Delivering Effector T Lymphocytes into Mice Carrying Human Brain Tumors
Abstract
Source: Jarry, U., et al. Stereotactic Adoptive Transfer of Cytotoxic Immune Cells in Murine Models of Orthotopic Human Glioblastoma Multiforme Xenografts. J. Vis. Exp. (2018).
This video demonstrates a stereotaxic injection technique for administering human effector T lymphocytes into a murine model carrying a human brain tumor. Upon positioning a tumor-bearing anesthetized mouse on a stereotactic frame and creating a burr hole at the injection location on the skull, effector T lymphocytes are injected at the tumor site.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines. All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Non-specific Expansion of Cytotoxic Effector T Lymphocytes
- Prepare and irradiate feeder cells at 35 Gy. For the stimulation of 2 x 105 – 4 x 105 effector cells, prepare 10 x 106 peripheral-blood mononuclear cells (PBMCs) and 1 x 106 B-lymphoblastoid cell lines (BLCLs) from three distinct, healthy donors.
- Resuspend both feeder cells and effector cells in 15 mL of Roswell Park Memorial Institute medium (RPMI) supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM L-glutamine, penicillin (100 IU/mL), and streptomycin (0.1 µg/mL) and 300 IU/mL recombinant interleukin-2 (IL-2).
- Add phytohemagglutinin (PHA-L) at a final concentration of 1 µg/mL, carefully mix, and distribute 150 µL of the cell suspension per well in 96-well U-bottomed plates.
- Incubate at 37 °C and with 5% CO2 in a humidified atmosphere.
- Daily check the plate until large cell clumps form in the culture wells (~day 7).
- Transfer the cells into a culture flask at 1 x 106 cells/mL in fresh culture medium.
- Determine the total cell number by counting (2x a week) and maintain them at 1 x 106 cells/mL in fresh culture medium.
NOTE: Effector immune cells should be ready for the therapeutic administration at a resting state (usually 3 weeks after initial amplification stimulus). The purity and the reactivity of these effector cells should be checked prior to in vivo injections (e.g., with in vitro assays).
2. Pre-operative Effector Cells Preparation
- After checking the effector cell count, collect the effector cells in a 50-mL tube by centrifugation (300 x g for 5 min).
NOTE: To compensate for any loss, prepare an excess of cells (e.g., 50 x 106). - Carefully remove the supernatant and resuspend the cells in 15 mL of sterile PBS and centrifuge for 5 min at 300 x g to perform the wash.
- Carefully and completely remove the supernatant and then resuspend the cell pellet in 1 mL of sterile PBS.
- Transfer the resuspended cells in a 1.5-mL microtube for centrifugation at 300 x g for 5 min.
- Carefully and completely remove the supernatant by pipetting slowly.
- Resuspend the cell pellet in 8 µL of sterile PBS per mouse.
NOTE: This is a critical step. - Measure the volume of the cell suspension by using a micropipette. If necessary, add sterile PBS (20 x 106 cells in 15 µL of PBS per dose) and mix carefully.
- Check, by using a micropipette, that the final volume per mouse is between 15 and 20 μL (imperatively < 20 µL).
- Keep the cells on ice until stereotactic injection.
NOTE: More than 3-h timepoints were not tested.
3. Stereotactic Injection
- Equipment set-up
- Assemble and calibrate a small animal stereotactic frame according to the manufacturer's instructions to ensure the accuracy of intracranial injections (e.g., syringe size, desired volume, and rate of injection).
NOTE: A slow infusion rate is recommended (i.e., 2 – 3 µL/min). - Install the material under a microbial safety cabinet (MSC) to maintain the sterility of the instruments and to protect the mice from infections.
NOTE: Place" isothermal blocs" in a water bath at 37 °C. This system limits the hypothermia of mice during the surgery. Heating pads, which are necessary for post-procedural care, must be used during the continuous temperature monitoring.
- Assemble and calibrate a small animal stereotactic frame according to the manufacturer's instructions to ensure the accuracy of intracranial injections (e.g., syringe size, desired volume, and rate of injection).
- Pre-operative animal preparation
- Anesthetize a mouse with an intraperitoneal injection of ketamine (10 mg/mL) and xylazine (0.1 mg/mL) at 10 µL/g of body weight of the mouse.
- Perform a toe pinch test to ensure the complete anesthesia and analgesia of the animal.
NOTE: Any movement is an indication of non-deep analgesia and, if that occurs, a few more minutes are required before repeating the operation. - Once the mouse is properly anesthetized, use scissors to remove the fur from the surgical site (between the two ears, up to the nose).
- Pre-operative cell preparation
- Carefully resuspend the cells with a pipette (several times) prior to each injection to prevent any cell clumping.
- Carefully draw the required cell suspension volume (15 – 20 µL) into the 22-G microsyringe to avoid the aspiration of bubbles.
NOTE: This cell-loading step into the microsyringe is important to minimize variances in injected volumes. Reload cells for each individual injection between procedures to prevent any cell clumping and to ensure an even number of effector cells administration in the cohort. - Then, place the syringe into the adapted syringe pump.
- Procedural care
- Disinfect the surgical site with swabs soaked in povidone-iodine 5% solution at least 3x.
- Place a lubricating ophthalmic ointment in the mouse's eyes to prevent any drying of the corneas.
- Position the anesthetized mouse on the stereotactic frame, on a warm isothermal block covered with a sterile plastic wrap to maintain the mouse's temperature during surgery and limit the mortality.
