Method for Novel Anti-Cancer Drug Development using Tumor Explants of Surgical Specimens
Summary
Here, we established a method for drug efficacy testing with surgical specimens of brain tumors, termed “tumor explant method”. With this method, we can evaluate drug efficacy without breaking the microenvironment of solid tumors. To validate reliability of this method, we describe representative data with our glioma specimen treated with the current first-line chemotherapeutic agent, temozolomide.
Abstract
The current therapies for malignant glioma have only palliative effect. For therapeutic development, one hurdle is the discrepancy of efficacy determined by current drug efficacy tests and the efficacy on patients. Thus, novel and reliable methods for evaluating drug efficacy are warranted in pre-clinical phase. In vitro culture of tumor tissues, including cell lines, has substantial phenotypic, genetic, and epigenetic alterations of cancer cells caused by artificial environment of cell culture, which may not reflect the biology of original tumors in situ. Xenograft models with the immunodeficient mice also have limitations, i.e., the lack of immune system and interspecies genetic and epigenetic discrepancies in microenvironment. Here, we demonstrate a novel method using the surgical specimens of malignant glioma as undissociated tumor blocks to evaluate treatment effects. To validate this method, data with the current first-line chemotherapeutic agent, temozolomide (TMZ), are described.
We used the freshly-removed surgical specimen of malignant glioma for our experiments. We performed intratumoral injection of TMZ or other drug candidates, followed by incubation and analysis on surgical specimens. Here, we sought to establish a tumor tissue explant method as a platform to determine the efficacy of novel anti-cancer therapies so that we may be able to overcome, at least, some of the current limitations and fill the existing gap between the current experimental data and the efficacy on an actual patient’s tumor. This method may have the potential to accelerate identifying novel chemotherapeutic agents for solid cancer treatment.
Protocol
1. Preparation of media
- The media was prepared using 10% FBS (16140-071- GIBCO) in DMEM/F-12 (1X), Liquid 1:1 (10565-042- Invitrogen) with 0.6 % Agar solution Purified, Granulated (1.01614.1000- EMD). Sterile conditions were maintained throughout the experiment.
- For the preparation of media 5mL of FBS (16140-071- GIBCO) was added to 45mL of DMEM/F-12 (1X), Liquid 1:1 (10565-042- Invitrogen) making final volume of 50mL. The prepared media was kept in water bath at 37°C temperature.
- The agar solution was prepared using heat microwave method. 0.3 grams granulated agar was dissolved in 50mL distilled water using microwave. The agar solution was cool down to 37°C temperature by keeping it into the water bath.
- We used equal volume of media from step 1.2 and 1.3 and prepared stock solution. The ratio of 0.6 % agar (0.5mL) and 10% FBS-D-MEM/F-12 media (0.5mL) was 1:1, making the total volume of 1 mL in each well of 6 well plates.
2. Tissue processing
- Glioblastoma Multiforme (GBM) tissues were received immediately after the surgery from Department of Pathology. The tissue was classified as GBM from Department of Pathology.
- The tissue was transferred to patri-plate using forceps carefully and washed with ice-cold 5mL 1XPBS for 3 times.
- Serial sections of the specimens were cut from the dissected samples to create tumor blocks (approximate 10 mm in diameter) with a surgical blade (Feather-2976#10) and forcep (fisher scientific).
- Blocks were transferred into a 6 well plate. Each well was containing 1mL of media mention in step 1.4. The tissue had enough exposure to air to keep tissue viable.
- Tumor blocks were then injected with either DMSO (5%) or TMZ (2.5 nM) and incubated for 16 hours at 37 °C in humidified air containing 5% CO2. 0.1mL of DMSO (5%) or TMZ was injected 3 times in tumor blocks at different places for consistent treatment. The drug was injected using Insulin Syringe 1mL 0.37X12.7mm 28G1/2 (Comfort point-26027).
