Abstract
Source: Pylaeva, E., et al. Transfer of Manipulated Tumor-associated Neutrophils into Tumor-Bearing Mice to Study their Angiogenic Potential In Vivo. J. Vis. Exp. (2019).
This video demonstrates an assay to generate anti-angiogenic tumor-associated neutrophils (TANs). Upon taking a murine melanoma tumor containing pro-angiogenic TANs, the single cells are isolated using fluorescence-activated cell sorting (FACS) and treated with an inhibitor of nicotinamide phosphoribosyl transferase (NAMPT) to reduce the capacity to stimulate angiogenesis.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
NOTE: The overall scheme of the protocol is shown in the Figure 1.
1. Preparation of B16F10 melanoma cell line
- Prepare mycoplasma-negative cells grown to a 90% confluent monolayer (approximately 10 x 106 cells/T75 flask) in complete Iscove's Modified Dulbecco's Medium (IMDMc: IMDM + 10% Fetal Bovine Serum (FBS) + 1% penicillin-streptomycin).
- Remove the medium, and rinse the cells with phosphate buffered saline (PBS). Apply 6 mL of a cell detachment solution containing proteolytic and collagenolytic enzymes (see the Table of Materials), and incubate at 37 °C for 2 min.
- Knock the flask gently to mobilize remaining adherent cells from the bottom. Collect the cell suspension in 15 mL tubes and centrifuge at 300 x g for 7 min and 20 °C.
- Remove the supernatant, and resuspend the pellet well in 1 mL of PBS. Add 14 mL of PBS and centrifuge (300 x g and 20 °C for 7 min).
- Remove the supernatant and resuspend the pellet in 1 mL of PBS.
- Count the cells, and resuspend them to the concentration of 3 x 106/mL PBS (for the step 2) or 6 x 106/mL PBS (for the step 6) for injection. Keep cells on ice for a maximum of 30 min.
2. Allogenic tumor model in mice
- Use 10 female Ifnar1-/- mice 8-12 weeks old that are kept under specific-pathogen-free (SPF) conditions.
NOTE: Female mice are preferable in a subcutaneous model of tumor growth, since males are more aggressive and thus prone to infractions of the tumor site, which influences tumor growth. - Shave the skin of the mouse on the flank with an electrical shaver, and disinfect the skin with tissue wet with 70% ethanol.
- Collect the prepared B16F10 melanoma cells at a concentration of 3 x 106/mL PBS (see the step 1) in a 1 mL syringe and 0.4 x 19 mm needle. Inject 100 μL of the suspension subcutaneously.
- Mix the cells well before every injection. Use needles not less than 0.4 mm in diameter as to not disturb tumor cells.
- Place up to 5 mice in one cage, and control tumor size (length, width and depth) with a caliper for 14 days.
NOTE: According to the animal regulations, the tumor size should not exceed 15 mm in diameter, mice with bigger or necrotic/open tumors should be sacrificed beforehand. - At day 14, sacrifice the mice in the CO2 chamber.
- Disinfect the skin with 70% ethanol and remove tumors with scissors and forceps in a sterile Petri dish. Keep tumors in a 50 mL tube in complete Dulbecco's Modified Eagle Medium (DMEMc: DMEM + 10% FBS + 1% penicillin-streptomycin) on ice.
3. TAN isolation
- Place tumors into sterile 6-well plates, 5 tumors per well. Cut tumors into 2-3 mm pieces with sterile scissors.
- Digest with 1 mL of dispase/collagenase D/DNase I solution (0.2 mg/0.2 mg/100 mg in 1 mL of DMEMc) per tumor. Incubate at 37 °C, 5% CO2 in a humid incubator, and mix with a 10 mL syringe without a needle every 15 min 3 times.
- To remove undigested fibers, mesh cells through 100 µm filters into 15 mL tubes (one well per filter per tube). Add PBS to 15 mL, centrifuge tubes at 460 x g, 4 °C for 5 min, and remove the supernatant.
- Lyse erythrocytes with a lysis buffer (NH4Cl 150 mM, KHCO3 10 mM, EDTA 0.1 mM, pH 7.3, 20 °C) by adding 1 mL into each tube. Mix well, and combine the solution from all tubes into one. Stop the reaction after 2 minutes with 11 mL of ice-cold (4 °C) DMEMc.
- Centrifuge at 460 x g, 4 °C for 5 min, and remove the supernatant. Resuspend the pellet with 15 mL of cold PBS. Centrifuge at 460 x g, 4 °C for 5 min, and remove the supernatant.
- Resuspend the pellet in 1 mL of PBS. Add 3 μL of Fc-block antibodies (CD16/CD32, stock 0.5 mg/mL), and incubate on ice for 15 min.
- Add antibodies: 10 μL of Ly6G-phycoerythrin (Ly6G-PE, stock 0.2 mg/mL) and 10 μL of CD11b-allophycocyanin (CD11b-APC, stock 0.2 mg/mL). Add 20 μL of 6-Diamidin-2-phenylindol viability dye (DAPI, stock 5 mg/mL) and incubate on ice in darkness for 30 min.
