A Serum Bactericidal Assay for the Complement-Mediated Bactericidal Activity of Antibodies
Abstract
Source: Weerts, H. P., et al. A High-throughput Shigella-specific Bactericidal Assay. J. Vis. Exp. (2019).
This video demonstrates a technique to evaluate the complement-mediated bactericidal activity of antibodies present in serum. This approach combines isolated serum with pathogenic bacteria and exogenous complement proteins. Upon incubation, the bacteria are grown on an agar plate, and the produced colonies are quantified to measure the serum bactericidal titer.
Protocol
1. Prepare Complement and Target Bacteria
- Prepare baby rabbit complement (BRC)
NOTE: Detailed criteria for complement lot selection can be found here: https://www.vaccine.uab.edu/uploads/mdocs/UAB-MOPA.pdf- Obtain frozen BRC and thaw with running cold water. Physically mix the BRC every ~10-20 min until completely thawed. Do not subject BRC to repeated freeze-thaw cycles.
- While BRC is thawing, label 1.5 mL, 5 mL, or 15 mL centrifuge tubes. Place labeled tubes on ice to pre-cool. Aliquot proper volume BRC to the pre-cooled centrifuge tubes (after filling, immediately return each tube to the ice). Store aliquots at ≤ -70 °C in the freezer.
NOTE: Approximately 1 mL of complement is needed for each assay plate. Complement aliquots are for single use and should be aliquoted in volumes appropriate to assay layouts. - To prepare Heat-inactivated BRC, thaw one aliquot of active BRC. Prepare a 56 °C water bath. After BRC is completely thawed, transfer it to the water bath and incubate for 30 min.
- After incubation, remove heat-inactivated BRC from the water bath and allow it to cool at room temperature (RT) for 10-15 min. Mix vigorously, and aliquot ~150 µL to 1.5 mL microcentrifuge tubes. Store aliquots at ≤ -10 °C.
- Prepare target bacteria stock
NOTE: The procedure below is used to prepare 48 aliquots of target bacteria stock; if more aliquots are needed the protocol can be scaled up.- Remove the bacteria master stock vial from the freezer and scrape the frozen bacterial surface to remove a small amount of ice from the vial onto a blood agar plate. Immediately return the master stock vial to the freezer.
- Streak this small aliquot of bacterial stock onto the blood agar plate and cover it with the plate lid. Incubate the plate upside down overnight in a 37 °C/5% CO2 incubator.
- Transfer ~10 isolated smooth colonies to a 50 mL tube containing 30 mL of LB broth. Incubate for 3-5 h at 37 °C with gentle shaking until the culture broth has an OD600 of ~0.6-0.7.
- Harvest the top 12.5 mL of the culture and transfer to a fresh 50 mL tube. Centrifuge the culture at 15,000 x g for 2 min using a tabletop micro-centrifuge. Discard the supernatant and re-suspend the pellet in 25 mL of 15% sterile glycerol-LB.
- Mix well and dispense 0.5 mL aliquots into sterile 1.5 mL microcentrifuge tubes (~48 tubes). Store aliquots at ≤ -70 °C in freezer.
- Confirm the bacterial identity using the agglutination test before use.
- Determination of optimal dilution factor for target bacteria stock
NOTE: Each batch of target bacteria stock must be titrated in assay conditions to determine the dilution necessary to yield ~120 CFU/spot on LB plates.- Get a microtiter plate (Dilution Plate) and add 135 µL of Assay Buffer to well 1A. Add 120 µL of Assay butter to wells 1B-1H.
- Remove a vial of frozen target bacteria from the freezer and thaw at room temperature. Add 15ul of thawed bacterial stock to well 1A to make a 10-fold dilution of the bacterial stock.
- Transfer 30 µL of bacterial solution from well 1A to well 1B to perform a 5-fold serial dilution. Continue 5-fold serial dilutions to well 1H for a total of 8 dilutions (1:10; 1:50; 1:250; 1:1 250; 1:6 250; 1: 31 250; 1: 156 250; 1:781 250).
- Get another microtiter plate (Assay Plate) and add 20 µL of Assay Buffer to all wells in columns 1 and 2 in the Assay Plate.
