Diagnosing Pulmonary Tuberculosis with the Xpert MTB/RIF Test
Summary
The Xpert MTB/RIF test integrates sample decontamination, hands-free operation, on-board sample processing, and ultra-sensitive hemi-nested PCR for the simultaneous detection of Mycobacterium tuberculosis and rifampicin resistance, either in expectorated sputum or concentrated sputum sediments, in approximately two hours. Testing is standardized and requires only moderate laboratory infrastructure and training.
Abstract
Tuberculosis (TB) due to Mycobacterium tuberculosis (MTB) remains a major public health issue: the infection affects up to one third of the world population1, and almost two million people are killed by TB each year.2 Universal access to high-quality, patient-centered treatment for all TB patients is emphasized by WHO’s Stop TB Strategy.3 The rapid detection of MTB in respiratory specimens and drug therapy based on reliable drug resistance testing results are a prerequisite for the successful implementation of this strategy. However, in many areas of the world, TB diagnosis still relies on insensitive, poorly standardized sputum microscopy methods. Ineffective TB detection and the emergence and transmission of drug-resistant MTB strains increasingly jeopardize global TB control activities.2
Effective diagnosis of pulmonary TB requires the availability – on a global scale – of standardized, easy-to-use, and robust diagnostic tools that would allow the direct detection of both the MTB complex and resistance to key antibiotics, such as rifampicin (RIF). The latter result can serve as marker for multidrug-resistant MTB (MDR TB) and has been reported in > 95% of the MDR-TB isolates.4, 5 The rapid availability of reliable test results is likely to directly translate into sound patient management decisions that, ultimately, will cure the individual patient and break the chain of TB transmission in the community.2
Cepheid’s (Sunnyvale, CA, U.S.A.) Xpert MTB/RIF assay6, 7 meets the demands outlined above in a remarkable manner. It is a nucleic-acids amplification test for 1) the detection of MTB complex DNA in sputum or concentrated sputum sediments; and 2) the detection of RIF resistance-associated mutations of the rpoB gene.8 It is designed for use with Cepheid’s GeneXpert Dx System that integrates and automates sample processing, nucleic acid amplification, and detection of the target sequences using real-time PCR and reverse transcriptase PCR. The system consists of an instrument, personal computer, barcode scanner, and preloaded software for running tests and viewing the results.9 It employs single-use disposable Xpert MTB/RIF cartridges that hold PCR reagents and host the PCR process. Because the cartridges are self-contained, cross-contamination between samples is eliminated.6 Current nucleic acid amplification methods used to detect MTB are complex, labor-intensive, and technically demanding. The Xpert MTB/RIF assay has the potential to bring standardized, sensitive and very specific diagnostic testing for both TB and drug resistance to universal-access point-of-care settings3, provided that they will be able to afford it. In order to facilitate access, the Foundation for Innovative New Diagnostics (FIND) has negotiated significant price reductions. Current FIND-negotiated prices, along with the list of countries eligible for the discounts, are available on the web.10
Protocol
Standard 2 of the International Standards for Tuberculosis Care states that all patients suspected of having pulmonary TB should submit at least two sputum specimens for bacteriological examination. When possible, at least one early-morning specimen should be obtained, as sputum collected at this time has the highest yield.11 The Xpert MTB/RIF assay can be applied to sputum samples or concentrated sputum sediments prepared from induced or expectorated sputa that are either acid-fast bacilli (AFB) smear positive or negative.6, 12, 13 The assay is intended for use with specimens from patients for whom there is clinical suspicion of pulmonary TB who have: 1) not received anti-tuberculosis therapy, 2) had < 7 days of therapy, or 3) have not received therapy in the last 60 days. Do note that it should not be used for monitoring the effects of drug therapy7 because bacterial DNA might persist following antimicrobial therapy.
Treat clinical specimens, including used cartridges, as if capable of transmitting infectious agents. Use proper precautions, such as wearing protective disposable gloves, laboratory coats and eye protection, and follow your institution’s safety procedures and guidelines.