- After 16 hours blocks were washed with 5 mL 1XPBS for 3 times. After washing the blocks were fixed with 10mL of 10 % v/v formalin for 24 hours (Ricca chemical company) in 50 mL tubes (Basix-5539802) and processed for paraffin embedded 4μm sections.
- The formalin fixed section was placed in beaker on the stir plate with “NO HEAT” and processed through the following solutions for 30 minutes in each solution. 30% ethanol, 50% ethanol, 75% ethanol, 80% ethanol, 95% ethanol, 100% ethanol, and Xylene.
- The tissue was blotted on paper towels and placed in melted paraffin (58 to 60 °C, Fisher Paraplast paraffin) for 30 minutes.
- The tissue was embedded in molds using Surgipath Blue Ribbon paraffin, embedded tissue was cooled to room temperature.
- Paraffin embedded 4μm tissue sections were taken and placed on Fisher Plus slides.
3. Immunohistochemistry
Immunohistochemistry was performed as described previously. 1
- Deparaffinization and rehydration Place the slides in a rack and perform following steps. Xylene for 3×5 minutes (3 times for 5 minutes), 100% ethanol for 3×5 minutes, 95% ethanol for 1×5 minutes, 70% ethanol for 1×5 minutes, rinse in running tap water for 3 minutes.
- Antigen retrieval with heat induced epitope retrieval Immerse slides in 1X citrate buffer (Thermo scientific-AP9003-500) diluted in distilled water in a glass beaker. Boil for 15 minutes on a hot plate (make sure sections don”t fall off the slide). Cool the solution for 30 minutes at room temperature. Rinse the slides with 1X PBS for 3×5 minutes.
- Inhibition of internal peroxide Immerse the slides in methanol (Fisher Scientific-A-452-4) with 0.3% hydrogen peroxide (Fisher Scientific- BP2633-500- diluted in distilled water) in a glass beaker for 15 minutes. Rinse in 1X PBS for 3×5 minutes.
- Blocking Cover the tissues with 10% normal goat serum. Transfer the slides in a humid box and incubate for 1 hour at room temperature. Wash the slides with 1X PBS for 3×5 minutes.
- Primary antibody Sections were incubated overnight at 4°C with primary antibody at following concentrations: human specific Caspase-3 (1:1,000, R&D systems, AF835), Ki67 (1:1, Dako, 15626) diluted with 1X PBS. Rinse the slides in 1X PBS 3×10 minutes on the following day.
- Secondary antibody reaction Cover the tissues with 2-3 drops of HRP labeled secondary antibody (envision systems). Put slides in a humid box and incubate for 1 hour at room temperature. Wash with 1X PBS for 3×10 minutes.
- Detection Develop for the chromogen DAB kit (Vector laboratories-SK-4100) for detection of primary antibody following the manufacturer’s protocol.
- Counter staining Immerse the slides with hematoxylin (10-15 seconds, make sure don”t overrun DAB signal). Rinse with running tap water for 3 minutes.
- Dehydration Immerse the slides in following order, 70% ethanol for 5 minutes, 95% ethanol for 5 minutes, 100 % ethanol for 3×5 minutes, Xylene for 3×5 minutes.
- Cover slip the slides with permount mounting reagent (Fisher Scientific-SP-15-100).
- Images were taken using Olympus fluorescence microscope (DP-72).3.11). In all experiments, specific labeling was confirmed by the negative control without primary antibody.