- Transfer 10 µL of diluted bacteria from each well in column 1 of the Dilution plate into the corresponding wells in columns 1 and 2 of the Assay Plate. 10 µL of bacteria is transferred from well 1A of the dilution plate into well 1A and 2A in the Assay Plate, etc.
- Continue with the assay as described in the Serum Bactericidal Assay (SBA) below for Control A and Control B, steps 3.6-3.12.
- After the plates have been incubated on ice, use a multichannel pipette with 8 pipette tips to spot 10 µL from the wells in column 1 onto an LBA plate. Also, spot the wells from column 2 onto the LBA plate.
- Continue with the assay as described below in steps 3.14-4.6.
- Determine the bacteria dilution that yields ~120 CFU/spot in Control B, this dilution will be used in the assay.
2. Serum Bactericidal Assay (SBA)
NOTE: The procedure described below is for one assay plate, but the number of Assay Plates can be increased.
- Heat-inactivate test samples by incubating samples in a 56 °C water bath for 30 min.
NOTE: Test samples must be heat-inactivated prior to the test to abrogate any endogenous complement activity. This can be done ahead of the assay and inactivated samples can be re-frozen or stored at 4 °C until tested. - Get an Assay Plate and add 20 µL of Assay Buffer to columns 1 through 12 of rows A through G. Add 20 µL of Assay Buffer to columns 1 and 2 of row H, see Table 1.
- Load 30 µL of each test sample, in duplicate, to row H of the Assay Plate. For example, dispense 30 µL of sample 1 into wells 3H and 4H, and dispense 30 µL of sample 2 into wells 5H and 6H, etc.
- Perform 3-fold serial dilutions of test samples using a multichannel pipette.
- Remove 10 µ of the sample from wells 3H-12H and transfer it to corresponding wells in row G and mix the sample well by pipetting up and down 8-10 times.
- Then remove 10 µ from wells 3G-12G and transfer to corresponding wells in row F and mix well.
- Continue these serial dilutions through row A. After mixing the wells in row A, remove and discard 10 µl from wells 3A-12A so that all wells contain 20 µl.
NOTE: Because 20 µL of serum is used in a total assay volume of 80 µL, there is a 4-fold additional dilution in the assay. This dilution must be taken into account during the calculation of an SBA titer by multiplying the dilution of serum by 4. For example, if a starting dilution of 1:2 is used, the actual dilution being tested is 1:8.
- Remove one vial of frozen Target Bacteria Stock and thaw at room temperature. Dilute the bacteria in 20 mL of Assay Buffer according to the pre-determined optimal dilution factor (this dilution factor was determined in section 1.3). Add 10 µL of diluted bacteria to each well of the assay plate using a multichannel pipette.
- Remove one vial of frozen BRC and one vial of frozen Heat-inactivated BRC, thaw at room temperature with running cold water, or place on the grate of a biological safety cabinet with blowing air to thaw quickly.
- Prepare a 20% solution of heat-inactivated BRC. Mix 100 µL of heat-inactivated BRC with 400 µL of Assay Buffer. Add 50 µL of this 20% heat-inactivated BRC solution to all wells in column 1 (Control A wells).
NOTE: Heat-inactivated BRC is used as a control to monitor non-specific killing (NSK) in the assay. - Prepare a 20% solution of native BRC. Mix 1 mL of native BRC with 4 mL of Assay Buffer. Add 50 µL of this mixture to all wells in columns 2 through 12 (Control B and test sample wells).
NOTE: The final concentration of BRC in the reaction mixture is 12.5%. - Briefly mix the Assay Plate by shaking gently for 10-15 s on a plate shaker or mix by pipetting up and down 8 times using a multichannel pipette.
- Put the Assay Plate in a 37 °C microbiological incubator for 2 h (without shaking).
- Dry 2 LBA plates by removing lids and placing plates face up in the biological safety cabinet for 40-60 min.
- When the 2 h incubation is complete, move the Assay Plate to wet ice and incubate for 10-20 min to stop the reaction.
