One-day Workflow Scheme for Bacterial Pathogen Detection and Antimicrobial Resistance Testing from Blood Cultures
Summary
The design of a straightforward one-day workflow scheme for bacterial pathogen diagnostics enables the rapid recognition of bloodstream infections. The inclusion of eight clinically relevant bacterial targets and their antibiotic resistance profiles offers the clinician an initial insight on the same day, which can lead to more adequate therapy.
Abstract
Bloodstream infections are associated with high mortality rates because of the probable manifestation of sepsis, severe sepsis and septic shock1. Therefore, rapid administration of adequate antibiotic therapy is of foremost importance in the treatment of bloodstream infections. The critical element in this process is timing, heavily dependent on the results of bacterial identification and antibiotic susceptibility testing. Both of these parameters are routinely obtained by culture-based testing, which is time-consuming and takes on average 24-48 hours2, 4. The aim of the study was to develop DNA-based assays for rapid identification of bloodstream infections, as well as rapid antimicrobial susceptibility testing. The first assay is a eubacterial 16S rDNA-based real-time PCR assay complemented with species- or genus-specific probes5. Using these probes, Gram-negative bacteria including Pseudomonas spp., Pseudomonas aeruginosa and Escherichia coli as well as Gram-positive bacteria including Staphylococcus spp., Staphylococcus aureus, Enterococcus spp., Streptococcus spp., and Streptococcus pneumoniae could be distinguished. Using this multiprobe assay, a first identification of the causative micro-organism was given after 2 h.
Secondly, we developed a semi-molecular assay for antibiotic susceptibility testing of S. aureus, Enterococcus spp. and (facultative) aerobe Gram-negative rods6. This assay was based on a study in which PCR was used to measure the growth of bacteria7. Bacteria harvested directly from blood cultures are incubated for 6 h with a selection of antibiotics, and following a Sybr Green-based real-time PCR assay determines inhibition of growth. The combination of these two methods could direct the choice of a suitable antibiotic therapy on the same day (Figure 1). In conclusion, molecular analysis of both identification and antibiotic susceptibility offers a faster alternative for pathogen detection and could improve the diagnosis of bloodstream infections.
Protocol
PART I: PATHOGEN IDENTIFICATION
1. Sample Preparation
Note: The entire molecular workflow as is described in the following protocol should be performed according to recommendations for quality assurance in molecular diagnostics3.
- Add an 0.1 ml aliquot of blood culture to 0.9 ml 0.9% NaCl into a 1.5 ml reaction tube to become a 1:10 diluted sample. (Dilute in order to prevent qPCR inhibition).
- Centrifuge sample at 13400 x g for 5 min to pellet the bacterial DNA.
- Resuspend the bacterial pellet in 100 μl sterile demineralised H2O.
- Store the DNA sample at 4 °C until further use.
2. Identification Assay: Real-time 16s rDNA PCR
- Prepare the reaction mixtures as follows. The assay consists of four separate reactions per sample. Each mixture includes 12.50 μl master mix, 0.9 μM forward primer (5-TCCTACGGGAGGCAGCAGT-3)7, 0.6 μM reverse primer (5-GGACTACCAGGGTATCTAATCCTGTT-3)8 and a panel of probes. The amount of probes is given for each of the four separate reactions in below.
- The first reaction includes:
- 0.2 μM universal probe (5-FAM-CGTATTACCGCGGCTGCTGGCAC-3-BHQ1)8
- 0.2 μM P. aeruginosa probe (5-JOE-CCAAAACTACTGAGCTAGAGTACG-3-BHQ1)
- The second reaction includes:
- 0.2 μM E. coli probe (5-JOE-GGAGTAAAGTTAATACCTTTGCTCATT-3-BHQ1)
- 0.2 μM Pseudomonas spp. probe (5-NED-CCTTCCTCCCAACTTAAAGTGCTT-3-MGBNFQ)
- The third reaction includes:
- 0.2 μM Staphylococcus spp. probe (5-NED-AATCTTCCGCAATGGGCGAAAGC-3-MGBNFQ)
- 0.2 μM S. aureus probe (5-FAM-AGATGTGCACAGTTACTTACACATAT-3-BHQ1)
- 0.2 μM Enterococcus spp. (5-JOE-TCCTTGTTCTTCTCTAACAACAGAG-3-BHQ1)
- The fourth reaction includes:
- 0.2 μM universal probe (5-FAM-CGTATTACCGCGGCTGCTGGCAC-3-BHQ1)
- 0.3 μM Streptococcus spp. probe (5-NED-CCAGAAAGGGACSGCTAACT-3-MGBNFQ)
- 0.2 μM S. pneumoniae probe (5-JOE-CCAAAGCCTACTATGGTTAAGCCA-3-BHQ1)
- Add sterile demineralised H2O to reach a total volume of 20 μl. Add 20 μl of each reaction mixture to the wells of a 96-well PCR plate.
