-assisted formation of giant unilamellar vesicles. Biophysical Journal. 105 (1), 154-164 (2013).
- Numata, T., Tatsuta, H., Morita, Y., Otani, Y., Umeda, N. Localized thermal processing with a laser-trapped and heated metal nanoparticle. IEEJ Transactions on Electrical and Electronic Engineering. 2, 398-401 (2007).
- Bendix, P. M., Reihani, S. N. S., Oddershede, L. B. Direct measurements of heating by electromagnetically trapped gold nanoparticles on supported lipid bilayers. ACS Nano. 4 (4), 2256-2262 (2010).
- Kyrsting, A., Bendix, P. M., Stamou, D. G., Oddershede, L. B. Heat profiling of three-dimensionally optically trapped gold nanoparticles using vesicle cargo release. Nano Letters. 11 (2), 888-892 (2011).
- Andersen, T., Kyrsting, A., Bendix, P. M. Local and transient permeation events are associated with local melting of giant liposomes. Soft Matter. 10 (24), 4268-4274 (2014).
- Bahadori, A., Oddershede, L. B., Bendix, P. M. Hot-nanoparticle-mediated fusion of selected cells. Nano Research. 10, 2034-2045 (2017).
- Rørvig-Lund, A., Bahadori, A., Semsey, S., Bendix, P. M., Oddershede, L. B. Vesicle fusion triggered by optically heated gold nanoparticles. Nano Letters. 15 (6), 4183-4188 (2015).
- Moreno-Pescador, G., Arastoo, M. R., Ruhoff, V. T., Chiantia, S., Daniels, R., Bendix, P. M. Thermoplasmonic vesicle fusion reveals membrane phase segregation of influenza spike proteins. Nano Letters. 23 (8), 3377-3384 (2023).
- Bahadori, A., Lund, A. R., Semsey, S., Oddershede, L. B., Bendix, P. M. Controlled cellular fusion using optically trapped plasmonic nano-heaters. SPIE Proceedings. SPIE 9922, Optical Trapping and Optical Micromanipulation XIII. 992211, (2016).
- Bahadori, A., Moreno-Pescador, G., Oddershede, L. B., Bendix, P. M. Remotely controlled fusion of selected vesicles and living cells: a key issue review. Reports on Progress in Physics. 81 (3), 32602 (2018).
- Moreno-Pescador, G., Arastoo, M. R., Chiantia, S., Daniels, R., Bendix, P. M. Thermoplasmonic induced vesicle fusion for investigating membrane protein phase affinity. bioRxiv. , (2022).
- Pescador, G. S. M., et al. Investigating plasma-membrane repair employing thermoplasmonics. Biophysical Journal. 120 (3), 45A (2021).
- Moreno-Pescador, G. S., Qoqaj, I., Thusgaard Ruhoff, V., Iversen, J., Nylandsted, J., Bendix, P. M. Effect of local thermoplasmonic heating on biological membranes. SPIE 11083, Optical Trapping and Optical Micromanipulation XVI. 110830M, (2019).
- Bement, W. M., Mandato, C. A., Kirsch, M. N. Wound-induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Current Biology. 9 (11), 579-587 (1999).
- Weisleder, N., et al. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Science Translational Medicine. 4 (139), 139ra85 (2012).
- Sudji, I. R., Subburaj, Y., Frenkel, N., García-Sáez, A. J., Wink, M. Membrane disintegration caused by the steroid saponin digitonin is related to the presence of cholesterol. Molecules. 20 (11), 20146-20160 (2015).
- Babiychuk, E. B., Monastyrskaya, K., Potez, S., Draeger, A. Intracellular Ca2+ operates a switch between repair and lysis of streptolysin O-perforated cells. Cell Death & Differentiation. 16, 1126-1134 (2009).
- Nygård Skalman, L., Holst, M. R., Larsson, E., Lundmark, R. Plasma membrane damage caused by listeriolysin O is not repaired through endocytosis of the membrane pore. Biology Open. 7 (10), bio035287 (2018).
- Swaggart, K. A., et al. Annexin A6 modifies muscular dystrophy by mediating sarcolemmal repair. Proceedings of the National Academy of Sciences of the United States of America. 111, 6004-6009 (2014).
- Yeheskely-Hayon, D., Minai, L., Golan, L., Dann, E. J., Yelin, D. Optically induced cell fusion using bispecific nanoparticles. Small. 9 (22), 3771-3777 (2013).
- Minai, L., Yeheskely-Hayon, D., Golan, L., Bisker, G., Dann, E. J., Yelin, D. Optical nanomanipulations of malignant cells: Controlled cell damage and fusion. Small. 8 (11), 1732-1739 (2012).
- Lukianova-Hleb, E., et al. Plasmonic nanobubbles as transient vapor nanobubbles generated around plasmonic nanoparticles. ACS Nano. 4 (4), 2109-2123 (2010).