NOTE: The mouse's nose and teeth should be appropriately positioned above the tooth bar, to ensure adequate respiratory flow during the procedure. - Once the mouse is positioned above the tooth bar, tighten the ear bars firmly under the mouse's ears to immobilize the head.
NOTE: Be careful to not damage the eardrums or to compromise the respiration. - Make a 1 – 2 cm midline sagittal skin incision with sterile scissors along the upper part of the cranium from anterior to posterior to expose the skull.
- Identify the intersection of the sagittal and coronal sutures (Bregma) to serve as landmarks for stereotactic localization prior to the injection (shown in Figure 1).
- Place the microsyringe above this point.
- Move the microsyringe 2 mm right lateral and 0.5 mm anterior of the Bregma.
- Using a microdrill, make a small hole in the skull with a sterile drill bit at predetermined coordinates. Be careful to remain superficial in order to avoid any traumatic injury of the brain.
NOTE: In this protocol, immune cells were injected within an established tumor (one week after tumor cell injection). The skin should be reopened (scar) and the injection is performed at the same coordinates used for the tumor cell implantation (the hole is generally still present up to 2 weeks after the injection). Coordinates were selected for injecting tumor and effector cells in the brain parenchyma.
- Injection of immune effector cells
- Carefully insert the microsyringe into the drilled hole and, moving slowly, forward the needle 3 mm down in the dura and then backward 0.5 mm to a final depth of 2.5 mm prior to injecting the effector cells.
- Run the effector cell injection at 2 – 3 µL/min and monitor the mice all along the injection time.
- Once the injection is complete, withdraw the needle for only 1 mm and keep the microsyringe in place for one additional minute before slowly withdrawing completely the microsyringe, to prevent any leakage from the infusion site.
NOTE: Following the removal of the animal from the stereotactic device, immediately clean the injection equipment for upcoming experiments.
- Post-operative care and follow-up of mouse
- Remove the animal from the stereotactic frame and immediately apply povidone-iodine 5% solution on the incision site and close the skin with an appropriate surgical suture.
- Apply 2% lidocaine gel directly on the wound and administer 0.15 µg/g of buprenorphine by a subcutaneous injection for post-procedural analgesia.
- Place back the anesthetized mouse to its cage above a heating pad set to 37 °C to maintain an appropriate mouse body temperature and to avoid any hypothermia.
NOTE: Separate housing is not necessary. - Monitor the mouse until it is fully recovered from anesthesia and transfer it to a housing room.
NOTE: To date, this protocol is well supported as few unforeseen complications have occurred (< 5% of injected mice). - Daily monitor the mice and euthanize them when any declining health signs are observed (e.g., hunched posture, reduced mobility, prostration, or significant body weight loss [≥ 15%]).
Representative Results
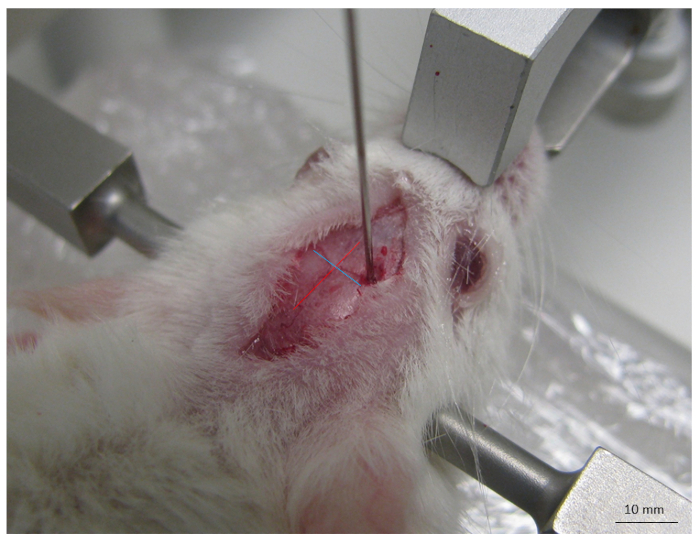
Figure 1: Picture of the main anatomical landmarks on the mouse skull. This includes sagittal (red line) and coronal (blue line) sutures and their intersection (Bregma) used to orient the site of injection. The scale bar is indicated.
Divulgaciones
The authors have nothing to disclose.
Materials
| PBMCs | from 3 different healthy donors | ||
| BLCLs | from 3 different donors | ||
| Roswell Park Memorial Institute medium (RPMI) | Gibco | 31870-025 | |
| FCS | Dutscher | S1810-500 | |
| L-glutamine | Gibco | 25030-024 | |
| penicillin/streptomycin | Gibco | 15140-122 | |
| IL-2 | Novartis | proleukin | |
| PHA-L | Sigma | L4144 | |
| Stereotaxic frame | Stoelting Co. | 51600 | |
| Mouse adaptator for stereotaxic frame | Stoelting Co. | 51624 | |
| microsyringe pump injector | WPI | UMP3-4 | |
| NanoFil Syringe | WPI | NF34BV-2 | |
| NSG mice | Charles River | NSGSSFE07S | |
| Ketamine | Merial | Imalgène 1000 | |
| Xylazine | Bayer | Rompur 2% | |
| Scissors | WPI | 201758 | |
| Forceps | WPI | 501215 | |
| OmniDrill 115/230V | WPI | 503598 | |
| Vicryl 4-0 | Ethicon | VCP397H | |
| Xylocaine | Astrazeneca | 3634461 |
Tags

.
