Multiparametric Optical Mapping of the Langendorff-perfused Rabbit Heart
Summary
This article describes the basic procedures for conducting optical mapping experiments in the Langendorff-perfused rabbit heart using the panoramic imaging system, and the dual (voltage and calcium) imaging modality.
Abstract
Optical imaging and fluorescent probes have significantly advanced research methodology in the field of cardiac electrophysiology in ways that could not have been accomplished by other approaches1. With the use of the calcium- and voltage-sensitive dyes, optical mapping allows measurement of transmembrane action potentials and calcium transients with high spatial resolution without the physical contact with the tissue. This makes measurements of the cardiac electrical activity possible under many conditions where the use of electrodes is inconvenient or impossible1. For example, optical recordings provide accurate morphological changes of membrane potential during and immediately after stimulation and defibrillation, while conventional electrode techniques suffer from stimulus-induced artifacts during and after stimuli due to electrode polarization1.
The Langendorff-perfused rabbit heart is one of the most studied models of human heart physiology and pathophysiology. Many types of arrhythmias observed clinically could be recapitulated in the rabbit heart model. It was shown that wave patterns in the rabbit heart during ventricular arrhythmias, determined by effective size of the heart and the wavelength of reentry, are very similar to that in the human heart2. It was also shown that critical aspects of excitation-contraction (EC) coupling in rabbit myocardium, such as the relative contribution of sarcoplasmic reticulum (SR), is very similar to human EC coupling3. Here we present the basic procedures of optical mapping experiments in Langendorff-perfused rabbit hearts, including the Langendorff perfusion system setup, the optical mapping systems setup, the isolation and cannulation of the heart, perfusion and dye-staining of the heart, excitation-contraction uncoupling, and collection of optical signals. These methods could be also applied to the heart from species other than rabbit with adjustments to flow rates, optics, solutions, etc.
Two optical mapping systems are described. The panoramic mapping system is used to map the entire epicardium of the rabbit heart4-7. This system provides a global view of the evolution of reentrant circuits during arrhythmogenesis and defibrillation, and has been used to study the mechanisms of arrhythmias and antiarrhythmia therapy8,9. The dual mapping system is used to map the action potential (AP) and calcium transient (CaT) simultaneously from the same field of view10-13. This approach has enhanced our understanding of the important role of calcium in the electrical alternans and the induction of arrhythmia14-16.
Protocol
1. Preparation
- Prepare freshly made Tyrodes’ solution (in mM, 128.2 NaCl, 1.3 CaCl2, 4.7 KCl, 1.19 NaH2PO4, 1.05 MgCl2, 20.0 NaHCO3, and 11.1 glucose). To expedite the daily preparation of solutions, prepare two stock solutions in advance and store them at +4°C refrigerator: (1) Stock I (in g/2L, 374.6 NaCl, 9.56 CaCl2, 17.52 KCl, 8.21 NaH2PO4, 10.67 MgCl2) and (2) Stock II (in g/2L, 84.01 NaHCO3). To make 2L of Tyrodes’ solution sufficient for one experiment take 1840mL of deionized water and mix in it 80mL of Stock I, 80mL of Stock II, and 4g of glucose.
- Prepare stock solutions of dyes and uncouplers: (1) excitation-contraction uncoupler blebbistatin stock solution (Tocris Bioscience, 2mg/mL solution in DMSO); (2) voltage-sensitive dye di-4-ANEPPS stock solution (Invitrogen, 1mg/mL solution in DMSO); (3) voltage-sensitive dye RH 237 stock solution (Invitrogen, 1.25mg/ml solution in DMSO); (4) calcium indicator Rhod-2AM stock solution (Invitrogen, 1mg/ml solution in DMSO). A single rabbit experiment requires approximately 30 μL of the di-4-ANEPPS stock solution, 30 μL of the RH237 stock solution, and 200 μL of the Rhod-2AM stock solution. To avoid repeated freezing and thawing, we store 100 μL aliquots of di-4-ANEPPS at -20°C. Other dyes are also stored at -20°C. Store the dissolved blebbistatin in +4°C refrigerator.
- Prior to harvesting the heart, transfer Tyrodes’ solution into the 2L bottle and place it into a water bath (Fisher Scientific) which maintains the solution temperature at 37 °C. The solution is oxygenated with 95% O2 – 5% CO2. The pH is maintained at 7.35±0.05 by adjusting the oxygenation level. The solution is circulated in the Langendorff perfusion system and is filtered by a nylon net filter (pore size: 11μm, Millipore) placed in the perfusion line before the cannula.
