Measuring Immunoglobulin G Levels Against a Test Vaccine in a Mouse Serum
Abstract
Source: Zeng, X. et al., Evaluating the Immune Response of a Nanoemulsion Adjuvant Vaccine Against Methicillin-Resistant Staphylococcus aureus (MRSA) Infection. J. Vis. Exp. (2023)
This video demonstrates a method for assessing the antibody immune response to a novel nanoemulsion adjuvant vaccine in pre-immunized mice. Immunization enhances antibody response as confirmed by determining the IgG antibody titers.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Preparation of the MRSA HI antigen protein
- Obtain Fe ion surface determining factor B (IsdB) and α-hemolysin mutant (Hla) clones from commercial sources (see Table of Materials), perform polymerase chain reaction (PCR) amplification to amplify IsdB and Hla genes, and ligate their products to the PGEX-6P-2 plasmid (obtained commercially; see Table of Materials). Screen with the vector Xl/blue strain of Escherichia coli.
- Transform the positive clone into E. coli BL21 competent cells (see Table of Materials) and amplify with shark culture.
- Express the antigen and isolate after induction with isopropyl-β-D-1-mercaptogalactopyranoside and multimodal chromatography (MMC) isolate kits (see Table of Materials).
- Characterize the antigen protein and purify it with a full-automation purifier.
- Affinity-purify gutathione S-transferase (GST)-tagged proteins from cleared lysates using a commercially available affinity chromatography resin (see Table of Materials).
- Purify the recombinant proteins by isopropyl-β-D-1-mercaptogalactopyranoside and multimodal chromatography (MMC).
- Remove the endotoxin by the Triton X-114 phase separation method. Briefly, mix 1% Triton X-114 with recombinant protein eluate at 0 °C for 5 min to ensure a homogenous solution, and incubate at 37 °C for 5 min to allow two phases to form.
- Use the bacterial endotoxin test method with Turtle kits (see Table of Materials) to detect the endotoxin concentration. The endotoxin for protein antigen is 0.06 EU/µg, lower than the international standard (1.5 EU/µg).
- Perform an interference test to eliminate the possible influence of the test product on endotoxin detection.
NOTE: According to the Appendix 2020 of Chinese pharmacopeia, the endotoxin content should be less than 5 EU/dose. The sensitivity of Limulus lysate (λ0.25 EU/mL) and the maximum effective dilution ratio of 33.3 were calculated based on maximum valid dilution, MVD = cL/λ, where L is the limit value of bacterial endotoxin for the test article, c is the concentration of the test article solution, and when L is expressed in EU/m, c is equal to 1.0 mg/mL. λ is the labeled sensitivity (EU/mL) of the horseshoe crab reagent in the gel method.
- Perform an interference test to eliminate the possible influence of the test product on endotoxin detection.
- Recombine the antigen (48 kDa, determined by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE]) at 1.5 mg/mL in sterile water without endotoxin or phosphate-buffered saline (PBS) (sodium dichlorate method).
NOTE: The protein peak time was 13.843 min, the purity was 99.4% (High-performance liquid chromatography method), and the protein was stored at -70 °C. Genomic DNA extracted from S. aureus strain MRSA252 (see Table of Materials) was used as the PCR template. The IsdB gene was amplified using the forward primer 59-CGCGGATCCATGAATGGCGAAGCAAAAGCAG
C-39 and the reverse primer 59-TTTTCCTTTTGCGG
CCGCCTATGTCATATCTTTATTAGATTCTTC-39.
The Hla gene was amplified using the forward primer PHLAF (GCGGATCCGATTCTGATATTAATATTAAAACC) and the reverse primer PHLAR (TAAGCGGCCGCTTATCAATTTGTCATTTCTTC).
2. Preparation of the nanoemulsion vaccine
- According to the mass ratio of 1:6:3(w/w), add three kinds of excipients in sequence (isopropyl myristate, polyoxyethylene castor oil, and propylene glycol; see Table of Materials) to the HI antigen protein solution (10 mg/mL) and stir well. Then, add gradually sterilized pure water to reach a final volume of 10 mL and stir at 500 rpm and 25 °C for approximately 20 min.
NOTE: The nanoemulsion prepared in this protocol has a volume of 10 mL, and the masses of the three excipients are 0.34 g, 2 g, and 1 g, respectively. The protein solution here is the working solution, 10 times the concentration of the final solution. - Prepare the nanoemulsion adjuvant vaccine (1 mg/mL protein) by low-energy emulsification methods to obtain a clear and transparent liquid solution.
NOTE: The blank nanoemulsion (BNE) was prepared by similar methods, except that water was replaced with HI. The usual emulsification method involves heating the outer and inner phases to approximately 80 °C for emulsification and then stirring and cooling them gradually. In this process, a large amount of energy is consumed, and finally, the emulsion is obtained.