- Using a 12-channel pipette, mix the wells in row H, and spot plate 10 µL of the reaction mixture onto the bottom of an LBA plate. Immediately tilt the plate and allow the spots to run for ~1.5-2 cm. Repeat this procedure for rows G, F, and E, spotting them above the previous row on the LBA plate. Row E, F, G, and H are spotted on one LBA plate and rows A, B, C, and D are spotted on a second LBA plate in the same manner, see Figure 1.
- Incubate the LBA plates at room temperature until the solution is adsorbed into the LBA plates (10-15 min). Put the lids on the LBA plates and place the LBA plates in the microbiological incubator upside-down to incubate overnight (~16-18 h). Incubate Shigella flexneri 2a and 3a at 29 °C and incubate S. sonnei is at 26 °C.
NOTE: These temperatures yield smaller "micro-colonies" with sizes suitable for accurate counting by a colony counter9. - After overnight incubation, add 25 mL of Overlay Agar (at ~55 °C) containing 100 µg/mL TTC and 0.1% NaN3 to each LBA plate.
- Incubate the LBA plates at 37 °C for 2 h to allow the surviving bacteria to develop a red color, see Figure 1 below.
- Photograph plates using a digital camera and transfer images to a computer where NIST's Integrated Colony Enumerating Software (NICE) has been installed, see Figure 2.
NOTE: NICE colony-counting software is available at no charge. See Materials List for details and installation instructions.
Table 1: Assay plate layout. Columns 1 and 2 contain the complement control wells. Control A is located in column 1 and is the heat-inactivated complement control, containing SBA buffer, bacteria, and heat-inactivated complement. Control B is located in column 2 and is the active complement control, containing SBA buffer, bacteria, and complement. Columns 3-12 contain serum samples. Each sample is run in duplicate and serially diluted 3-fold from row H to row A
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| A | Control A | Control B | Dilution 8 | Dilution 8 | Dilution 8 | Dilution 8 | Dilution 8 | Dilution 8 | Dilution 8 | Dilution 8 | Dilution 8 | Dilution 8 |
| B | Control A | Control B | Dilution 7 | Dilution 7 | Dilution 7 | Dilution 7 | Dilution 7 | Dilution 7 | Dilution 7 | Dilution 7 | Dilution 7 | Dilution 7 |
| C | Control A | Control B | Dilution 6 | Dilution 6 | Dilution 6 | Dilution 6 | Dilution 6 | Dilution 6 | Dilution 6 | Dilution 6 | Dilution 6 | Dilution 6 |