- Add 5 μl of sample to each well.
- Use an adhesive film to seal the 96-well PCR plate.
- Run the plate on the ABI PRISM 7900HT Real Time PCR System using the following optimal thermal cycling conditions:
- Pre-heating at 50 °C for 10 min
- Initial denaturation at 95 °C for 15 min
- 42 cycles of
- Denaturation at 95 °C for 15 s
- Annealing at 60 °C for 1 min
3. Analysis of the Results
Adjust the threshold of the Ct Analysis to 0.1 in the tab Analysis Settings. Narrow the baseline configurations to Start (Cycle):6 and End (Cycle):15.- Record the cycle threshold (Ct) value for all samples. The cut-off value to consider a PCR result as positive can be set to a Ct-value of 35. The amount of bacteria present in blood cultures ranged from 107 to 1011 CFU/ml, generating Ct-values below 35.
PART II: ANTIBIOTIC SUSCEPTIBILITY TESTING
4. Isolation of Bacteria from Positive Blood Cultures9
- Aspirate 5 ml of broth from a positive blood culture bottle and transfer it into a serum separator tube.
- Centrifuge the serum separator tube at 2000 x g for 10 min.
- Discard the supernatant from the serum separator tube.
- Transfer bacteria from the gel layer of the tube with a sterile cotton swab into 0.9% saline until a 0.5 McFarland standard suspension is obtained.
5. Inoculation of Micro Titre Plates
- Dilute the 0.5 McFarland suspension in double concentrated Mueller Hinton II broth to form a suspension of 5 x 105 CFU/ml.
- Add this suspension to the wells of a micro titre plate containing a selection of antibiotics (Table 1).
- Incubate the micro titre plate at 37 °C for 6 h.
- Store an aliquot of the suspension at 4 °C (as negative growth control).
- After 6 h of incubation, transfer the content of each well into a sterile tube, as well as the negative growth control sample that was stored at 4 °C.
- Centrifuge the tubes at 16000 x g for 5 min.
- Carefully remove the supernatant, without disturbing the bacterial pellet.
- Resuspend the pellet in sterile demineralised H2O.
- Dilute the samples 10-fold in sterile demineralised H2O.
6. Real-time 16s rDNA PCR10
- Prepare the PCR mixture as follows:
- 12.50 μl iQ SYBR Green Supermix
- 0.5 μM forward primer 16S-1 (5-TGGAGAGTTTGATCCTGGCTCAG-3)11
- 0.25 μM reverse primer 16S-2 (5-TACCGCGGCTGCTGGCAC-3)11
- sterile demineralised H2O to a total volume of 20 μl
- Add 20 μl of PCR mixture to the wells of a 96-well PCR plate.
- Add 5 μl of sample to each well.
- Use an adhesive film to seal the 96-well PCR plate.
- Run the plate on the MyiQ Single-Color Real-Time PCR Detection System, using the following optimal thermal cycling conditions:
- Initial denaturation at 95 °C for 4 min
- Initial annealing at 65 °C for 30 s
- 35 cycles of
- Denaturation at 95 °C for 15 s
- Annealing at 60 °C for 1 min
- Melt curve analysis (from 60-95 °C in 20 min with increments of 0.57 °C)
7. Analysis of the Results
- Calculate the cut-off Ct value using one of the following formulas (depending on type of antibiotic).
- In general:
Cut-off Ct value = Ct value positive growth control + 0.5 x (Ct value negative growth control – Ct value positive growth control) - Piperacillin, piperacillin/tazobactam and ceftazidime in Gram-negative rods; Amoxicillin, oxacillin and trimethoprim/sulfamethoxazole in S.aureus; Amoxicillin in Enterococcus spp.: Cut-off Ct value = Ct value positive growth control + 0.25 x (Ct value negative growth control – Ct value positive growth control)
- In general:
- Use the sample incubated with sterile demineralised H2O as positive growth control.
