Mast Cell Degranulation Assay to Study the Effect of a Target Chemical
Abstract
Source: Tsvilovskyy, V., et al. Isolation of Peritoneum-derived Mast Cells and Their Functional Characterization with Ca2+-imaging and Degranulation Assays. J. Vis. Exp. (2018).
This video demonstrates a colorimetric assay to determine the degranulation activity of mast cells. The enzyme released by the mast cells hydrolyzes the substrate and produces a colored product. The degranulation activity of the mast cells was measured as the percentage release of enzymes outside the cell normalized with the total enzyme content in the cell.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. PMC Isolation and Cultivation by Intraperitoneal Lavage
- PMC isolation by intraperitoneal lavage
- Prior to the cell isolation, prepare the materials listed in Table 1.
- Use 8 to 14-week-old male mice for PMC isolation. Euthanize a mouse by CO2 inhalation, and confirm the death by loss of reflexes. Spray the mouse with 70% ethanol and fix it on a foam block using pins (dorsal side down). Remove the ventral skin of the mouse using blunt-edge scissors. Avoid damaging the peritoneal cavity.
NOTE: We use typically this protocol for mice with a C57BL/6N strain genetic background. - Inject 7 mL of the ice-cold RPMI medium and 5 ml of air in the peritoneal cavity using a 10 mL syringe equipped with a 27 G needle. Push the needle carefully in the peritoneum and do not perforate any organs. Use a spot in the region of the epididymal fat to reduce the risk of organ perforation.
- After injection, shake the mouse for 1 min to detach peritoneal cells into the RPMI medium. Do not shake the mouse too strongly, to avoid damaging the internal organs and contaminating the peritoneal cavity with the blood.
- Reuse the 10-mL syringe by equipping it with a new 20 G cannula. Shift the inner organs to one side by tilting the foam block and gently tapping it on the bench to make medium aspiration easier from the other side. Insert a 20 G needle, bevel up, and aspirate the fluid from the abdomen gently and slowly (~0.5 mL/s) to avoid clogging by the inner organs. Collect as much fluid as possible (typically 5–6 mL).
- Remove the needle from the syringe and transfer the collected cell suspension to a collection tube on ice. Discard a sample tube if there is visible blood contamination.
- Centrifuge the tubes with the cell suspension at ~300 x g for 5 min. Under a sterile hood aspirate the supernatant. For each mouse, combine the sample pellets in 4 mL of cold (4–10 °C) PMC Medium and transfer the cell suspension to a 25 cm2 culture flask. Add the growth factors IL-3 and SCF to the final concentrations of 10 ng/mL and 30 ng/mL, respectively. Place the flask containing the cells in an incubator (37 °C and 5% CO2) and incubate for ~48 h until the next procedure.
- PMC culture
- Day 2 (~48 h after isolation): Medium change
- To remove the supernatant and non-adherent cells (erythrocytes and dead cells), aspirate the medium from the flask by placing a Pasteur pipette tip at the edge of the flask and holding the flask almost horizontally. Add 4 mL of pre-warmed PMC Medium per mouse. Add the growth factors IL-3 (10 ng/mL) and SCF (30 ng/mL). Place the flask containing the cells in an incubator (37 °C and 5% CO2).
- Day 9: Cell splitting
- Transfer the cell suspension from the cell culture flask into a 50 mL plastic centrifuge tube. Wash the flask three times with 10 mL pre-warmed (37 °C) Dulbecco's phosphate-buffered saline (DPBS), collecting the cell suspension with the detached cells in the same 50 mL plastic centrifuge tube. Count the cell concentration by a hemocytometer, and calculate the total number of cells.
- Centrifuge the cell suspension at ~300 x g for 5 min and aspirate the supernatant. Recover the pellet in pre-warmed PMC Medium (37 °C) to obtain the cell concentration 1 x 106 cells/mL. Transfer the cell suspension to a new 25 cm2 culture flask. Discard the old flask with fibroblasts adhering to the bottom.
- Add the growth factors IL-3 (10 ng/mL) and SCF (30 ng/mL), and place the flask containing the cells in an incubator (37 °C and 5% CO2).
- Days 12–15: Cell measurements
- If any experiments with stimulation of FcɛRI receptors are planned, pretreat the cells overnight with 300 ng/mL of IgE anti-DNP in a standard culture medium.