- Prepare monitoring equipment prior to harvesting the heart. A pressure transducer (WPI) is used to monitor the aortic pressure during the experiment. Baseline of the pressure transducer is adjusted to zero mmHg when the heart is not attached to the perfusion system. Pseudo-ECG electrodes are placed in the chamber to approximate Lead I, II, and III of the Einthoven triangle ECG.
2. Harvesting and Perfusion of Rabbit Hearts
- Fix the rabbit in the rabbit restrainer. Euthanize the rabbit by an intravenous injection of sodium pentobarbital (50 mg/kg) with 2000 U heparin. When the rabbit is completely euthanized, which is determined by the lack of pain reflex, the thoracic cavity is quickly opened and the heart and lungs are excised.
- Make a cut at the upper end of ascending aorta before all the branches of aortic arch. Flush out the air from the ascending aorta and then quickly cannulate the heart to a 16-gauge cannula, which has been previously attached to a bubble trapper that is very important for keeping air out of coronaries. Once the heart is retrogradely perfused in a non-recirculating Langendorff perfusion system, make a cut to open the pericardium quickly.
- Remove the lung, trachea, fat, and connective tissues, while the blood is flushed out by the perfusion.
- Very important! A silicone tubing (˜3cm long, and 2mm in diameter) is inserted through a pulmonary vein and mitral valve into the left ventricle (LV) and kept there throughout the experiment. This tube releases the solution that is trapped in the LV. Without circulation for hours during the Langedorff-perfusion experiment in a mechanically immobilized heart, it is likely to cause severe ischemia in the LV cavity and to produce arrhythmia.
- Move the heart with the cannula to the recirculating Langendorff-perfusion system with the optical mapping apparatus.
3. Conducting Experiments using the Panoramic Optical Mapping System
- Place the heart in a custom-made hexagon chamber and connect the cannula to the perfusion system. Maintain the aortic pressure at 60 ± 5 mmHg by adjusting the flow rate of the perfusion pump. Monitor lead I pseudo-ECG. Maintain the pH at around 7.35 ± 0.05.
- Turn off the room light, because blebbistatin is photoinactivated by UV and low end (450-490 nm)17 of the visible part of the spectrum. Slowly inject blebbistatin stock solution through the injection port in the air-bubble trapper located above the cannula. Slowly inject 0.1ml blebbistatin for every bolus injection. Wait for the pressure to stabilize before the next injection.
- Gently place the pacing electrode onto the location specific to your experimental design.
- Focus the image of the heart in the frosted glass located at the image plane of each photo-diode array (PDA) from three evenly spaced angles surrounding the heart. Adjust the position of the cannula and the distances between each PDA and the heart in order to fit the heart in the field-of-view of all three PDAs. Take a picture of each focused image in the frosted glass.
- Slowly inject 10˜20μL di-4-ANEPPS stock solution through the injection port in the air-bubble trap into the perfusion solution. Wait 1˜3 minutes before taking optical recordings.
- For the first recording, turn the green LED light (no excitation filter, LED FLOOD, LUMILEDS) on, take optical recordings simultaneously from three PDAs connected with the custom-made data acquisition system,5 and turn off the LED light. Check the quality of the signals from different pixels of all three PDAs. Add 0.1˜0.2mL blebbistatin stock solution if motion artifacts in the optical action potential are noticed. Add another 5 μL di-4-ANEPPS stock solution if signal-to-noise ratio is low.
- Finish the designed experimental protocol for the functional study. Re-stain the heart with additional 5μL di-4-ANEPPS stock solution if the signal deteriorates during the experiments due to photobleaching or washout.
- Turn on the room light after the completion of the functional study. Take pictures of the heart from 36 evenly spaced angles. This is achieved by rotating the heart at a step of 10° with a digital camera fixed at the location of one PDA.
- Take the heart out of the chamber. Drain all the solutions. Wash the perfusion system in the sequence of DI water, 70% reagent alcohol, and again DI water.
- Data analysis includes the reconstruction of the heart geometry from the 36 digital photographs, registration of the optical signal onto the surface of the reconstructed geometry, and quantification of action potential duration (APD), conduction velocity (CV), phase, etc.6
4. Conducting Experiments using the Dual Mapping System
- (Continue after part 2) Place the cannulated heart in a glass chamber (Radnoti) and connect the cannula to the perfusion system. Pin down the heart to the silicon bottom of the chamber at the ventricular apex and atria.