- Depending on the microorganism, use the appropriate negative growth control:
- Gram-negative rods Sample incubated with mixture of antibiotics
- Enterococcus spp. Sample stored at 4 °C
- S.aureus Sample stored at 4 °C
- Determine the susceptibility (S) or resistance (R) of the strain for the tested antibiotic as follows:
- A Ct value higher than the cut-off Ct value indicates susceptibility
- A Ct value lower than the cut-off Ct value indicates resistance
8. Representative Results
Two model organisms, i.e. a Gram-negative E. coli and a Gram-positive S. aureus, are chosen to visualize the combined procedure for the detection and identification of bacterial pathogens and the determination of their antimicrobial profile. The first part of the protocol comprises the pathogen identification. Specific probes are designed for the detection of eight clinically relevant microorganisms. In presence of a target included in the bacterial panel, amplification curves are generated and Ct values are calculated (Figure 2). The cut-off value to consider a PCR result as positive is set to a Ct value of 35. In Figure 2A, the identification profile of an E.coli-infected blood culture is shown. The 16S universal probe is included in two separate reaction mixtures and consequently generates two amplification curves (Ct of 25.20 and 25.95). The third signal is derived from the probe specific for E. coli (Ct of 27.04). The identification of a S. aureus-infected blood culture is shown in Figure 2B. The 16S universal probe has amplification signals of 33.35 and 33.71. The two remaining signals are derived from the probes specific for Staphylococcus spp. and S. aureus (Ct of 32.48 and 30.59).
After the first part of the protocol, the causative microorganism is known and the antimicrobial profile can be determined. Figure 3 is an example of an antibiotic susceptibility testing amplification plot, representing the E.coli strain that was also shown in Figure 2A. Each line represents one antibiotic that the bacterial sample was incubated with. A sample with a low Ct value is a sample in which growth has occurred in the presence of an antibiotic, indicating resistance to the tested antibiotic. On the contrary, a high Ct value represents a sample in which no growth has occurred because of the effective working of the antibiotic, indicating susceptibility to the tested antibiotic. Table 1 illustrates the determination of the antimicrobial profile of the E. coli and S. aureus isolates. All Ct values are reported and, using the formulas mentioned in the protocol text (7.1), two cut-off Ct values are calculated to distinguish between resistance and susceptibility. The strain is resistant to the antibiotic if the reported Ct value is lower than the calculated cut-off Ct value (and vice versa).
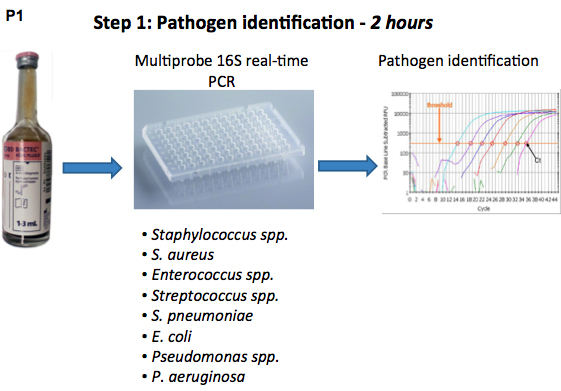
Figure 1. Flow chart of the pathogen identification and antibiotic susceptibility testing procedure using real-time 16S rDNA PCR.
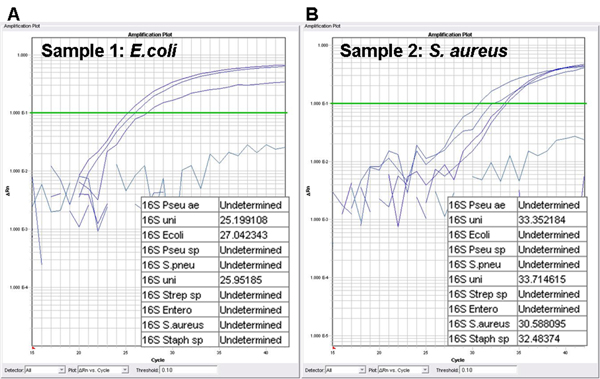
Figure 2. Identification assay: Amplification plots and cycle threshold values (Ct values). A positive blood culture is detected by the universal 16S rDNA probe, while the specific probes are used for the identification of the causal pathogen. A. amplification plot of blood culture containing E. coli; B. amplification plot of blood culture containing S. aureus; Pseu ae, Pseudomonas aeruginosa; uni, 16S universal probe; Ecoli, Escherichia coli probe; Pseu sp, Pseudomonas spp. probe; S. pneu, Streptococcus pneumoniae probe; Strep sp, Streptococcus spp. probe; Entero, Enterococcus spp. probe; S. aureus, Staphylococcus aureus probe; Staph sp, Staphylococcus spp. probe.