- Day 2 (~48 h after isolation): Medium change
2. Beta-hexosaminidase Release Measurement in PMCs
- Prior to the measurements, prepare the materials listed in Table 2.
NOTE: β-hexosaminidase released from MCs after their stimulation hydrolyzes p-nitrophenyl-acetyl-D-glucosamine (pNAG) into p-nitrophenol and N-acetyl-D-glucosamine. The amount of β-hexosaminidase in the sample is proportional to the amount of p-nitrophenol that will be formed. In a high pH environment of "Stop Solution", p-nitrophenol exists as fully deprotonated p-nitrophenolat and can be detected by its light absorbance at 405 nm. - Prepare Tyrode's solution containing 130 mM NaCl, 5 mM KCl, 1.4 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 5.6 mM glucose, and BSA 0.1%. Adjust the pH to 7.4 with 3 M NaOH.
- Prepare the lysis solution by adding a 1% volume of Triton X-100 to the Tyrode's solution.
- Prepare the stop solution composed of 200 mM glycine and adjust the pH to 10.7 with NaOH.
- Prepare the pNAG solution (4-Nitrophenyl 2-acetamido-2-deoxy-β-D-glucopyranoside) solution by dissolving pNAG in 0.4 M citric acid to a final concentration of 4 mM.
- Calculate the amount of the cells needed for the assay (perform the assay in duplicate). Use 200,000 PMCs per well. Use 2 wells as negative controls (Tyrode's solution without any secretagogues such as DNP or compound 48/80) and 2 wells as positive controls (Tyrode's solution with 10 µM Ionomycin) for every genotype.
- Transfer the cells into a 15-mL plastic centrifuge tube, adjust the volume to 15 mL with Tyrode's solution, and centrifuge at 200 x g for 4 min. Remove the supernatant with a Pasteur pipette and resuspend the cells in Tyrode's solution to 2 x 106 cells/mL.
- Transfer 100 µL of the cell suspension per well to a 96-well V-bottom well plate. Add 25 µL of either stimulation or control solution to each well (according to the pipetting scheme illustrated in Figure 1A). Incubate the cells for 45 min at 37 °C and 5% CO2.
- Stop the reaction by placing the 96-well plate on ice for 5 min. Then, centrifuge the plate at 4 °C for 4 min at 120 x g. Transfer 120 µL of the supernatant using a multichannel pipette to a flat-bottom 96-well plate and place it on ice. Carefully avoid touching the cell pellets, but completely aspirate the supernatant.
- Add 125 µL of lysis buffer to the cell pellets. Incubate the cell pellets for 5 min at room temperature and resuspend them after the incubation by repeated pipetting (approximately 5 times).
- Pipette 25 µL of the pNAG solution (4 mM) in the required wells of a new flat-bottom 96-well plate. Add 25 µL from each supernatant to the prepared pNAG solution as well as 25 µL of each cell lysate.
- Incubate the reaction batches for 1 h at 37 °C. After the incubation, pipette 150 µL of stop solution to each well to stop the reaction.
- Analyze the plate with a microplate reader using a dual-wavelength setting at 405 nm with reference 630 nm for automatic background subtraction. In the acquisition program, tick the box "Reference" and in the "Absorbance" program element menu, select "630 nm" from the drop-down list. If the optical density of the samples is too high, prepare an appropriate dilution of the probe before re-measuring.
Table 1: Materials for Step 1.
| 1 | Ice |
| 2 | 10 mL syringes |
| 3 | 27 G needles |
| 4 | 20 G needles |
| 5 | Styrofoam block and pins |
| 6 | Collection tubes (50 mL Plastic Centrifuge Tubes) |
| 7 | 70% ethanol |
| 8 | RPMI Medium (Pre chilled and kept on ice) |
| 9 | PMC Medium: RPMI Medium + 20% FCS (Fetal Calf Serum) + 1% Penicillin Streptomycin solution (Pen-Strep) |
| 10 | Dulbecco’s phosphate buffered saline – DPBS (without Ca2+ and Mg2+) |
| 11 | Culture Flasks (25 cm) |
| 12 | Growth factors stock solutions (IL-3: 1 μg/μL; SCF: 2.5 μg/mL) |
| 13 | Serological pipettes (10 mL) |
| 14 | Pipettes tips sterile (20–200 µL) |
| 15 | Hemocytometer |
| 16 | Bench Centrifuge |
| 17 | Scissors and forceps |
| 18 | CO2 chamber for mice |
| 19 | Open sterile hood |
| 20 | Closed sterile hood |
| 21 | Cell incubator (37 °C and 5% CO2) |
| 22 | Transfer pipettes (20–200 µL) |
Table 2: Materials for Step 2.
