A Microtiter Plate Method to Assess the Minimum Inhibitory Concentration of an Antibiotic
Abstract
Source: Ketelboeter, L. M., et al. Methods to Inhibit Bacterial Pyomelanin Production and Determine the Corresponding Increase in Sensitivity to Oxidative Stress. J. Vis. Exp. (2015).
This video demonstrates a microtiter plate-based method for determining the Minimum Inhibitory Concentration (MIC) of test antibiotics against bacteria. Upon adding a suspension of the bacterial pathogen Pseudomonas aeruginosa to a microtiter plate containing serial dilutions of a test antibiotic, and an inhibitor of the production of pyomelanin by the bacteria, the optical density of the wells is measured to compute the minimum inhibitory concentration of the antibiotic, and the impact of the pyomelanin production inhibitor the antibiotic sensitivity of the bacteria.
Protocol
1. Antibiotic Minimum Inhibitory Concentration (MIC) Assay in 96-well Plates
- Set up overnight cultures of the strains to be tested in LB (Luria-Bertani) with and without NTBC (2-[2-nitro-4-(trifluoromethyl)benzoyl]-1,3-cyclohexanedione).
NOTE: This protocol is described using the representative level of 300 µM NTBC.- Add 300 µM NTBC to 2 ml LB. Add an equivalent volume of vehicle (Dimethyl sulfoxide, DMSO) to 2 ml LB for the no NTBC condition.
- Using sterile toothpicks, inoculate tubes with one isolated colony of bacteria. There will be one culture with NTBC and one culture without NTBC for each strain. Incubate overnight at 37 °C with aeration using a tissue culture rotator in an air incubator.
- The following day, make LB + NTBC and LB + DMSO master solutions for setting up the MIC assay. Add NTBC at a concentration of 600 µM as this will be diluted two-fold when inoculum is added, yielding a final concentration of 300 µM.
- To test one antibiotic for one strain, add 600 µM NTBC to 2 ml LB and mix to make the NTBC master solution. Add an equivalent volume of vehicle (DMSO) to 2 ml LB and mix to make the no NTBC master solution. Use these solutions for creating antibiotic stock solutions as well as for setting up the dilution series in 96-well plates.
NOTE: The master solution formulations will yield extra solutions to account for pipetting errors. The master solutions can be scaled up or down as required depending on the number of antibiotics and strains tested.
- To test one antibiotic for one strain, add 600 µM NTBC to 2 ml LB and mix to make the NTBC master solution. Add an equivalent volume of vehicle (DMSO) to 2 ml LB and mix to make the no NTBC master solution. Use these solutions for creating antibiotic stock solutions as well as for setting up the dilution series in 96-well plates.
- Prepare the antibiotic solutions in the LB + NTBC or LB + DMSO master solutions.
NOTE: The antibiotic concentration in these solutions should be double the final desired concentration. Enough solution should be made to transfer 100 µl to four wells in a 96-well plate.- Prepare gentamicin +/- NTBC stock solution at 64 µg/ml. To make this solution, add 0.288 µl of gentamicin stock (100 mg/ml) to 450 µl LB + NTBC or LB + DMSO master solution.
NOTE: The maximum concentration of gentamicin for Pseudomonas aeruginosa PAO1 is 32 µg/ml. - Make the kanamycin +/- NTBC stock solution at 256 µg/ml. To make this solution, add 3.84 µl of kanamycin stock (30 mg/ml) to 450 µl LB + NTBC or LB + DMSO master solutions.
NOTE: The maximum concentration of kanamycin for P. aeruginosa PAO1 is 128 µg/ml. - Prepare tobramycin +/- NTBC stock solution at 8 µg/ml. To make this solution, add 0.36 µl of tobramycin stock (10 mg/ml) to 450 µl LB + NTBC or LB + DMSO master solutions.
NOTE: For P. aeruginosa PAO1, the maximum concentration of tobramycin is 4 µg/ml.
NOTE: The antibiotics and concentrations can be adjusted for the bacteria to be tested.
- Prepare gentamicin +/- NTBC stock solution at 64 µg/ml. To make this solution, add 0.288 µl of gentamicin stock (100 mg/ml) to 450 µl LB + NTBC or LB + DMSO master solution.
- Add 100 µl of each 2x antibiotic solution to four wells in a 96-well plate. Place these solutions in row A. For example, gentamicin should be placed in A1 through A4, kanamycin should be placed in A5 through A8, and tobramycin should be placed in A9 through A12. See Figure 1A for a diagram of a 96-well plate setup.
NOTE: Multiple antibiotics can be tested in one plate, but only one strain should be tested per plate to eliminate the potential for cross-contamination from other strains. - Add 50 µl of the LB + NTBC or LB + DMSO master solution to rows B through H of the 96-well plate. Ensure that one plate is LB + NTBC and one plate is LB + DMSO. See Figure 1A.
- Use LB + NTBC for the antibiotics in LB + NTBC. Use LB + DMSO for the antibiotics in LB + DMSO.