4. Representative Results:
We collected surgical specimens of glioblastoma multiforme (GBM). The patients underwent the first surgery without prior chemotherapies including temozolomide (TMZ). The T1-weighted image of MRI with Gadolinium enhancement in Figure-1a demonstrated an enhanced tumor in the right frontal lobe before surgery. The postoperative image (Figure-1b) confirmed subtotal removal of the lesion after surgery. The surgical tissues were sent to the Department of Pathology for the clinical diagnosis purpose, and the remaining specimens were processed to the tissue procurement under the approved Institutional Review Board (IRB) protocol at the Ohio State University Medical Center. Hematoxylin and Eosin (H&E) staining demonstrated the presence of necrosis, pseudopallisading cells, and microvascular proliferation (Figure 1-c). Thus, these tumors were histopathologically diagnosed as GBM. Of note, tumor explants following incubation without TMZ up to 48 hours maintained the cytoarchitecture of GBM .In contrast, with incubation for 72 hours or longer, we noticed the increased number of condensed nuclei in the specimens, suggesting some of the tumor cells starting to undergo apoptosis. As a result, tumor tissues following longer incubation contained patchy regions that lost tumor cells, which were preferentially observed at and around necrotic areas (data not shown).
In order to investigate if these tumor explant samples can reliably be utilized for the purpose of drug efficacy test, we tested the effect of TMZ treatment. Figure-2 represents the flow of the experiments in this study. A recent study indicated that intratumoral injection of TMZ has more potent effect on growth control of malignant glioma than that of the systemic administration of this drug 2. Following incubation for 16 hours, these explants were embedded with paraffin and serial sections were prepared for staining. Immunohistochemistry of TMZ-treated GBM tissues demonstrated a substantial reduction of Ki-67-positive tumor cells in comparison with the control samples. In turn, immunohistochemistry with an apoptosis marker, Caspase-3, did not yield any significant difference between TMZ-treated glioma specimens and the control samples (Figure-3).
Treatment effect with TMZ was further characterized by combining this explant assay together with either in vitro culture or flow cytometry. Specifically, we sought to determine the effect on stem cell-like tumor cells (SCLTC). Therefore, following intratumoral treatment with TMZ, we performed sphere forming assay and flow cytometry of these tumor explants. The TMZ-treated specimens were dissociated into individual single cells and these single cells were seeded in a low density (1 cell per microliter or lower) in 96-well plates in serum-free medium supplemented with bFGF and EGF. In the control group, formation of tumor spheres from the tumor explants was observed after 7 day incubation. Given that sphere formation is a property of SCLTC3 4, alterations in the number of tumor spheres by a drug treatment indicates the effect on SCLTC. Similarly, flow cytometry of the dissociated tumor explants with a cell surface marker, CD133, was carried out to verify the effect on SCLTC. If SCLTC in heterogeneous tumor cells in GBM are selectively eradicated by a drug treatment, one might predict that flow cytometry should detect decrease of the CD133-positive fraction by treatment (data not shown).
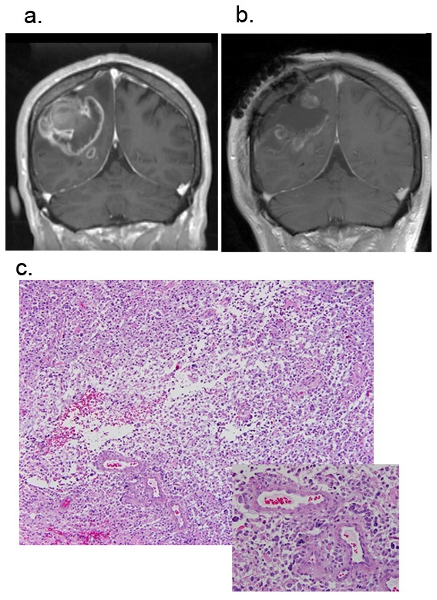
Figure.1 Magnetic resonance images (MRI) of a patient GBM. Representative figures of pre-operation Figure-1a and post-operation Figure-1b. Figure-1c is H&E staining of fresh GBM tissue sample. Original magnification, 40X.