1. Expectorated Sputum Samples
Note: Process only as many specimens at one time, as there are GeneXpert Dx System modules available to run the test!
- Label each Xpert MTB/RIF cartridge with the corresponding specimen ID.
- Transfer 1.0 ml expectorated sputum to a conical, screw-capped tube using a sterile transfer pipette. Alternatively, the entire specimen may be processed in the original leak-proof sputum collection container.
- Add 2.0 ml Xpert MTB/RIF Sample Reagent (2:1; v/v) to the expectorated sputum using a sterile transfer pipette.
- Replace the lid, and shake the tube vigorously 10-20 times. Alternatively, the mixture may be vortexed (30 sec).
- Allow the tube to stand upright for 5 min at room temperature.
- Shake the tube vigorously 10-20 times. Alternatively, use a vortex (30 sec).
- Allow the tube to stand upright for another 10 min at room temperature.
- Inspect the specimens: samples should be liquefied with no visible clumps of sputum.
2. Concentrated Sediments
Note: Process only as many specimens at one time as there are GeneXpert Dx System modules available to run the tests!
- Label each Xpert MTB/RIF cartridge with the corresponding specimen ID.
- Transfer 1.5 ml Xpert MTB/RIF Sample Reagent to a conical, screw-capped tube using a sterile transfer pipette.
- Add at least 0.5 ml concentrated sediment to the Sample Reagent containing tube using a sterile transfer pipette.
- Replace the lid, and shake the tube vigorously 10-20 times. Alternatively, use a vortex (30 sec).
- Allow the tube to stand upright for 5 min at room temperature.
- Shake the tube vigorously 10-20 times. Alternatively, use a vortex (30 sec).
- Allow the tube to stand upright for another 10 min at room temperature.
- Inspect the specimens: samples should be liquefied with no visible clumps of sputum.
3. Loading the Xpert MTB/RIF Cartridge
Note: Start the test within 30 minutes of adding the sample to the cartridge!
- Check the labels on the Xpert MTB/RIF cartridge and the specimen ID.
- Open the cartridge lid.
- Using the sterile transfer pipette provided, aspirate the liquefied specimen into the transfer pipette until the meniscus is above the minimum mark and transfer the sample into the open port of the Xpert MTB/RIF cartridge.
Note: Dispense slowly to minimize the risk of aerosol formation!
- Close the cartridge lid.
4. Starting the Test
Note: Before starting the test, ensure that the GeneXpert Dx System is equipped with the GX2.1 software, and the Xpert MTB/RIF assay is imported into the software! 9
- Turn on the computer, and then turn on the GeneXpert Dx instrument.
- On the Windows desktop, double-click the GeneXpert Dx shortcut icon.
- Log on to the GeneXpert Dx System software using your user name and password.
- In the GeneXpert Dx System window, click Create Test. The Scan Cartridge Barcode dialog box appears.
- In the Sample ID box, scan or type the sample ID.
- Scan the barcode on the Xpert MTB/RIF cartridge. The Create Test window appears.
- Click Start Test, and enter your password in the dialog box that appears.
- Open the instrument module door with the blinking green light, and load the cartridge.
- Close the door. The test starts and the light stops blinking and remains constantly green. When the test is finished, the light turns off.
- Wait until the system releases the door lock at the end of the run, then open the module door and remove the cartridge.
- Dispose of used cartridges in the appropriate waste containers according to your institution’s standard practices.