| D | Control A | Control B | Dilution 5 | Dilution 5 | Dilution 5 | Dilution 5 | Dilution 5 | Dilution 5 | Dilution 5 | Dilution 5 | Dilution 5 | Dilution 5 |
| E | Control A | Control B | Dilution 4 | Dilution 4 | Dilution 4 | Dilution 4 | Dilution 4 | Dilution 4 | Dilution 4 | Dilution 4 | Dilution 4 | Dilution 4 |
| F | Control A | Control B | Dilution 3 | Dilution 3 | Dilution 3 | Dilution 3 | Dilution 3 | Dilution 3 | Dilution 3 | Dilution 3 | Dilution 3 | Dilution 3 |
| G | Control A | Control B | Dilution 2 | Dilution 2 | Dilution 2 | Dilution 2 | Dilution 2 | Dilution 2 | Dilution 2 | Dilution 2 | Dilution 2 | Dilution 2 |
| H | Control A | Control B | Dilution 1 | Dilution 1 | Dilution 1 | Dilution 1 | Dilution 1 | Dilution 1 | Dilution 1 | Dilution 1 | Dilution 1 | Dilution 1 |
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | ||||||||
Representative Results
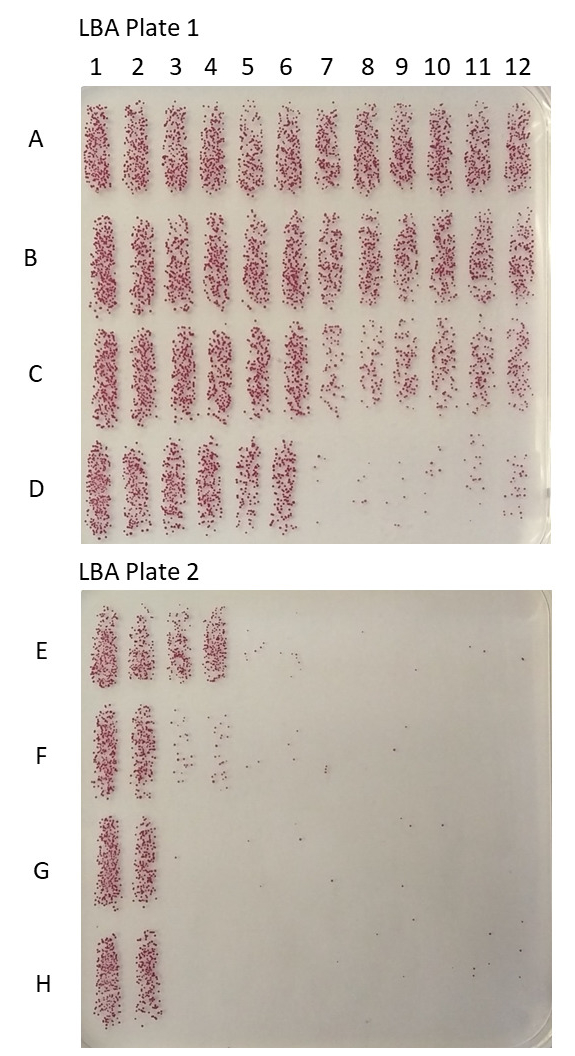
Figure 1: LBA plates after color development. Representative S. flexneri 3a bacterial micro-colonies have grown overnight to the appropriate size. Overlay agar has been added and colonies have developed a red color by reduction of the TTC compound in overlay agar.
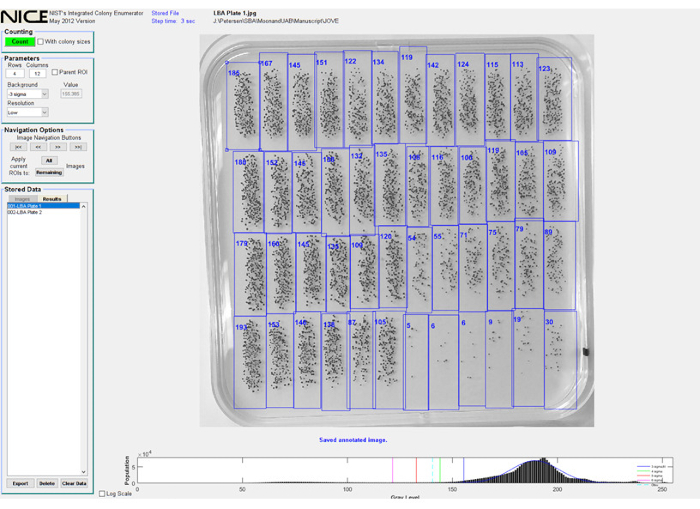
Figure 2: NIST's Integrated Colony Enumerator (NICE) software interface. Graphical representation of the NICE software interface. Regions of Interest (ROIs) are centered over colonies for each spot before counting. Data can be exported directly from the NICE window.
Divulgaciones
The authors have nothing to disclose.
Materials
| Gelatin | Sigma | G9391 | Type B, powder, BioReagent, suitable for cell culture |
| TTC (2,3,5-Triphenyltetrazolium chloride) | Sigma | T8877 | ≥98.0% (HPLC) |
| Sodium azide (NaN3) | Sigma | S2002 | ≥99.5% |
| Baby Rabbit Complement | PelFreez | 31061-3 | 3-4 week old |
| HBSS with Ca2+/Mg2+ | Invitrogen | 14065-56 | Without Phenol Red |
| LB Agar (Lennox) | Sigma | L2897 | Powder microbial growth medium |
| Bacto Agar | BD | 214010 | Powdered, (C12H18O9)n |
| Glycerol | Sigma | G5516 | For molecular biology, ≥99% |
| LB Broth (Lennox) | Sigma | L3022 | Powder microbial growth medium |
| Square Petri Dish | Sigma | Z617679-240EA | 120 mm x 120 mm |
| Assay Plate | Costar | 3799 | 96 well u-bottom plate with lid |
| NICE Software | University of Alabama at Birmingham | ftp://ftp.nist.gov/pub/physics/mlclarke/NICE/ |