- Turn off the room light. Slowly inject blebbistatin stock solution (15˜20 min to reach 10μM) through the injection port prior to perfusion cannula to immobilize the heart.
- Put a plastic Petri dish, or other glass window cover, above the epicardial surface to reduce the motion of the solution surface.
- Focus two CMOS cameras in the dual mapping system (Ultima-L, SciMedia) at the same field of view. Emitted fluorescence is separated by a dichroic mirror (635nm cutoff, Omega Optical), and filtered by a 700nm longpass filter (Thorlabs) for voltage signals and by a 590/30 nm bandpass filter (Omega Optical) for calcium signals.
- Aim the light guides of two halogen lamps (Newport Oriel Instruments, Stratford, CT; SciMedia, Costa Mesa, CA) towards the mapping field of view to achieve even illumination. Excitation filters (531/40 nm, SemRock) are used.
- Stain the heart with the voltage-sensitive dye RH 237 stock solution (10˜30 μL) through the injection port.
- Mix the Rhod-2AM (0.2mL) stock solution with Pluronic F-127 (Invitrogen, 1:1 mixture). Sonicate for 1min in a water-bath sonicator. Inject the mixture through the bubble trapper’s port. Wait for approximately 20 minute to allow the de-esterification of the Rhod-2 AM before mapping starts.
- For one recording, turn off the superfusion pump to avoid motion at the surface of the solution; turn on the excitation light source (the halogen lamps); take optical recordings using both cameras connected to the data acquisition system (Ultima-L, SciMedia); turn off the excitation light; and turn on the superfusion pump. Check the quality of the optical signals. Re-stain the tissue if necessary.
- Finish the rest of the designed experimental protocol for a study.
- Turn on the room light and take a photograph of the heart containing the field of view. Take the heart out of the chamber. Drain all the solutions. Wash the perfusion system in the sequence of DI water, 70% reagent alcohol (Fisher Scientific), and DI water.
- Data analysis contains measurements of APD, CV, calcium transient duration (CaTD), the delay between AP upstroke and CaT rise, the rise time of the calcium transient, and the time constant of a monoexponential fit of the CaT decay.
Representative Results:
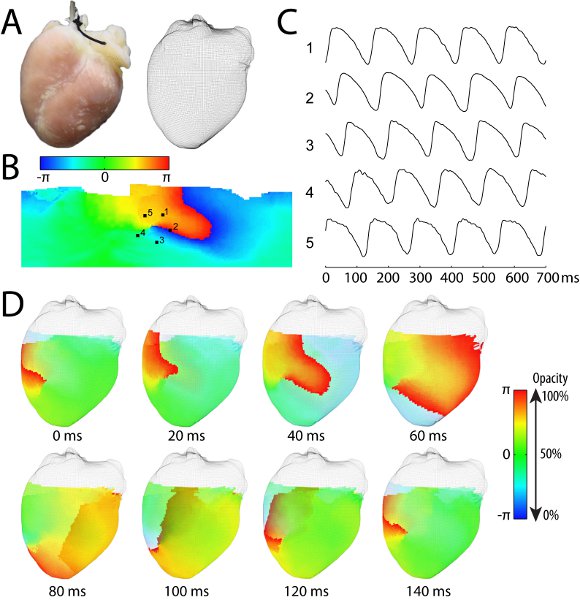
Figure 1. Representative results of a Langendorff-perfused rabbit experiment using the panoramic optical mapping system. (A) The anterior view of the rabbit heart and the reconstructed rabbit heart geometry in the form of a three-dimensional mesh-grid surface. (B) The unwrapped epicardial surface color-coded by the phase (obtained from the phase plane analysis18) indicating the wavefront shown in red during an episode of tachyarrhythmia. (C) The optical action potential recordings from five locations around the phase singularity marked by 1-5 in panel B. (D) Eight snapshots of activation wavefront (red color) propagation during a cycle of a stable reentrant arrhythmia. The wavefront circles clockwise around a phase singularity, which is visible at the anterior surface of the heart. The color for repolarization (blue) is set to be partially transparent so that the posterior wavefront is visible (e.g., at 80ms, 100ms, and 120ms). A movie of this reentrant arrhythmia is provided in the supplemental video 1. The methods for geometry reconstruction, signal registration, calculation of phase map, and surface unwrapping are described in details elsewhere6.