3. Physical characterization and stability of the nanoemulsion adjuvant vaccine
- Particle size, zeta potential, and polymer dispersion index characterization (PDI).
- Prepare 100 µL of the nanoemulsion adjuvant vaccine and dilute 50-fold with water for injection. Add 2 mL of sample for detection.
- Observe the particle sizes, zeta potential, and PDI at 25 °C using a Nano Zetasizer (ZS; see Table of Materials).
- Transmission electron microscopy (TEM)
- Dilute 10 µL of nanoemulsion vaccine 50-fold with water, place on a carbon-coated 100-mesh copper grid, and stain with 1% phosphotungstic acid (see Table of Materials) for 5 min at room temperature.
- Remove excess phosphotungstic acid solution with filter paper.
- Observe the morphology under a transmission electron microscope (see Table of Materials). Examine whether the finished products are nearly stable and circular within the field of view of the electron microscope and whether they exhibit good dispersion.
- Scanning electron microscopy (SEM)
- Dilute 10 µL of the nanoemulsion adjuvant vaccine 50-fold with water, drop 10 µL of prediluted samples on a silicon wafer, and place in a 37 °C thermostat-controlled incubator for 24 h.
- Spray the prepared platinum on the silicon for 40 s and cool to room temperature.
- Observe the morphological properties of the prepared samples under a high-resolution scanning electron microscope (see Table of Materials). Determine whether the samples are nearly stable, spherical, and well dispersed within the field of view under the electron microscope.
- Morphological stability
- Place 1 mL of nanoemulsion vaccine samples in microcentrifuge tubes, and evaluate the stability of fresh samples by centrifuging for 30 min at 13,000 x g at room temperature (25 ° C).
- Observe the appearance of these fresh samples and then perform a thermodynamic stability test of six temperature cycles (one cycle: store at 4 °C for 48 h, 25 °C for 48 h, and 37 °C for 48 h).
- After centrifugation, allow the samples to stand for 15 min to determine whether there is a Tyndall effect (whether the sample is clear and transparent under light) and whether demulsification has occurred (shown by clear stratification after demulsification).
- SDS-PAGE and western blot
- Shake the samples, mix a solution of 0.6 mL of absolute ethanol and 0.3 mL of vaccine, and centrifuge (13,000 x g, 30 min at room temperature, 25 °C).
- Transfer the supernatant of the solution to a clean tube and dissolve the precipitate with 0.3 mL of water. Obtain the supernatant and precipitate solutions (both blank nanoemulsion and vaccine) after treatment with absolute ethanol and distilled water solution, and perform the same 50-fold dilution.
- Mix 2.5 mL of separation gel A and 2.5 mL of separation gel B (see Table of Materials), add 50 µL of 10% ammonium persulfate, and quickly place the solution in the glass plate with a pipette. Mix 1 mL of concentrated gel A and 1 mL of concentrated gel B, add 20 µL of 10% ammonium persulfate into the glass plate, insert an appropriately sized comb, and wait for solidification.
- Add a total of 5 µL of 5x protein loading buffer solution to the diluted sample obtained in step 3.5.2, and boil the sample for 5 min.
- Add 10 µL of heated protein to the corresponding well, and add 5 µL of protein marker to the marker well.
- Connect the electrode, turn on the electrophoresis meter (see Table of Materials), and adjust the voltage to 300 V. Turn off the power when the electrophoresis reaches 1 cm away from the bottom of the PAGE rubber.
- Remove the gel and rinse with double-distilled water (ddH2O). Add Coomassie bright blue G-250 staining solution (see Table of Materials) for 10 min. Pour out the staining solution, and rinse the gel twice with solution and the commercially available decolorization solution and microwave the gel for 1 min. Shake the gel for 10 min, and repeatedly change the decolorization solution until the gel background is clear. Then, perform gel imaging (see Table of Materials).
NOTE: The western blot was pre-processed, as described in steps 3.5.1-3.5.6. - Soak three filter paper blocks with an electrophoresis transfer buffer. Soak the polyvinylidene fluoride (PVDF) membrane in methanol for 30 s and transfer with a semidry gel transfer instrument (two layers of filter paper, PVDF membrane, electrophoresis gel, and one layer of filter paper). Use a voltage of 23 V to transfer the membrane for 23 min.
- Remove the PVDF membrane and wash once with tris-buffered saline-Tween 20 (TBST) after transfer.
- Place the PVDF membrane in sealing solution (5% skimmed milk powder solution) and seal overnight at 4 °C.
- Remove the PVDF membrane and wash three times with TBST or 10 min each.
