Intracerebroventricular Injection in Mouse: An Ommaya Mediated Direct Drug Administration Method to Deliver Drugs to Cerebral Ventricles of Mouse Brain
Abstract
Source: Law, V. et al. A Murine Ommaya Xenograft Model to Study Direct-Targeted Therapy of Leptomeningeal Disease. J. Vis. Exp. (2021)
This video demonstrates ommaya mediated intracerebroventricular injection in a mouse model. The method enables localized delivery of potential drug candidates and evades the blood-brain barrier.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Murine Ommaya Assembly and Implantation
- Preparation of station
- Disinfect the station surface. Place a blue covering over the surface and keep ready all sterilized tools and supplies indicated in Figure 1.
- Presurgical procedure
- Apply analgesia (Bup-SR) and maintain sterile surgical environment.
- Surgical implantation of Murine Ommaya
- Assemble the Murine Ommaya injection device using a 25G (0.51 mm outer diameter) miniature injection port and a 1 mm spacer disc. Use a cyanoacrylate sterile adhesive to ensure the penetration of approximately 2.5 mm of the metal cannula into the right cerebral hemisphere (Figure 2A–C).
NOTE: An MRI-compatible Mouse Ommaya prototype was developed in this laboratory, which already has both parts (miniature injection port and spacer) 3D-printed together as a single unit (Figure 2D). The single unit version was tested by MRI and can save time by eliminating the assembly step. - Anesthetize the mouse with 2–3% isoflurane until there are no signs of the righting reflex. Further, check for tail and/or paw pinch reflex to confirm the state of anesthesia.
- Shave the entire ventral surface of the head of fur and prepare the skin according to the sterile technique. Place the mouse in the stereotactic apparatus with a nose cone to continue isoflurane administration during the procedure; decrease the isoflurane to 1.5%. Gently tighten the ear bars to secure the head and apply eye lubricant to cover the eyes of the mouse.
- Make a small skin incision (3 mm), followed by blunt dissection of the underlying subcutaneous tissues to expose the skull. Dry the skull using hydrogen peroxide-soaked cotton-tipped applicator sticks.
- Drill a burr hole in the skull 0.5 mm posterior and 1.1 mm lateral of the bregma-the anatomical point on the skull where the coronal suture is intersected perpendicularly by the sagittal suture (Figure 2A), as well as a 0.9 mm burr hole to expose the dura mater. Move the microdrill aside, and gently score the bone immediately surrounding the burr hole prior to inserting an injection port (depth of approximately 2.5 mm), affixed to the skull using a cyanoacrylate sterile adhesive. State suture around the injection spot using 4-0 no-absorbance nylon sutures in an interrupted stitch pattern or purse string suture.
- House post-surgery mice in individual cages for surgery recovery.
NOTE: It is possible that the Murine Ommaya may come off when multiple surgery-recovered mice are housed in the same cage, possibly due to constant tampering interactions. It is recommended that Murine Ommaya-implanted mice are housed individually (or no more than 2 mice per cage) over the course of the drug efficacy trial.
- Assemble the Murine Ommaya injection device using a 25G (0.51 mm outer diameter) miniature injection port and a 1 mm spacer disc. Use a cyanoacrylate sterile adhesive to ensure the penetration of approximately 2.5 mm of the metal cannula into the right cerebral hemisphere (Figure 2A–C).
2. Murine Ommaya Treatment
- Dosing mice using the Murine Ommaya
- Anesthetize the mouse with 2–3% isoflurane, until there are no signs of righting reflex. Further, check for tail and/or paw pinch reflex to confirm maintenance of anesthesia.
- Access the Murine Ommaya using a port injection adapter and a Hamilton syringe. Using forceps, hold the top of the miniature injection port and gently insert the port injector adaptor fully into the port's septum.
NOTE: The port injector pierces the septum of the port in a manner that reduces dead space to a minimum and allows for controlled injection via a motorized syringe pump (Figure 3A). Targeted/novel treatments are injected at a flow rate of 1 µL/min while under anesthesia. The treatment is to be given at specified intervals (daily/weekly) for a set duration (weeks/months), according to the researcher's discretion.