NOTE: Another combination of viability dyes and fluorescent conjugates of antibodies can be used. - Add PBS up to 15 mL, centrifuge at 460 x g, 4 °C for 5 min, and remove the supernatant.
- Resuspend the pellet in DMEMc to the concentration approximately 10 x 106/mL, and keep on ice.
- Sort CD11b+ Ly6Ghi alive (DAPI-negative) neutrophils with a fluorescence-activated cell sorter (gating strategy see Figure 2).
NOTE: Keep the tube with cell suspension and the tube with DMEMc for sorted cells at 4 °C. Use the following optimal sorting settings: a 70 μm nozzle, a threshold rate of maximal 22,000 events/second and a flow rate of 1-3. - Check the purity of the sorted neutrophils using a cytometer for a recommended purity of >95%.
- Centrifuge sorted neutrophils at 460 x g, 4 °C for 5 min, and remove the supernatant. Resuspend the sorted cells in DMEMc to the concentration of 1 x 106/mL.
NOTE: The expected number of neutrophils in one 14-day B16F10 tumor (10 mm diameter) is approximately 3 x 104 cells.
4. NAMPT inhibition in TANs in vitro
- Prepare FK866 (NAMPT inhibitor) stock in dimethylsulfoxide (DMSO) at a final concentration of 100 mM.
- Seed sorted neutrophils (step 3.11) into 2 wells of a 96-well U-bottom plate (1.5 x 105 neutrophils/well). Add FK866 into the intervention well (final concentration of 100 nM), and an equal amount of DMEMc with DMSO into the control well. Incubate for 2 h at 37 °C, 5% CO2 in a humid incubator.
- Centrifuge at 460 x g, 4 °C for 5 min, and remove the supernatant. Resuspend in 200 μL of PBS in each well. Repeat 2 times.
- Centrifuge at 460 x g, 4 °C for 5 min, and remove the supernatant. Resuspend in commercial endothelial cell growth medium (supplemented with 4 µL/mL endothelial cell growth supplement, 0.1 ng/mL recombinant human epidermal growth factor, 1 ng/mL recombinant human basic fibroblast growth factor, 90 µg/mL heparin and 1 µg/mL hydrocortisone) to a final concentration of 0.2 x 106 cells/mL (in 0.75 mL) (for step 5) or in PBS to the final concentration of 0.6 x 106 cells/mL (in 0.25 mL).
Representative Results
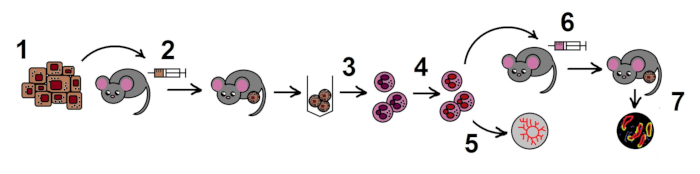
Figure 1. The scheme of the protocol. Step 1. Preparation of B16F10 melanoma cell line; 2. Allogenic tumor model in mice; 3. Isolation of TANs from the tumors; 4. Inhibition of NAMPT in TANs in vitro; 5. Estimation of angiogenic properties of TANs in the aortic ring assay; 6. Adoptive transfer of treated neutrophils in the allogenic tumor model; 7. Tumor growth monitoring, histological examination.
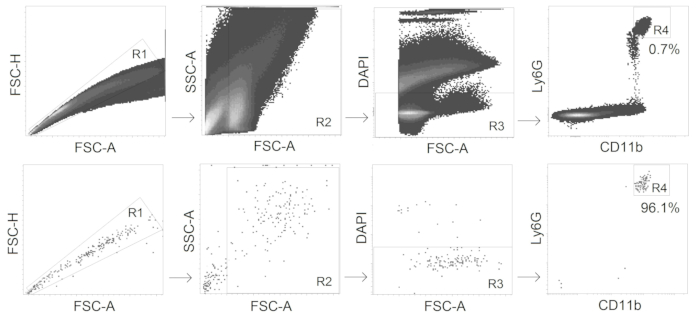
Figure 2. Gating strategy for TANs sorting. CD11b+ Ly6Ghi alive neutrophils are sorted from tumors with the purity ≥95%.
Divulgaciones
The authors have nothing to disclose.