- Vogel, A., Noack, J., Hüttman, G., Paltauf, G. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Applied Physics B. 81, 1015-1047 (2005).
- Baffou, G., Polleux, J., Rigneault, H., Monneret, S. Super-heating and micro-bubble generation around plasmonic nanoparticles under cw illumination. Journal of Physical Chemistry C. 118 (9), 4890-4898 (2014).
- Sasikumar, K., Liang, Z., Cahill, D. G., Keblinski, P. Curvature induced phase stability of an intensely heated liquid. Journal of Chemical Physics. 140 (23), 234506 (2014).
- Jauffred, L., Samadi, A., Klingberg, H., Bendix, P. M., Oddershede, L. B. Plasmonic heating of nanostructures. Chemical Reviews. 119 (13), 8087-8130 (2019).
- Neuman, K. C., Nagy, A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nature Methods. 5 (6), 491-505 (2008).
- Bendix, P. M., Jauffred, L., Norregaard, K., Oddershede, L. B. Optical trapping of nanoparticles and quantum dots. IEEE Journal of Selected Topics in Quantum Electronics. 20, 15-26 (2014).
- Samadi, A., Bendix, P. M., Oddershede, L. B. Optical manipulation of individual strongly absorbing platinum nanoparticles. Nanoscale. 46, 18449-18455 (2017).
- Jørgensen, J. T., Norregaard, K., Tian, P., Bendix, P. M., Kjaer, A., Oddershede, L. B. Single particle and PET-based platform for identifying optimal plasmonic nano-heaters for photothermal cancer therapy. Scientific Reports. 6, 30076 (2016).
- Goldenberg, H., Tranter, C. J. Heat flow in an infinite medium heated by a sphere. British Journal of Applied Physics. 3 (9), 296-298 (1952).
- Eustis, S., El-Sayed, M. A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chemical Society Reviews. 35, 209-217 (2006).
- Landau, L. D., Lifshitz, E. M. . Fluid Mechanics: Landau and Lifshitz: Course of Theoretical Physics. 6, (2013).
- Niederauer, C., Seynen, M., Zomerdijk, J., Kamp, M., Ganzinger, K. A. The K2: Open-source simultaneous triple-color TIRF microscope for live-cell and single-molecule imaging. HardwareX. 13, e00404 (2023).
- Richardson, A. C., Reihani, N., Oddershede, L. B. Combining confocal microscopy with precise force-scope optical tweezers. SPIE Proceedings:SPIE 6326, Optical Trapping and Optical Micromanipulation III. 632628, (2006).
- Samadi, A., Klingberg, H., Jauffred, L., Kjær, A., Bendix, P. M., Oddershede, L. B. Platinum nanoparticles: a non-toxic, effective and thermally stable alternative plasmonic material for cancer therapy and bioengineering. Nanoscale. 10 (19), 9097-9107 (2018).
- . Available from: https://www.thermofisher.com/order/catalog/product/A7816 (2023)
- Kreibig, U., Vollmer, M. Theoretical considerations. In: Optical Properties of Metal Clusters. 25, (1995).
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Annalen der Physik. 330 (3), 377-445 (1908).
- Rueden, C. T., et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 18 (1), 529 (2017).
- Schindelin, J., et al. Fiji: An open-source platform for biological-image analysis. Nature Methods. 9, 676-682 (2012).
- Klenow, M. B., Heitmann, A. S. B., Nylandsted, J., Simonsen, A. C. Timescale of hole closure during plasma membrane repair estimated by calcium imaging and numerical modeling. Scientific Reports. 11, 4226 (2021).
- Li, T., Wu, X., Liu, F., Li, N. Analytical methods based on the light-scattering of plasmonic nanoparticles at the single particle level with dark-field microscopy imaging. Analyst. 142 (2), 248-256 (2017).
- Gibbs-Flournoy, E. A., Bromberg, P. A., Hofer, T. P. J., Samet, J. M., Zucker, R. M. Darkfield-Confocal Microscopy detection of nanoscale particle internalization by human lung cells. Particle and Fibre Toxicology. 8 (1), 2 (2011).
- Taylor, R. W., Sandoghdar, V. Interferometric scattering microscopy: Seeing single nanoparticles and molecules via Rayleigh scattering. Nano Letters. 19 (8), 4827-4835 (2019).
- Wu, Y., Ali, M. R. K., Chen, K., Fang, N., El-Sayed, M. A. Gold nanoparticles in biological optical imaging. Nano Today. 24, 120-140 (2019).
- Klingberg, H., Oddershede, L. B., Loeschner, K., Larsen, E. H., Loft, S., Møller, P. Uptake of gold nanoparticles in primary human endothelial cells. Toxicology Research. 4 (3), 566-666 (2015).