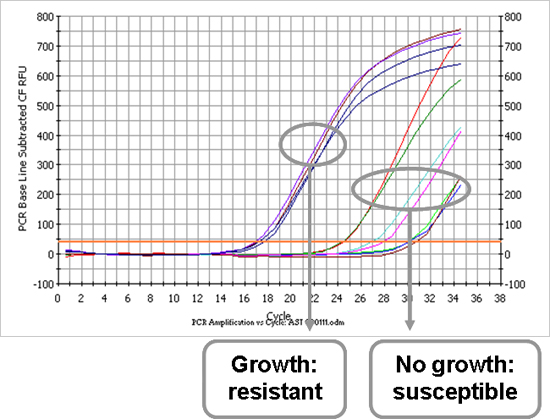
Figure 3. Amplification plot of antibiotic susceptibility testing of an E. coli isolate (sample 1). Each curve represents one antibiotic that the strain was incubated with. An early signal is caused by a high bacterial load, which means that the strain has grown in the presence of the tested antibiotic and is thus resistant to the antibiotic. Late signals indicate that the strain has not grown in the presence of the antibiotic, in other words, it is susceptible.
| Sample 1: E. coli | Sample 2: S. aureus | ||||
| AST | Ct | R/S | AST | Ct | R/S |
| Amoxicillin 8 mg/L | 16,83 | R | Amoxicillin 0.25 mg/L | 21,03 | R |
| Amoxicillin-clavulanate 8/4 mg/L | 17,36 | R | Oxacillin 2 mg/L | 25,80 | S |
| Piperacillin 16 mg/L | 16,67 | R | Vancomycin 2 mg/L | 25,20 | S |
| Piperacillin-tazobactam 16/4 mg/L | 24,15 | S | Gentamicin 4 mg/L | 25,86 | S |
| Ciprofloxacin 1 mg/L | 29,72 | S | Trimethoprim-sulfamethoxazole 2/38 mg/L | 24,62 | S |
| Ceftazidime 1 mg/L | 24,03 | S | |||
| Ceftazidime 8 mg/L | 26,58 | S | |||
| Gentamicin 4 mg/L | 29,83 | S | |||
| Trimethoprim-sulfamethoxazole 2/38 mg/L | 27,60 | S | |||
| Negative growth control (mixture of antibiotics) | 30,41 | Negative growth control (sample stored at 4 °C) | 27,42 | ||
| Positive growth control | 16,90 | Positive growth control | 20,22 | ||
| Cut-off Ct-value 1* | 21,76 | Cut-off Ct-value 1*** | 23,82 | ||
| Cut-off Ct-value 2** | 18,75 | Cut-off Ct-value 2**** | 22,02 | ||
| * For amoxicillin, amoxicillin-clavulanate, ciprofloxacin, gentamicin, trimethoprim-sulfamethoxazole ** For piperacillin, piperacillin-tazobactam, ceftazidime |
*** For vancomycin and gentamicin **** For amoxicillin, oxacillin and trimethoprim-sulfamethoxazole |
Table 1. Determination of antibiotic susceptibility testing of the two samples (E.coli and S.aureus). Ct values of the PCR-assay were copied to this excel file, as which can automatically calculates the two cut-off Ct alues from the positive and negative growth control, using the formulas shown in the protocol text. If an antibiotic shows a Ct value lower than the cut-off Ct value, the strain is resistant to the antibiotic, if the Ct value was higher than the cut-off, the strain is susceptible.
Discussion
The protocol described here enables the rapid identification of pathogens and provides a functional antimicrobial profile that could lead to the early administration of adequate antibiotics thereby improving the prognosis of patients with bloodstream infections. Depending on the requested conditions of a test, i.e. low cost, high throughput, minimal turn-around time, testing conditions can be adjusted. The whole procedure can be performed within one working day. Moreover, the two parts of the protocol can be performed simultaneously, which reduces the turn-around time significantly. As presented here, the identification panel is a selection of the most clinically relevant bacteria in our hospital. Since the main principle is targeting the 16S gene region, specific probes for other microorganisms can be designed and added to the assay. The complete assay was originally intended for the rapid analysis of blood cultures, but can also be used for the processing of other sample materials. This is also the case for the antibiotics that were used for antibiotic susceptibility testing: more or other antibiotics can be added, based on local resistance patterns and guidelines.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Profileringsfonds azM (PF245).