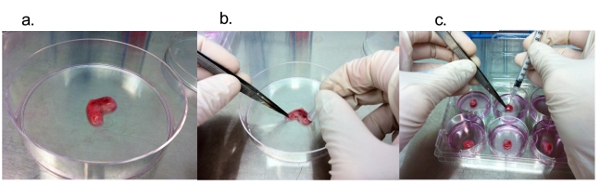
Figure. 2 The experimental flow of block culture experiment. Figure-2a. Image of fresh surgical GBM received from department of pathology. Figure-2b shows Dissection of tumor block into 10mm small pieces with the help surgical blade. Figure-2c is the drug (TMZ) treatment of tumor blocks using insulin syringe.
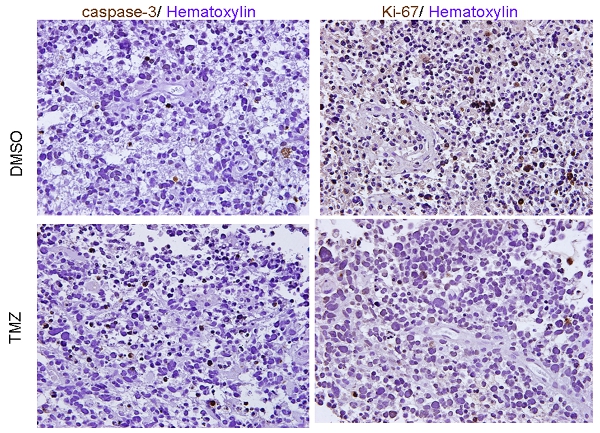
Figure. 3 Immunohistochemistry. Immunohistochemistry of GBM tissue blocks injected with DMSO or TMZ with activated Caspase-3 and Ki67. Hematoxylin was used for nuclear staining. Original magnification, 40X.
Discussion
There is a gap between the drug efficacy in the current pre-clinical experimental models and the efficacy in patients. More specifically, in the brain tumor research field, novel and reliable methods are required that would help filling the existing gap between the drug evaluation with the current methods and the efficacy in patients. The described assay in this study is an additional asset, if not a solution, to facilitate filling the discrepancy of the experimental data and outcome of affected patients.
In vitro cell cultures preferentially expand certain tumor cell types regardless of the culture conditions, and represent only a subpopulation of the entire tumor. 5 In addition, genetic and phenotypic transformation of the artificial cell expansion occurs and is inevitable with the long-term cell cultures. One of the major hurdles to treat malignant glioma is the heterogeneity of tumor cells, both within a single tumor and between tumor samples 6, 7. Therefore, selective enrichment of a certain population of tumor cells, regardless of one type or another, may not be an appropriate means to determine efficacy of any chemotherapeutic agents on heterogeneous tumor cells. 8 9 Any animal model of brain tumors derived from a subpopulation of tumor cells shed lights on drug efficacy on a subpopulation, but not the entire tumor.
Several limitations, on the other hand, still remain in this tumor explant method. One such limitation is the relatively short period of treatment. Since malignant tumor cells are considered to acquire resistance to most, if not all, therapies over time, initial control of the short-term tumor cell growth may not be reflected by the long-term patient prognosis, as was recently reported with an anti-angiogenic agent, bevacizumab 10. Thus, improvement of the protocol for this explant assay to preserve tissues for a longer period is required with further investigations. Another question is a specific effect of a tested drug on SCLTC in the tumor. Given that SCLTC are relatively resistant to the current therapies for malignant glioma, identification of novel anti-cancer drugs that potently eradicate SCLTC is warranted. In this regard, we currently seek to combine this explant assay with the subsequent in vitro experiments, such as neurosphere forming assay (data not shown). We expect to learn more about the effects, if any, of a drug candidate on SCLTC through these combined assays. Lastly, using small blocks of tumor samples may not reflect the entire tumor cell populations, as is the case with the conventional tumor cell lines or primary cultures from surgical specimens. These issues aside, preserving tumor stroma, including the vascular niche, is helpful in characterizing the environmental factors for tumor cells. Therefore, this assay may have potential to evaluate any therapeutic strategies under a more physiologically relevant condition.