Materials
| 15 ml tubes | Sarstedt AG & Co., Nümbrecht, Germany | 6,25,54,502 | |
| 50 ml tubes | Cellstar, Greiner Bio One International GmbH, Frickenhausen, Germany | 227261 | |
| 5ml / 10ml / 25ml sterile tipps for the automatic pipette | Cellstar, Greiner Bio One International GmbH, Frickenhausen, Germany | 6006180 / 607180 / 760180 | |
| 6 well flat-bottom cell culture plates | Sarstedt AG & Co., Nümbrecht, Germany | 8,33,920 | |
| 96 well flat-bottom cell culture plates | Cellstar, Greiner Bio One International GmbH, Frickenhausen, Germany | 655180 | |
| 96 well U-bottom cell culture plates | Cellstar, Greiner Bio One International GmbH, Frickenhausen, Germany | 65018 | |
| anti-mouse CD11b | BD Pharmigen, Becton Dickinson, Franklin Lakes, U.S. | 553312 | clone M1/70, APC-conjugated, 0.2mg/mL |
| anti-mouse Ly6G | BioLegend, California, U.S. | 127608 | clone 1A8, PE-conjugated, 0.2mg/mL |
| BD FACS AriaII | BD Biosciences, Becton Dickinson, Franklin Lakes, U.S. | cell sorter | |
| Caliper | Vogel Germany, Kevelaer, Germany | ||
| Casy cell counter | Innovatis, Roche Innovatis AG, Bielefeld, Germany | ||
| Cell Trics 50µm / 100 µm sterile filters | Sysmex Partec GmbH, Goerlitz, Germany | 04-004-2327 / 04-004-2328 | |
| Centrifuge Rotina 420 R | Andreas Hettich, Tuttlingen, Germany | 4706 | |
| Collagenase D | Sigma-Aldrich/Merck, Darmstadt, Germany | 11088858001 | |
| DAPI (4',6-Diamidino-2-Phenylindole, Dilactate) | BioLegend, California, U.S. | 422801 | Stock: 5mg/ml |
| Dispase I | Sigma-Aldrich/Merck, Darmstadt, Germany | D4818-2MG | |
| DMEM | Gibco, Life Technologies/Thermo Fisher Scientific, Massachusetts, U.S. | 41966-029 | DMEM complete: DMEM + 10% FBS + 1% penicillin-streptomycin |
| DMSO (Dimethylsufoxide) | WAK-Chemie Medical GmbH, Steinbach, Germany | WAK-DMSO-10 | CryoSure-DMSO |
| DNase I | Sigma-Aldrich/Merck, Darmstadt, Germany | DN25-100MG | |
| DPBS | Gibco, Life Technologies/Thermo Fisher Scientific, Massachusetts, U.S. | 14190-094 | |
| Endothelial cell growth medium | PromoCell, Heidelberg, Germany | c-22010 | |
| FBS (Fetal Bovine Serum) | Biochrom, Berlin, Germany | S0115 | |
| Fc-block (Anti-mouse CD16/32) | BD Pharmingen, Becton Dickinson,Becton Dickinson, Franklin Lakes, U.S. | 553142 | clone 2.4G2, Stock: 0.5mg/mL |
| FK 866 hydrochloride | Axon Medchem, Groningen, Netherlands | Axon 1546 | Stock: 100 mM |
| Goat Anti-Rabbit IgG H&L | Abcam, Cambridge, U.K. | ab97075 | Cy3-conjugated, Stock: 0.5 mg/mL |
| Heracell 240i CO2 Incubator | Thermo Fisher Scientific, Waltham, U.S. | 51026334 | |
| IMDM | Gibco, Life Technologies/Thermo Fisher Scientific, Massachusetts, U.S. | 12440-053 | IMDM complete: IMDM + 10% FBS + 1% penicillin-streptomycin |
| Isis GT420 shaver | B. Braun Asculap, Suhl, Germany | 90200714 | |
| Matrigel Matrix basement membrane | Corning Life Sciences, Amsterdam, Netherlands | 7205011 | |
| Monoclonal Anti-Actin, α-Smooth Muscle | Sigma-Aldrich/Merck, Darmstadt, Germany | F3777 | FITC-conjugated, no information about stock concentration |
| Needles 0.4 mm x 16 mm | BD Microlance, Becton Dicson, Becton Dickinson, Franklin Lakes, U.S. | 302200 | |
| Neomount | Merck, Darmstadt, Germany | HX67590916 | |
| Normal goat serum | Jackson ImmunoResearch Laboratories, West Grove, U.S. | 005-000-121 | |
| Penicillin Streptomycin | Gibco, Life Technologies/Thermo Fisher Scientific, Massachusetts, U.S. | 15140-122 | |
| Pipetus automatic pipette | Hirschmann Laborgeräte, Eberstadt, Germany | 9907200 | |
| ProLong Gold Antifade Mountant with DAPI | Invitrogen, Thermo Fisher Scientific, Massachusetts, U.S. | P36935 | |
| rabbit anti mouse Laminin gamma 1 chain | Immundiagnostik, Bensheim, Germany | AP1001.1 | No information about stock concentration |
| StemPro Accutase | Gibco, Life Technologies/Thermo Fisher Scientific, Massachusetts, U.S. | A1105-01 | |
| Sterile disposal scalpel (no. 15) | MedWare, Naples, U.S. | 120920 | |
| Syringes 1 ml | BD Plastipak, Becton Dickinson, Franklin Lakes, U.S. | 303172 | |
| Syringes 10 ml | BD Discardit II, Becton Dickinson, Franklin Lakes, U.S. | 309110 | |
| T75 sterile cell culture flasks | Sarstedt AG & Co., Nümbrecht, Germany | 83,39,11,302 |
Tags

.