| 1 | PMC (12–15 days old) 1 x 106 cells/mL |
| 2 | Anti-DNP-Antibody (IgE) stock solution |
| 3 | DNP-HSA stock solution |
| 4 | Compound 48/80 stock solution |
| 5 | Ionomycin |
| 6 | 96-Well plate, v-shaped bottom |
| 7 | 96-Well plates, flat bottom |
| 8 | Plastic centrifuge tubes (15 mL) |
| 9 | Serological pipettes |
| 10 | Cell incubator (37 °C and 5% CO2) |
| 11 | Bench Centrifuge |
| 12 | Microtiter plate reader for optical density measurements |
| 13 | Multichannel pipette (20–200 μL) |
| 14 | Hemocytometer |
Representative Results
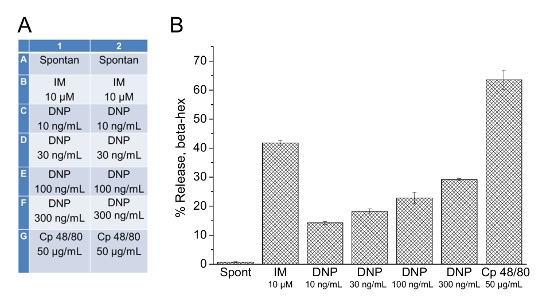
Figure 1: Beta-hexosaminidase release colorimetric multi-well measurements performed with PMCs isolated from wild-type mice. (A) Stimulation scheme of 96-well plate pipetting for performing the beta-hexosaminidase assay; results are presented in panel B. "Spontan" corresponds to pipetting of Tyrode's Solution without the addition of any secretagogues; IM = Ionomycin; Cp 48/80 = Compound 48/80. (B) Bar graph illustrating the percentage of ß-hexosaminidase release under basal conditions (Spont), after stimulation with Ionomycin (IM), dinitrophenol-human serum albumin (DNP), and Compound 48/80 (Cp 48/80) with indicated concentrations. The assay was performed in duplicate; error bars show the standard error of the mean.
Divulgaciones
The authors have nothing to disclose.
Materials
| RPMI Medium | Thermo Scientific | 21875034 | |
| DPBS | Sigma-Aldrich | 14190144 | |
| FCS | Thermo Scientific | R92157 | |
| IL-3 | R&D Systems | 403-ML | |
| SCF | Thermo Scientific | PMC2115 | |
| DNP-HSA | Sigma-Aldrich | A6661 | |
| Anti-DNP-Antibody | Sigma-Aldrich | D-8406 | |
| Penstrep | Thermo Scientific | 15140122 | |
| Compound 48/80 | Sigma-Aldrich | C2313 | |
| 4-(1,1,3,3-Tetramethylbutyl)phenyl-polyethylene glycol | Sigma-Aldrich | X100 | |
| 4-Nitrophenyl 2-acetamido-2-deoxy-β-D-glucopyranoside | Sigma-Aldrich | N9376 | |
| CoverWell Imaging Chamber | Sigma-Aldrich | GBL635051 | |
| 96-Well plate, v-shaped bottom | Corning | 3896 | |
| 96-Well plates, flat bottom | Greiner Bio-One | 655180 | |
| Microtiter plate reader with "i-control" software | Tecan | Nano Quant, infinite M200 Pro | |
| Flat bottom plate | Greiner Bio-One | 655180 | |
| Ionomycin | Sigma-Aldrich | I9657 | |
| 50 mL Plastic Centrifuge Tubes | Sarstedt | 62.547.254 | |
| Culture Flasks (25 cm) | Greiner Bio-One | 69160 | |
| Hemocytometer | VWR | 631-0696 | |
| Monochromatic Light Source | Sutter Instruments | Lambda DG-4 | |
| 15 mL Plastic Centrifuge Tubes | Sarstedt | 6,25,54,502 | |
| Bench Centrifuge | Heraeus | Megafuge 1.0R |