Materials
| Name of the reagent | Company | Catalogue number | Comments |
| Sodium Chloride (NaCl) | Merck Chemicals | 106404 | 0.9% in water |
| Vacutainer SST Serum Separator Tube 5 ml | BD Diagnostic Systems | 367986 | |
| Mueller Hinton II broth | BD Diagnostic Dystems | 212322 | 44 g/L in water |
| Centrifuge Rotixa 50 rs | Andreas Hettich GmbH & Co. KG | 4910 | |
| Centrifuge 5415 D | Eppendorf | Discontinued | |
| Primers | Sigma-Aldrich | n.a. | |
| Probes | Sigma-Aldrich/Applied Biosystems | n.a. | |
| TaqManEnvironmental master mix 2.0 | Applied Biosystems | 4396838 | |
| iQ SYBRGreen Supermix | Bio-Rad Laboratories BV | 170-8880 | |
| MicroAmp Optical 96-Well Reaction Plate | Applied Biosystems | N8010560 | |
| MicroAmp Optical Adhesive Film | Applied Biosystems | 4311971 | |
| iQ 96-Well PCR Plates | Bio-Rad Laboratories BV | 223-9441 | |
| Microseal B Adhesive Seals | Bio-Rad Laboratories BV | MSB-1001 | |
| Real-time PCR Detection System | Applied Biosystems | ABI PRISM 7900HT | |
| Real-Time PCR Detection System | Bio-Rad Laboratories BV | MyiQ Single-Color |
Referencias
- Wallet, F. Preliminary clinical study using a multiplex real-time PCR test for the detection of bacterial and fungal DNA directly in blood. Clin. Microbiol. Infect. 16, 774 (2010).
- Beekmann, S. E., Diekema, D. J., Chapin, K. C., Doern, G. V. Effects of rapid detection of bloodstream infections on length of hospitalization and hospital charges. J. Clin. Microbiol. 41, 3119 (2003).
- Raymaekers, M., Bakkus, M., Boone, E., de Rijke, B., Housni, H. E. l., Descheemaeker, P., De Schouwer, P., Franke, S., Hillen, F., Nollet, F., Soetens, O., Vankeerberghen, A. Molecular Diagnostics working group. Reflections and proposals to assure quality in molecular diagnostics. Acta. Clin. Belg. 66, 33 (2011).
- Peters, R. P. New developments in the diagnosis of bloodstream infections. Lancet Infect. Dis. 4, 751 (2004).
- Hansen, W. L., Beuving, J., Bruggeman, C. A., Wolffs, P. F. Molecular probes for the diagnosis of clinically relevant bacterial infections in blood cultures. J. Clin. Microbiol. 48, 4432-4432 (2010).
- Beuving, J. Antibiotic susceptibility testing of grown blood cultures by combining culture and real-time polymerase chain reaction is rapid and effective. PLoS ONE. 6, (2011).
- Rolain, J. M., Mallet, M. N., Fournier, P. E., Raoult, D. Real-time PCR for universal antibiotic susceptibility testing. J. Antimicrob. Chemother. 54, 538 (2004).
- Nadkarni, M. A., Martin, F. E., Jacques, N. A., Hunter, N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiol. 148, 257 (2002).
- Waites, K. B., Brookings, E. S., Moser, S. A., Zimmer, B. L. Direct susceptibility testing with positive BacT/Alert blood cultures by using MicroScan overnight and rapid panels. J. Clin. Microbiol. 36, 2052 ( ).
- Vliegen, I. Rapid identification of bacteria by real-time amplification and sequencing of the 16S rRNA gene. J. Microbiol. Meth. 66, 156 (2006).
- Hall, L., Doerr, K. A., Wohlfiel, S. L., Roberts, G. D. Evaluation of the MicroSeq system for identification of mycobacteria by 16S ribosomal DNA sequencing and its integration into a routine clinical mycobacteriology laboratory. J. Clin. Microbiol. 41, 1447 (2003).