A volume between 3 and 7 µL is optimal, as a volume <3 µL is unreliable, and a volume >7 µL may cause too much pressure. The size of the Hamilton syringe can range from 10 to 100 µL, depending on the number of mice in each treatment arm. Preloading the appropriate volume into the Hamilton syringe will prevent repeated replacement of the syringe, thereby minimizing errors. It is best to dedicate 1 Hamilton syringe per treatment arm. - Once the injection is made, detach the Murine Ommaya from the port injection adaptor using forceps, and return the mouse to the cage to recover from anesthesia.
Representative Results
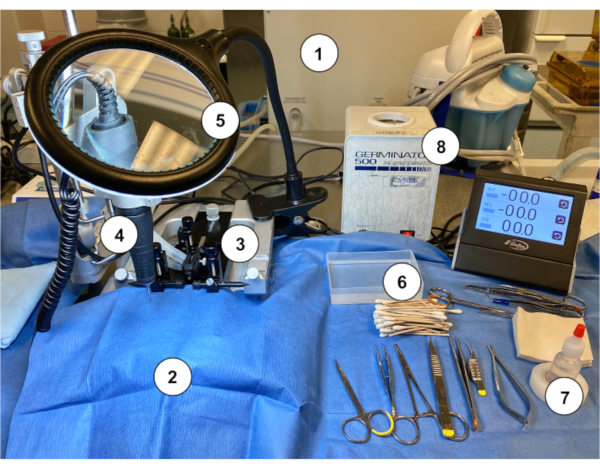
Figure 1: An example of a workstation setup for performing the Murine Ommaya implantation in mice. (1) Gas anesthesia machine/vaporizer. (2) Sterile blue paper drape covering a stereotaxic stand. (3) Stereotaxic device (stand/stage, ear bars, nose cone). (4) Microdrill. (5) Magnifying glass with light. (6) Sterile cotton-tipped applicator sticks with sterile saline rinse container. (7) Hydrogen peroxide. (8) Bead sterilizer.
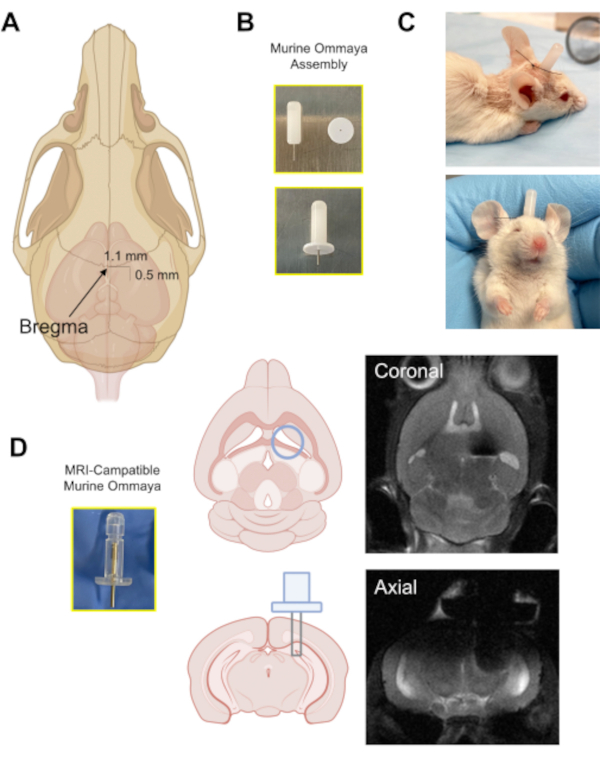
Figure 2: The implantation of the Murine Ommaya device. (A) An illustration with an arrow pointing at the location of the bregma on the skull, and the approximate distance at which a burr hole is drilled in the skull (0.5 mm posterior/1.1 mm lateral) from the bregma using a microdrill. (B) A Murine Ommaya is assembled by combining a metal cannula and a 1 mm spacer as the base for glue attachment to the skull. (C) Representative images of mice that had Murine Ommayas implanted; these mice are monitored to ensure they are bright, alert, and reactive before receiving any injections. (D) An example of the prototype magnetic resonance imaging-compatible Murine Ommaya and representative brain magnetic resonance imaging images of Murine Ommaya implants.