5. Representative Results
Each Xpert MTB/RIF cartridge includes reagents for the detection of MTB complex and RIF resistance as well as a sample processing control (SPC) to control for adequate processing of the target bacteria and to monitor the presence of inhibitor(s) in the PCR reaction. The Probe Check Control (PCC) verifies reagent rehydration, PCR tube filling in the cartridge, probe integrity, and dye stability.9 The primers in the Xpert MTB/RIF assay amplify a portion of the rpoB gene containing the 81 base pair “core” region. Five differently colored fluorogenic nucleic acid hybridization probes, called molecular beacons, interrogate the entire 81-bp core.14 Each molecular beacon was designed to be so specific that it does not bind to its target if the target sequence differs from the wild-type rpoB sequence by as little as a single nucleotide substitution. Since molecular beacons fluorescence only when they are bound to their targets, i.e. wild-type rpoB sequence, the absence of any one of the five colors in the assay differentiate between the conserved wild-type sequence and mutations in the core region that are associated with RIF resistance.6
The SPC should be positive in a negative sample and can be negative or positive in a positive sample. The test result will be “Invalid” if the SPC is not detected in a negative test. Before the start of the PCR reaction, the GeneXpert Dx System measures the fluorescence signal from the probes to monitor bead rehydration, reaction-tube filling, probe integrity and dye stability. The PCC passes if the fluorescence signal from the probes meets the assigned acceptance criteria. The results are interpreted by the GeneXpert Dx System from measured fluorescence signals and embedded calculation algorithms and are displayed in the Ver resultados window9, as indicated below:
MTB Detected: MTB target DNA is detected; both controls, SPC and PCC, meet the assigned acceptance criteria. Lower Ct values represent a higher starting concentration of DNA template; higher Ct values represent a lower concentration of DNA template. In MTB DETECTED results “RIF Resistance DETECTED” (Figure 1A), “RIF Resistance NOT DETECTED” (Figure 1B), or “RIF Resistance INDETERMINATE” will display on a separate line.
MTB Not Detected: MTB target DNA is not detected; both controls, SPC and PCC, meet the assigned acceptance criteria (Figure 2).
Invalid: Presence or absence of MTB cannot be determined: SPC does not meet acceptance criteria, i.e. the sample was not properly processed, or PCR was inhibited (Figure 3). Note: repeat test with extra specimen!
Error: One or more of the PCC results failed (FAIL). Both MTB and SPC display NO RESULT. Note: repeat test with extra specimen! If the PCC passed (PASS), the error is caused by a system component failure.
Figure 1: MTB target DNA is detected; both controls, SPC and PCC, meet the assigned acceptance criteria. Lower Ct values represent a higher starting concentration of DNA template; higher Ct values represent a lower concentration of DNA template. In MTB DETECTED results “RIF Resistance NOT DETECTED” (Figure 1A), “RIF Resistance DETECTED” (Figure 1B), or “RIF Resistance INDETERMINATE” will display on a separate line.

Figure 1A. MTB DETECTED; RIF Resistance NOT DETECTED, i.e. only the wild-type nucleic acid sequence of the 81-bp RIF resistance-determining region (RRDR) of the rpoB gene was present.6 Click here to view larger figure.
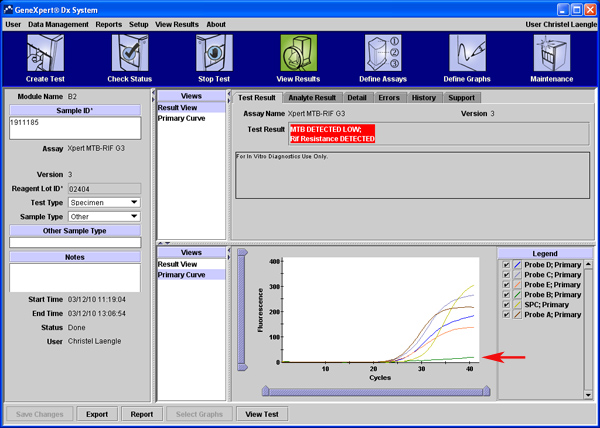
Figure 1B. MTB DETECTED; RIF Resistance DETECTED. Complete drop out of Probe B (arrow), which covers the positions 513 to 517 of the RRDR of the rpoB gene.6 In the example, partial sequencing revealed that the rpoB gene mutation D516V was detected.8 Click here to view larger figure.
