Cranial Ring Assembly Installation in Mouse Model: A Surgical Procedure to Implant Visualization Window Assembly on Murine Skull for High-resolution Skull Bone Marrow Imaging
Abstract
Source: Bornert, A. et. al., In Vivo Two-photon Imaging of Megakaryocytes and Proplatelets in the Mouse Skull Bone Marrow. J. Vis. Exp. (2021)
In this video, we describe a surgical procedure to implant a cranial ring assembly on the exposed skull bone and further immobilize the mouse’s head for high-resolution brain imaging for a long duration.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Surgery and installation of the cranial ring
NOTE: The full support for mouse installation comprises 4 pieces, a block and a plate, the ring to hold the mouse head, and a screw to fix the ring to the block (Figure 1A). All elements of the support have been obtained from i.materialise.com by 3D printing. The plate is made of acrylonitrile butadiene styrene (ABS) polymer, the block and screw of stainless steel, and the ring of high-grade stainless steel (high-detailed stainless steel) (See Figure 2 for dimensions and Materials for the 3D printing files). The block is fixed permanently to the support, either screwed or glued. Once the ring is fixed on the mouse skull, it should be screwed to the block holder (Figure 1A).
- Moisten the hair on the scalp with a paper towel wet with 70% ethanol for disinfection. Remove all the loose hair with a wet paper towel.
NOTE: For convenience, the scalp may be shaved before surgery. - Incise the scalp by forming a T at the midline up to 1 cm and between the ears to expose the calvarium using sterile fine scissors and tweezers.
NOTE: The ring will be positioned to overlap the frontal and parietal bones (Figure 1B). - Use sterile tweezers to expose the skull. Carefully remove the periosteum with scissors and tweezers. Use a sterile cotton swab soaked with physiological saline to remove all the periosteum and any debris or hair, which could alter imaging.
NOTE: To limit inflammatory reactions, take great care not to damage the bone. - Remove any blood traces by rinsing with saline, and rapidly dry the bone with a dry cotton swab. Apply the glue gel to the ring, position it on the exposed bone, and maintain it for a few seconds so that the ring is firmly attached to the skull.
NOTE: No glue must enter the observation field as it may lead to undesirable autofluorescence. - Lightly moisten the skull with a cotton swab immersed in physiological saline.
- Prepare the silicon dental paste by carefully mixing 1:1 blue and yellow components. Apply dental paste all around the ring for sealing to prevent leakage during imaging. Carefully remove any dental paste or glue that may have entered the ring.
- Fill the ring with saline and check for leakage.
NOTE: The saline serves as the immersion medium for the microscope objective. Bleeding may be more critical when using mice with hemostasis-related defects. It is essential to prevent blood from entering the ring as it may blur further imaging. - Place the mouse on the support and screw the ring on the block holder (Figure 1C). Place folded compresses under the animal's head to raise the head and prevent detachment of the ring from the skull.
NOTE: The head is directly fixed so that it prevents image movements due to breathing. - Position the support and mouse assembly in the heated microscope chamber (Figure 1D).
NOTE: Be careful not to dislodge the inserted catheter at any time.
Representative Results
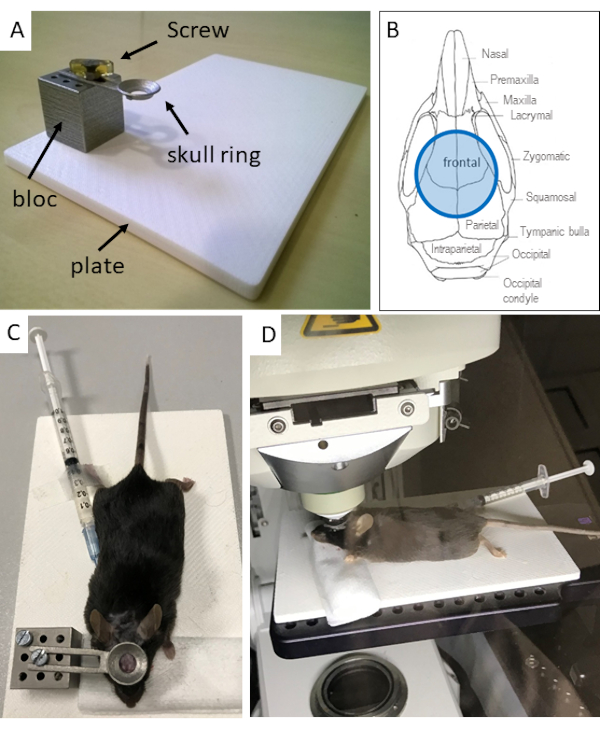
Figure 1: Experimental setup used for two-photon imaging through the skull bone. (A) The 3D printed stainless steel block is fixed on the ABS polymer support (see Figure 2 and Materials for the 3D designs). The block and the skull ring are designed so that the ring can be screwed to the block. Note that, for ease of use, the screw head has been embedded in Epon. (B) Schematic showing the positioning of the ring on the exposed skull bone. (C) Photographs illustrating the mouse with the ring glued to the skull, the head on a tissue pad, and (D) the positioning under the microscope objective. Abbreviations: 3D = three-dimensional; ABS = acrylonitrile butadiene styrene.
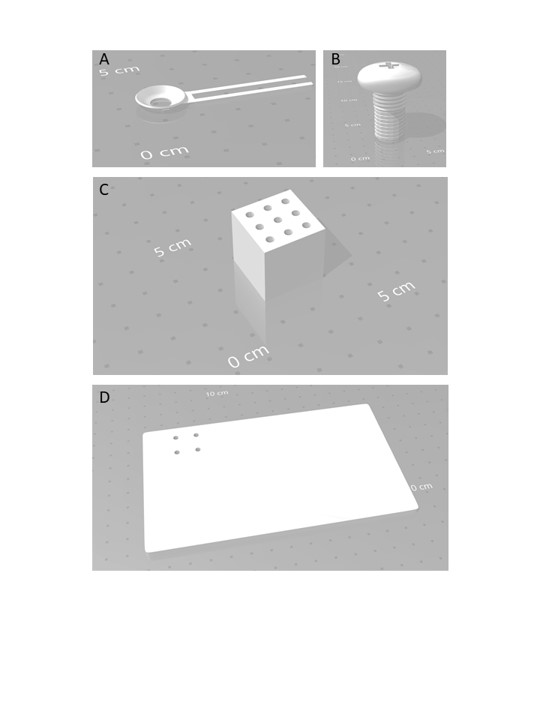
Figure 2: Design for the 3D printing of the skull ring and support. This design was used to 3D print (A) the skull ring in high-quality stainless steel, (B) the screw, (C) the block in stainless steel, and (D) the support in ABS polymer. Abbreviations: 3D = three-dimensional; ABS = ABS = acrylonitrile butadiene styrene.
Divulgaciones
The authors have nothing to disclose.
Materials
| Ocrygel 10 g | Laboratoires T.V.M. | 3.70045E+12 | Silicon dental paste blue and yellow |
| Superglue gel | To glue the ring to the bone | ||
| Histoacryl 5 x 0.5 mL | Braun | 1050052 | Injectable solution of surgical glue |