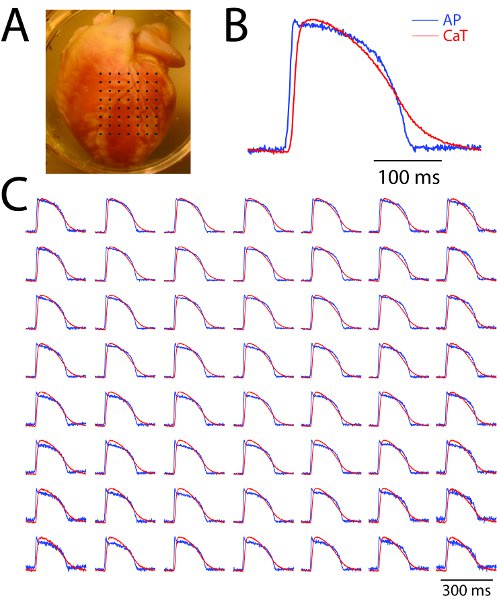
Figure 2. Representative results from a Langendorff-perfused rabbit heart experiment using the dual mapping system (simultaneous mapping of action potential and calcium transient). (A) The anterior surface of the heart with the mapping field of view covered by black dots. (B) A close-up view of recordings from one site. (C) Sample traces of action potential (blue) and calcium transient (red) from an array of evenly spaced locations marked by the black dots in panel A. Note that not all the pixel recordings are shown and the spatial resolution is 200μm.
Discussion
Based on our experience, the keys for a successful Langendorff-perfused rabbit heart experiment include well-prepared Tyrodes’ solution, quick harvest of the heart, well-maintained perfusion pressure, and appropriate pH of the oxygenated solution in the perfusion system. In order to record the signal with highest possible signal-to-noise ratio, we need to consider factors including light source, optical filters, focusing optics, photodetectors, etc19. Details of these aspects are discussed elsewhere19. Young rabbits (age: 4-5 months; weight: 7-9 pounds) could be used to avoid the epicardial fat, which decreases the signal to noise ratio of the optical signals.
The signal recorded by each pixel is a weighted integration of emitted light from a volume of tissue. The depth of this tissue volume depends on the excitation and emission wavelengths of the dye used. For di-4-ANEPPS, as an example, the estimated penetration depth is 300μm in the rabbit heart20. Thus, interpretation of the optical signal should be done with caution when the local heterogeneity of electrical function are present in sinoatrial node, atrioventricular node, and during ventricular arrhythmia 1,21,22.
One limitation of the optical mapping technique compared with electrode recording is that the repolarization phase of the optical action potential is often distored by motion artifact caused by cardiac contraction. Mechanical constraint could be used to reduce the artifact, but cannot completely eliminate it. In comparison, pharmacological excitation-contraction uncouplers are effective in removing the motion artifact. However, these uncouplers (e.g. 2,3-Butanedione Monoxime) could have significant electrophysiological side effects. Blebbistatin was demonstrated to have no adverse side-effects on the cardiac electrophysiology in the normal heart23, and is thus a promising uncoupler for optical mapping. It should be noted that the acceleration of edema due to the abolishment of the contraction could also affect the electrophysiology.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
NIH grants R01 HL085369, HL067322, HL082729, EB008999
Materials
| Reagent | Company | Catalogue Number |
| NaCl | Fisher Scientific, Fair Lawn, NJ | S271-1 |
| CaCl2 (2H2O) | Fisher Scientific, Fair Lawn, NJ | C79-500 |
| KCl | Fisher Scientific, Fair Lawn, NJ | S217-500 |
| MgCl2 (6H2O) | Fisher Scientific, Fair Lawn, NJ | M33-500 |
| NaH2PO4 (H2O) | Fisher Scientific, Fair Lawn, NJ | S369-500 |
| NaHCO3 | Fisher Scientific, Fair Lawn, NJ | S233-3 |
| D-Glucose | Fisher Scientific, Fair Lawn, NJ | D16-1 |
| Blebbistatin | Tocris Bioscience, Ellisville, MO | 1760 |
| Di-4-ANEPPS | Invitrogen, Carlsbad, CA | D1199 |
| RH237 | Invitrogen, Carlsbad, CA | S1109 |
| Rhod-2AM | Invitrogen, Carlsbad, CA | R1244 |
| Pluronic F127 | Invitrogen, Carlsbad, CA | P3000MP |
| Dimethyl sulphoxide (DMSO) | Sigma, St. Louis, MO | D2650 |
Referencias
- Efimov, I. R., Nikolski, V. P., Salama, G. Optical imaging of the heart. Circ Res. 95, 21-33 (2004).