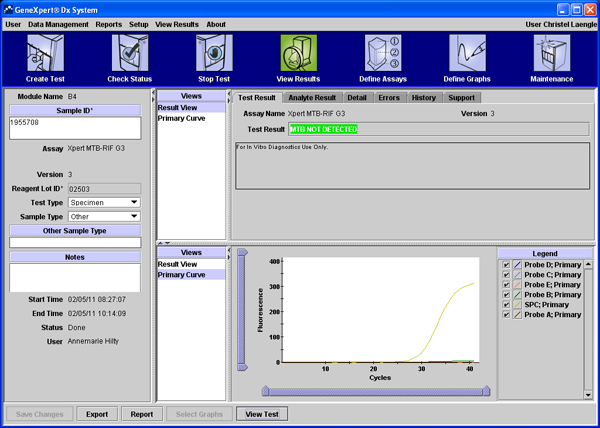
Figure 2. MTB target DNA was not detected; both controls, SPC and PCC, meet the assigned acceptance criteria. Click here to view larger figure.
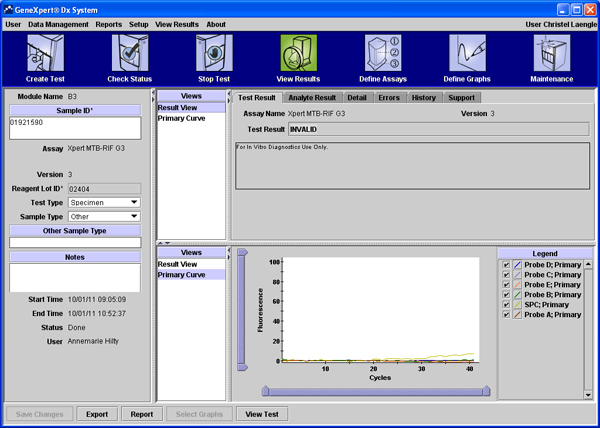
Figure 3. Invalid test result, i.e. presence or absence of MTB cannot be determined: SPC does not meet acceptance criteria, because the sample was not properly processed, or because PCR was inhibited. Click here to view larger figure.
Discussion
The Xpert MTB/RIF assay integrates sample decontamination, hands-free operation, on-board sample processing, and ultra-sensitive hemi-nested PCR for the simultaneous detection of MTB and RIF resistance, either in expectorated sputum or concentrated sediments, in a single, easy to use system. The assay’s analytical limit of detection was found to be 131 CFU/ml of sputum.6 The log-linear relationship between Ct values and the number of MTB cells present holds true from 102 to 107 CFU/ml of sputum. As clinically relevant MTB concentrations all fall within the linear range of the assay, the assay provides semi-quantitative estimates of the bacterial load.7 The assay requires only moderate laboratory infrastructure and training. The limit of detection lies at least two orders of magnitude lower than that of conventional microscopy.6 Specificity is excellent. Furthermore, the simultaneous detection of RIF-resistant MTB is a significant benefit in the setting of point-of-care testing in the era of drug-resistant TB.3
To date only limited published data on the assay’s performance for diagnosing pulmonary TB in clinical settings are available.12; 13 The Xpert MTB/RIF assay’s overall clinical sensitivity of detecting MTB in sputum of patients with suspected pulmonary TB was 97.6% when compared to culture as the reference. These were the findings of a prospective multi-center study that involved five study sites (Lima, Peru; Baku, Azerbaijan; Cape Town & Durban, South Africa; Mumbai, India) and a total of 1462 patients. The overall pre-test probability of culture-confirmed TB in the study population was 50.7%. The sensitivity was 99.8% for smear- and culture-positive cases and 90.2% for smear-negative, culture-positive cases. Among HIV-positive patients with pulmonary TB, the sensitivity of the MTB/ RIF test was 93.9%, as compared with 98.4% in HIV-negative patients.12 In a setting of low TB prevalence, the overall agreement for detecting MTB was 89% when compared to culture; the sensitivity was 98% for smear-positive and 72% for smear-negative specimens, respectively, and the specificity was excellent.13 There was no significant difference in sensitivity between tests on untreated sputum and those on concentrated sediments.12 In the same study, the MTB/RIF test correctly detected RIF resistance in 209 of 211 patients (99.1% sensitivity) and in all 506 patients with RIF susceptibility (100% specificity), when sequencing results were taken into account.12 The authors found that performance for both case detection, and discrimination of RIF resistance, was similar across diverse sites, suggesting that the findings are likely to be widely applicable.12 Furthermore, concentrated sediments of respiratory specimens other than sputum, such as broncho-alveolar lavage and bronchial secretions, were tested with comparable sensitivity and specificity.13 Data on the assay’s performance when testing extra-pulmonary TB is emerging.15
Global TB control urgently needs effective tools that permit rapid diagnosis of new TB cases and detection of RIF-resistant MTB in universal-access point-of-care settings.3 Current nucleic acid amplification methods used to detect MTB are complex, labor-intensive, and technically demanding. The Xpert MTB/RIF assay has the potential to bring standardized, sensitive and very specific diagnostic testing for both TB and drug resistance to these settings, provided that they will be able to afford it. In order to facilitate access, the Foundation for Innovative New Diagnostics (FIND) has negotiated significant price reductions for the Xpert MTB/RIF test. Current FIND-negotiated prices, along with the list of countries eligible for the discounts, are available on the web.10
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge W. J. Looney for carefully reviewing the manuscript.