- Incubate with the primary antibody (from immunized mice with positive serum). Dilute the primary antibody (see Table of Materials) to be tested 500-fold (100-fold) with TBST. Place the blocked PVDF membrane in the primary antibody and incubate at 37 °C for 1 h.
- Remove the PVDF membrane and wash three times with TBST for 10 min each.
- Incubate with the secondary antibody (see Table of Materials). Dilute alkaline phosphatase (AP)-labeled goat anti-mouse secondary antibody 5,000-fold with TBST. Place the PVDF membrane in the secondary antibody and incubate at 37 °C for 40 min.
- Remove the PVDF membrane and wash four times with TBST for 10 min each.
- Submerge the PVDF membranes in a mixture (just enough to soak the membrane) of the AP substrate nitro blue tetrazole (NBT) and BCIP (5-bromo-4-chloro-3'-indolyphosphate p-toluidine salt) (see Table of Materials) for staining. A positive result is purple.
NOTE: The results must be a clear purple band, and the sample strip and the molecular weight of the antigen should be at the same labeled position.
4. Assessment of antibody immune response to this vaccine after intramuscular administration
NOTE: The mice were immunized by intramuscular injection of the nanoemulsion vaccine following a published report. The mice were administered PBS as a negative control. At 1 week after three immunizations were completed, serum was collected from the mice. The serum levels of IgG and subclasses of IgG1, IgG2a, and IgG2b were quantitatively determined by enzyme-linked immunosorbent assay (ELISA).
- Antigen coating: Dilute HI protein antigen to 10 µg/mL with coating solution and add to the ELISA plate at 100 µL/well. Incubate at 4 °C overnight.
NOTE: The coating solution is prepared by dissolving 1.6 g of anhydrous sodium carbonate and 2.9 g of sodium bicarbonate in 1 L of deionized water. - Sealing: Wash the plate (three cycles, each cycle 300 µL). Add 300 µL of the sealing solution to each well, and seal the wells for 2 h at 37 °C.
NOTE: The sealing solution is prepared by adding Tween-20 and bovine serum albumin (BSA) to PBS. The content of BSA in the PBST solution is 1%. - Serum dilution: Dilute the sample serum of mice 100 times with PBST (take 3 µL of serum and add it to 297 µL of PBST and vortex), and use 100 µL for each serum sample. Dilute the positive serum and negative control serum 100 times with PBST by adding 4 µL to 396 µL of PBST, then mixing by vortexing.
- Double ratio dilution: Add 200 µL of the above diluted experimental serum to the first row of the coated ELISA plate, and add 100 µL of PBST to all wells except the first and 12th rows. Perform a 1:2 dilution successively from the first row: adjust the pipette to 100 µL, transfer 100 µL from the first row to the second row, and mix with the pipette 10 times.
- Dilute the serum to Row 11 at multiple ratios and discard 100 µL of liquid.
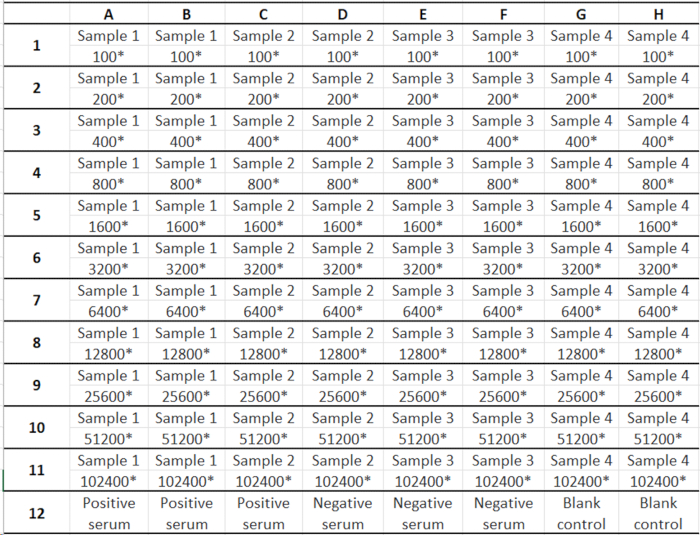
- Add diluted negative and positive serum to the corresponding wells in Row 12 according to the sample template (see Table 1)-100 µL/well.
- Seal the ELISA plates with plastic wrap and incubate at 37 °C for 1 h.
- Dilute secondary antibody anti-mouse IgG-HRP (see Table of Materials) with PBST at 1:10,000.
- Wash the plate (three cycles, each cycle 300 µL). Then, add 100 µL of the diluted secondary antibody to each well, and incubate the plate at 37 °C for 40 min.
- Staining and termination: Wash the plate (five cycles, each cycle 300 µL). Add 3,3′,5,5′-tetramethylbenzidine (TMB) staining solution (100 µL; see Table of Materials) to each well. Place the plates at 37 °C for 10 min away from light, and then terminate the reaction immediately with 50 µL of 2 M H2SO4.