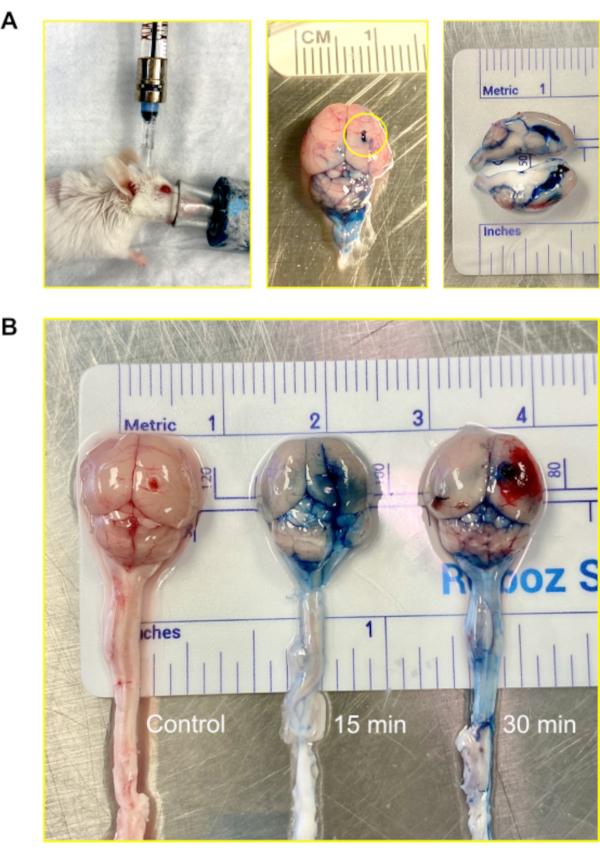
Figure 3: Intraventricular (central nervous system) injection using the Murine Ommaya. (A) An image of an injection, accessing the ventricle and central nervous system via the Murine Ommaya. Mice remain under anesthesia during the injection. In the example, the Murine Ommaya is connected to the miniature port that is attached to a prefilled Hamilton syringe. Injections are performed using an automatic injection set at an infusion rate of 1 µL/min and a volume of 5–7 µL. An image of a mouse brain injected with Evans Blue is shown. The circle shows where the Murine Ommaya was attached. No leakage of the dye was observed on the exterior of the brain. A cross section of the brain shows the lateral ventricles were filled with the dye; the dye did not penetrate brain parenchyma. (B) Images of mouse brains after 15 and 30 min following the injection of Evans Blue dye. The dye infiltrated the brain (15 min) and began to circulate on the spinal cord (30 min). Out of 5 mice, 4 received dye for visualization, and 1 served as control. The experiment was repeated in triplicate.
Divulgaciones
The authors have nothing to disclose.
Materials
| 1 mm spacer disc | Alzet, Durect Corporation | #0008670 | Spacer disc only |
| 4-0 ethilon nylon suture | Any vendor | ||
| Automatic syringe pumps | Harvard Syringe Pumps (or any vendor) | #70-4505 | Pump 11 Elite |
| Bead sterilizer | Braintree Scientific Inc. (or any vendor) | #GER 5287-120V | Germinator 500 |
| Buprenorphine Sustained-Release (Bup-SR) | Zoopharm | DEA controlled | |
| Cyanoacrylate sterile adhesive | Any vendor | ||
| Gas inhalation anestehsia system | VeteEquip | #901812 | COMPAC5 |
| Hamilton microliter syringes | Hamilton | 10, 25, 50, and 100 μL; 30G for cisterna magna injection |
|
| Hydrogen peroxide | Any vendor | ||
| IVIS 200 imaging system | Caliper Life Sciences | ||
| Magnifying glass with light | Any vendor | ||
| Microdrill | Stoelting (or any vendor) | #51555M | |
| MRI imaging | Bruker | BioSpec series | Optional |
| Murine Ommaya (MRI-compatible) prototype | Instech Laboratories, Inc. | #VAB620-25MRI-3.3 | |
| PinPort injector | Instech Laboratories, Inc. | #PNP3M-50 | |
| PinPort | Instech Laboratories, Inc. | #1-PNP3F28-50 | |
| Rodent Surgical Instruments (Scissors, Forceps) | Roboz Surgical Instrument (or any vendor) | ||
| Stereotaxic device | Stoelting (or any vendor) | #51730M | |
| Sterile blue paper/ drape covering | Any vendor | ||
| Sterile cotton sticks | Any vendor |