- Using a micro pipettor perform two-fold serial dilutions of the antibiotics by transferring 50 µl of the solution from row A to row B. Mix the solution, change the pipet tips, and transfer 50 µl of the solution from row B to row C. Repeat for the remaining rows. After diluting row G, remove 50 µl of the solution from that row and discard. Use row H as a no-antibiotic control for bacterial growth. See Figure 1B.
NOTE: Each well in rows A through G now contains 50 µl of antibiotic in LB + NTBC or LB + DMSO at 2X the final desired concentration. Row H contains LB + NTBC or LB + DMSO with no antibiotics. - Measure the OD600 of the overnight cultures. Wash all cultures before taking OD600 readings to eliminate pyomelanin present in the media.
- Wash the cultures by centrifuging 1 ml of culture in a microcentrifuge at 16,000 x g for 2 min. Remove the supernatant with a micro pipettor and resuspend the cell pellet in 1 ml LB.
- Dilute the overnight cultures to 2.75×105 CFU/ml in LB.
NOTE: Assume that one OD600 unit is the equivalent of 1×109 CFU/ml for P. aeruginosa. OD to CFU/ml conversions may be different in other bacteria. - Add 50 µl of the diluted bacterial culture to the appropriate well.
NOTE: Cultures grown in NTBC should be added to the wells containing NTBC and cultures grown in DMSO should be added to the wells containing DMSO. See Figure 1B.- Add bacteria to three wells for each strain and antibiotic concentration. Add 50 µl of LB to the fourth well to act as a control for bacterial contamination. See Figure 1B.
- Use a multi-channel micro pipettor to inoculate the wells. Ensure that pipet tips are near the bottom of the wells when adding inoculum to prevent contamination of neighboring wells.
NOTE: Adding bacterial culture to the wells will dilute the antibiotic and NTBC concentrations twofold.
- Cover the 96-well plates with parafilm and incubate for approximately 24 hr at 37 °C. Incubate 96-well plates statically, in an air incubator.
- Examine the plates for bacterial growth in the wells. The MIC is the lowest concentration of antibiotic in which no bacterial growth is seen for all three replicates of each strain.
- Visually examine the plate for growth or read using a plate reader set to OD600.
Representative Results
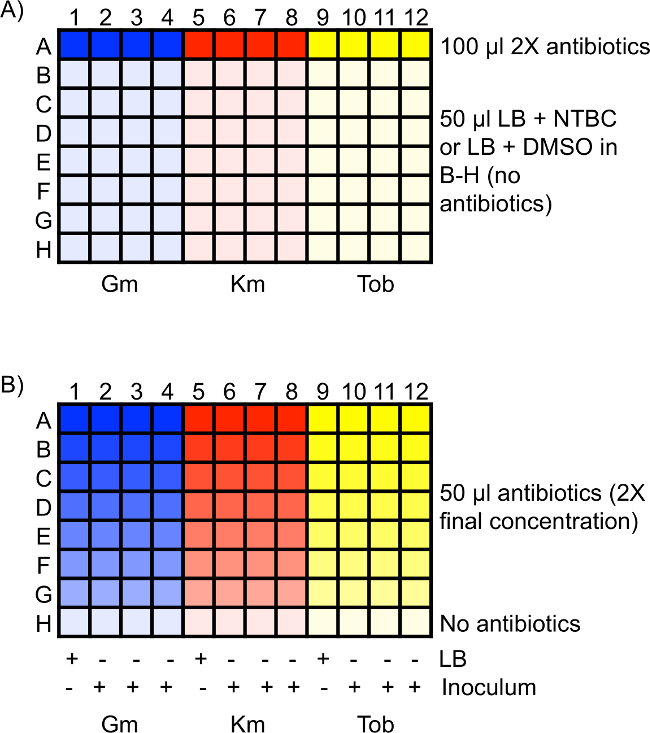
Figure 1: Schematic of antibiotic MIC assay 96-well plate setup. (A) 100 µl of 2x antibiotics of the highest starting concentration are in row A. Rows B through H are filled with 50 µl of either LB + NTBC or LB + DMSO without antibiotics. (B) Two-fold serial dilutions are performed in rows A through G, resulting in 50 µl of diluted antibiotic in each well at 2x the final desired concentration. Row H is a control well for bacterial growth without antibiotics. 50 µl of LB or inoculum is added to the appropriate wells, diluting the antibiotics two-fold to the final concentration. LB serves as a control for bacterial contamination in the antibiotics. Gm, gentamicin; Km, kanamycin; Tob, tobramycin
Divulgaciones
The authors have nothing to disclose.
Materials
| 2-[2-nitro-4-(trifluoromethyl)benzoyl]-1,3-cyclohexanedione (NTBC) | Sigma-Aldrich | SML0269-50mg | Also called nitisinone. Soluble in DMSO. |
| H2O2 | Sigma-Aldrich | 216763-100ML | 30 wt. % in H2O. Stabilized. |
| Gentamicin | Gold Bio | G-400-100 | Soluble in H2O. Filter sterilize. |
| Kanamycin | Fisher Scientific | BP906-5 | Soluble in H2O. Filter sterilize. |
| Tobramycin | Sigma-Aldrich | T4014-100MG | Soluble in H2O. Filter sterilize. |