- Detect the optical density (OD450) value by an enzyme marker (read the OD450 value within 15 min after termination).
Table 1: ELISA spiking sequence and dilution template.
Divulgaciones
The authors have nothing to disclose.
Materials
| 5424-Small high speed centrifugeFA-45-24-11 | Eppendorf, Germany | 5424000495 | |
| 96-well plates | Corning Incorporated, USA | CLS3922 | |
| AFM Dimension FastScan | BRUKER, Germany | null | |
| Balb/c mice | Beijing HFK Bioscience Co. Ltd. | ||
| BCIP/NBT | Fuzhou Maixin Biotechnology Development Company,China | BCIP/NBT | |
| Bio-Rad 6.0 microplate reader | Bio-Rad Laboratories Incorporated Limited Co., CA, USA | null | |
| BL21 Competent Cell | Merck millipore,Germany | 70232-3CN | |
| BSA-100G | Sigma-Aldrich, USA | B2064-100G | |
| Centrifuge 5810 R | Eppendorf, Germany | 5811000398 | |
| Coomassie bright blue G-250 staining solution | MIKX,China | DB236 | |
| Decolorization solution | BOSTER,China | AR0163-2 | |
| Electro-heating standing-temperature cultivator HH-B11-420 | Shanghai Yuejin Medical Device Factory, China | null | |
| Electrophoresis apparatus | Beijing Liuyi Instrument Factory, China | DYCZ-25D | |
| Gel image | Tanon, USA | null | |
| Glutathione-Sepharose Resin GST | Mei5bio,China | affinity chromatography resin | |
| H2SO4 | Chengdu KESHI Chemical Co., LTD,China | 7664-93-9 | |
| HI recombinant protein | Third Military Medical University,China | 110-27-0 | |
| HRP -Goat Anti-Mouse IgG | Biodragon, China | BF03001 | |
| HRP- Goat anti-mouse IgG1 | Biodragon, China | BF03002R | |
| HRP- Goat anti-mouse IgG2a | Biodragon, China | BF03003R | |
| HRP- Goat anti-mouse IgG2b | Biodragon, China | BF03004R | |
| Inoculation loop | Haimen Feiyue Co.,LTD,China | YR-JZH-1UL | |
| IsdB and Hla clones | Shanghai Jereh Biotechnology Co,China | null | |
| Isopropyl nutmeg (pharmaceutic adjuvant) | SEPPIC, France | null | |
| isopropyl- β-D-1-mercaptogalactopyranoside | fdbio,China | FD3278-1 | |
| LB bouillon culture-medium | Beijing AOBOX Biotechnology Co., LTD,China | 02-136 | |
| Low temperature refrigerated centrifuge | Eppendorf, Germany | null | |
| Malvern NANO ZS | Malvern Instruments Ltd., UK | null | |
| Micro plate washing machine 405 LSRS | Bio Tek Instruments,Inc Highland Park,USA | null | |
| Mini-TBC Compact Film Transfer Instrument | BeiJingDongFangRuiLi Co.,LTD,China | 1658030 | |
| MMC packing | TOSOH(SHANGHAI)CO.,LTD | 22818 | |
| MRSA252 | USA, ATCC | null | |
| Nanodrop ultraviolet spectrophotometer | Thermo Scientific, USA | null | |
| New FlashTM Protein any KD PAGE Protein electrophoresis gel kit | DAKEWE, China | 8012011 | |
| PBS | biosharp, China | null | |
| PCR, Amplifier | Thermal Cycler, USA | null | |
| pGEX-target gene recombinant plasmid | Shanghai Jereh Biotechnology Co,China | B3528G | |
| Phosphotungstic acid | G-CLONE, China | CS1231-25g | |
| Pipette | Eppendorf, Germany | 3120000844 | |
| Polyoxyethylated castor oil (pharmaceutic adjuvant) | Aladdin, China | K400327-1kg | |
| Primary antibody | Laboratory homemade:from immunized mice with positive sera | null | See Reference 11 for details |
| Propylene glycol (pharmaceutic adjuvant) | Sigma-Aldrich, USA | P4347-500ML | |
| Protein Marker | Thermo Scientufuc, USA | 26616 | |
| PVDF Transfer Membrane | Invitrogen,USA | 88518 | |
| Scanning Electron Microscope | JEOL,Japan | JSM-IT800 | |
| Talos L120C TEM | Thermo Fisher, USA | null | |
| TMB color solution | TIAN GEN, China | PA107-01 | |
| Turtle kits | Xiamen Bioendo Technology Co.,LTD | ES80545 | |
| Tween-20 | Macklin, China | 9005-64-5 |