- Panfilov, A. V. Is heart size a factor in ventricular fibrillation? Or how close are rabbit and human hearts?. Heart Rhythm. 3, 862-864 (2006).
- Maier, L. S., Bers, D. M., Pieske, B. Differences in Ca(2+)-handling and sarcoplasmic reticulum Ca(2+)-content in isolated rat and rabbit myocardium. J Mol Cell Cardiol. 32, 2249-2258 (2000).
- Bray, M. A., Lin, S. F., Wikswo, J. P. Three-dimensional surface reconstruction and fluorescent visualization of cardiac activation. IEEE Trans Biomed Eng. 47, 1382-1391 (2000).
- Qu, F., Ripplinger, C. M., Nikolski, V. P., Grimm, C., Efimov, I. R. Three-dimensional panoramic imaging of cardiac arrhythmias in rabbit heart. J Biomed Opt. 12, 044019-044019 (2007).
- Lou, Q., Ripplinger, C. M., Bayly, P. V., Efimov, I. R. Quantitative panoramic imaging of epicardial electrical activity. Ann Biomed Eng. 36, 1649-1658 (2008).
- Kay, M. W., Amison, P. M., Rogers, J. M. Three-dimensional surface reconstruction and panoramic optical mapping of large hearts. IEEE Trans Biomed Eng. 51, 1219-1229 (2004).
- Li, W., Ripplinger, C. M., Lou, Q., Efimov, I. R. Multiple monophasic shocks improve electrotherapy of ventricular tachycardia in a rabbit model of chronic infarction. Heart Rhythm. 6, 1020-1027 (2009).
- Ripplinger, C. M., Lou, Q., Li, W., Hadley, J., Efimov, I. R. Panoramic imaging reveals basic mechanisms of induction and termination of ventricular tachycardia in rabbit heart with chronic infarction: implications for low-voltage cardioversion. Heart Rhythm. 6, 87-97 (2009).
- Efimov, I. R., Rendt, J. M., Salama, G. Optical maps of intracellular [Ca2+]i transients and action-potentials from the surface of perfused guinea-pig hearts (abstract). Circulation. 90, 632-632 (1994).
- Choi, B. R., Salama, G. Simultaneous maps of optical action potentials and calcium transients in guinea-pig hearts: mechanisms underlying concordant alternans. J Physiol. 529, 171-188 (2000).
- Fast, V. G., Ideker, R. E. Simultaneous optical mapping of transmembrane potential and intracellular calcium in myocyte cultures. J Cardiovasc Electrophysiol. 11, 547-556 (2000).
- Laurita, K. R., Singal, A. Mapping action potentials and calcium transients simultaneously from the intact heart. Am J Physiol Heart Circ Physiol. 280, 2053-2060 (2001).
- Choi, B. R., Burton, F., Salama, G. Cytosolic Ca2+ triggers early afterdepolarizations and Torsade de Pointes in rabbit hearts with type 2 long QT syndrome. J Physiol. 543, 615-631 (2002).
- Hwang, G. S. Intracellular calcium and vulnerability to fibrillation and defibrillation in Langendorff-perfused rabbit ventricles. Circulation. 114, 2595-2603 (2006).
- Lou, Q., Efimov, I. R. Enhanced susceptibility to alternans in a rabbit model of chronic myocardial infarction. Conf Proc IEEE Eng Med Biol Soc. , 4527-4530 (2009).
- Kolega, J. Phototoxicity and photoinactivation of blebbistatin in UV and visible light. Biochem Biophys Res Commun. 320, 1020-1025 (2004).
- Bray, M. A., Wikswo, J. P. Considerations in phase plane analysis for nonstationary reentrant cardiac behavior. Phys Rev E Stat Nonlin Soft Matter Phys. 65, 051902-05 (2002).
- Fast, V. Recording action potentials using voltage-sensitive dyes. Practical methods in cardiovascular research. , 233-255 (2005).
- Knisley, S. B. Transmembrane voltage changes during unipolar stimulation of rabbit ventricle. Circ Res. 77, 1229-1239 (1995).
- Bishop, M. J. The role of photon scattering in optical signal distortion during arrhythmia and defibrillation. Biophys J. 93, 3714-3726 (2007).
- Efimov, I. R., Fedorov, V. V., Joung, B., Lin, S. F. Mapping cardiac pacemaker circuits: methodological puzzles of the sinoatrial node optical mapping. Circ Res. 106, 255-271 (2010).
- Fedorov, V. V. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm. 4, 619-626 (2007).