Materials
| Reagent/Equipment | Company | Catalogue number |
| Xpert MTB/RIF test | Cepheid | GXMTB/RIF-10 |
| Transfer pipettes | Sarstedt | 86.1172.001 |
| Conical tubes, 15 ml, 120 x 17 mm, PS | Sarstedt | 62.553.542 |
Referencias
- Sudre, P., Dam, t. e. n., Chan, G., C, A., Kochi, Tuberculosis in the present time: a global overview of the tuberculosis situation. World Health Organisation Document WHO/TUB/91. 158, 1-47 (1991).
- World Health Organisation. Global tuberculosis control: epidemiology, strategy, financing. WHO report 2010. , (2010).
- . . The global plan to stop TB, 2006-2015. , (2006).
- Morris, S., et al. Molecular mechanisms of multiple drug resistance in clinical isolates of Mycobacterium tuberculosis. J. Infect. Dis. 171, 954-960 (1995).
- Traore, H., Fissette, K., Bastian, I., Devleeschouwer, M., Portaels, F. Detection of rifampicin resistance in Mycobacterium tuberculosis isolates from diverse countries by a commercial line probe assay as aninitial indicator of multidrug resistance. Int. J. Tuberc. Lung Dis. 4, 481-484 (2000).
- Helb, D., et al. Rapid Detection of Mycobacterium tuberculosis and Rifampin Resistance by Use of On-Demand Near-Patient Technology. J. Clin. Microbiol. 48, 229-237 (2010).
- Blakemore, R., et al. Evaluation of the Analytical Performance of the Xpert MTB/RIF. Assay. J. Clin. Microbiol. 48, 2495-2501 (2010).
- Telenti, A., et al. Detection of rifampicin-resistance in Mycobacterium tuberculosis. Lancet. 341, 647-650 (1993).
- . . GeneXpert Dx System Operator Manual. , (2011).
- . . International Standards for Tuberculosis Care (ISTC). , (2009).
- Boehme, C. C., et al. Rapid Molecular Detection of Tuberculosis and Rifampin Resistance. N. Engl. J. Med. 363, 1005-1015 (2010).
- Marlowe, E. M., et al. Evaluation of the Cepheid Xpert MTB/RIF assay for the Direct Detection of Mycobacterium tuberculosis Complex from Respiratory Specimens. J. Clin. Microbiol. 49, 1621-1623 (2011).
- El-Hajj, H. H., Marras, S. A. M., Tyagi, S., Kramer, F. R., Alland, D. Detection of rifampin resistance in Mycobacterium tuberculosis in a single tube with molecular beacons. J. Clin. Microbiol. 39, 4131-4137 (2001).
- Hillemann, D., Ruesch-Gerdes, S., Boehme, C., Richter, E. Rapid molecular detection of extrapulmonary tuberculosis by automated GeneXpert MTB/RIF system. J. Clin. Microbiol. 49, 1202-1205 (2011).