Here, we established an assay for drug efficacy testing with explants of surgical specimens of GBM. In fact, with the tested surgical specimens, we found that a direct injection of TMZ reduces proliferation in the GBM samples. This tissue explant method is theoretically applicable to other solid cancers and provides asset to determine drug efficacy on a patient’s tumor, which will hopefully help us avoiding another failure in clinical trials for cancers.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
The American Cancer Society (MRSG-08-108-01), Vincent J. Sgro/The American Brain Tumor Association, and the Khan Family Foundation for I Nakano.
Materials
| Name of the reagent | Company | Catalogue number |
| Fetal Bovine Serum, Qualified, Heat-Inactivated | GIBCO | 16140-071 |
| D-MEM/F-12 (1X) Liquid 1:1 | Invitrogen | 10565-042 |
| Agar-Agar Purified, Granulated | EMD | 1.01614.1000 |
| DPBS for staining | Invitrogen | 14190 |
| Hematoxyllin | Richalrd allan sci | 7211 |
| 10% w/v formalin Caspase-3 | Ricca chemical company | 3810 |
| Caspase-3 | R&D systems | AF835 |
| Ki67 | Dako | 15626 |
| DMSO | Sigma | D2650 |
| Envision system- anti-mouse | Dako | 2012-07 |
| Envision system- anti-rabbit | Dako | 2011-12 |
| DAB-kit | Vector Lab. | SK-4100 |
Table 1. Materials.
| Name of the equipment | Company | Catalogue number |
| 6 Well Plate | Greiner-bio one | 657160 |
| Petri dish | VWR | 25384-302 |
| Serological pipette | Axygen | |
| Filter tips | USA scientific | |
| 500 μm polyester mesh Netwell insert | Costar | 3480 |
| Matrigel Matrix | BD Biosciences | 354230 |
| BD 10 mL syringe | BD biosciences | 309604 |
| Hypodermic needle Aluminum Hub. | Tyco Health Care | 2000029 |
| Tissue culture flask | Corning | |
| Centrifuge tubes | Basix | 5539800 |
| Thin wall tubes | Eton | 1107A |
| Surgical Blade stainless steel | Feather | 2976#10 |
| Insulin Syringe | Comfort point | 26027 |
| 50mL flat top screw cap tube | Basix | 5539802 |
| Dissection microscope | Tritech | |
| Fluorescence microscope | Olympus | DP-72 |
| Forcep | Fisher Sci |
Table 2. Equipment.
Referencias
- Hemmati, H. D. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 100, 15178-15183 (2003).
- Heimberger, A. B. Temozolomide delivered by intracerebral microinfusion is safe and efficacious against malignant gliomas in rats. Clin Cancer Res. 6, 4148-4153 (2000).
- Nakano, I. Maternal embryonic leucine zipper kinase is a key regulator of the proliferation of malignant brain tumors, including brain tumor stem cells. J Neurosci Res. 86, 48-60 (2008).
- Laks, D. R. Neurosphere formation is an independent predictor of clinical outcome in malignant glioma. Stem Cells. 27, 980-987 (2009).
- Phillips, H. S. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 9, 157-173 (2006).
- He, J. Glycoproteomic Analysis of Glioblastoma Stem Cell Differentiation. J Proteome Res. , (2010).
- Ma, Y. H., Xu, Q. S., Zheng, J. S., Zhou, Y. Q., Zhan, R. Y. Expression of stem cell markers and proliferative labeling on glioma cell lines]. Zhonghua Bing Li Xue Za Zhi. 37, 333-334 (2008).
- Vik-Mo, E. O. Brain tumor stem cells maintain overall phenotype and tumorigenicity after in vitro culturing in serum-free conditions. Neuro Oncol. 12, 1220-1230 (2010).
- Hovinga, K. E. Inhibition of notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells. 28, 1019-1029 (2010).
- de Groot, J. F. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 12, 233-242 (2010).

