Experimental Methods for Testing the Effects of Neurotrophic Peptide, ADNF-9, Against Alcohol-induced Apoptosis during Pregnancy in C57BL/6 Mice
Summary
The experimental designs proposed here focus on studying the effects of alcohol exposure in apoptosis and the application of neurotrophic peptide during pregnancy in fetal brain. A detailed description from the breeding to the collection of fetal brains is described. Techniques for determination of apoptosis are also described in detail.
Abstract
Experimental designs for investigating the effects of prenatal alcohol exposure during early embryonic stages in fetal brain growth are challenging. This is mostly due to the difficulty of microdissection of fetal brains and their sectioning for determination of apoptotic cells caused by prenatal exposure to alcohol. The experiments described here provide visualized techniques from mice breeding to the identification of cell death in fetal brain tissue. This study used C57BL/6 mice as the animal model for studying fetal alcohol exposure and the role of trophic peptide against alcohol-induced apoptosis. The breeding consists of a 2-hr matting window to determine the exact stage of embryonic age. An established fetal alcohol exposure model has been used in this study to determine the effects of prenatal alcohol exposure in fetal brains. This involves free access to alcohol or pair-fed liquid diets as the sole source of nutrients for the pregnant mice.
The techniques involving dissection of fetuses and microdissection of fetal brains are described carefully, since the latter can be challenging. Microdissection requires a stereomicroscope and ultra-fine forceps. Step-by-step procedures for dissecting the fetal brains are provided visually. The fetal brains are dissected from the base of the primordium olfactory bulb to the base of the metencephalon.
For investigating apoptosis, fetal brains are first embedded in gelatin using a peel-away mold to facilitate their sectioning with a vibratome apparatus. Fetal brains embedded and fixed in paraformaldehyde are easily sectioned, and the free floating sections can be mounted in superfrost plus slides for determination of apoptosis or cell death.
TUNEL (TdT-mediated dUTP Nick End Labeling; TdT: terminal deoxynucleotidyl transferase) assay has been used to identify cell death or apoptotic cells. It is noteworthy that apoptosis and cell-mediated cytotoxicity are characterized by DNA fragmentation. Thus, the visualized TUNEL-positive cells are indicative of cell death or apoptotic cells.
The experimental designs here provide information about the use of an established liquid diet for studying the effects of alcohol and the role of neurotrophic peptides during pregnancy in fetal brains. This involves breeding and feeding pregnant mice, microdissecting fetal brains, and determining apoptosis. Together, these visual and textual techniques might be a source for investigating prenatal exposure of harmful agents in fetal brains.
Introduction
The goal of the experimental methods described here is to assess the neuroprotective effects of trophic peptides in a fetal alcohol exposure model using several techniques involving breeding, feeding, microdissecting, and detecting cell death. Work from our laboratory has involved a liquid diet mixed with alcohol as a model of moderate alcohol drinking, which might be similar to a human drinking paradigm in term of the amount of alcohol consumed 1-5. We and others have identified three peptide derivatives that are neuroprotective against the deleterious effects of fetal alcohol exposure. Studies investigating alcohol exposure during embryonic stages using animal models may lead to the identification of potential mechanisms of neuroprotection and allow for the development of intervention procedures. This may provide ample information about the neuroprotective effects and attenuation of the deleterious effects of alcohol exposure during pregnancy.
Possible prevention of the effects of prenatal alcohol exposure may involve the treatment of pregnant mice with peptides that have been shown to be involved in neuroprotection in vitro 6,7 and in vivo 1,2,4,8,9. Among these peptides, SALLRSIPA, known as ADNF-9 or SAL, is derived from activity-dependent neurotrophic factor (ADNF) 7,10. Another peptide with the sequence NAPVSIPQ peptide, termed NAP, derived from activity-dependent neuroprotective protein (ADNP) 6,11, demonstrated a potent protective effect against oxidative stress associated with alcohol exposure 12,13. In addition, we have recently identified a new synthesized peptide, colivelin that appears to play a key neuroprotective role in the fetal alcohol exposure model 2. Colivelin is composed of ADNF-9 and AGA-(C8R)HNG17 (PAGASRLLLLTGEIDLP). The importance of the use of these peptides is that they have the capability to cross the brain-blood barrier to prevent the effects of alcohol-induced apoptosis and brain growth deficits.
The experimental methods used C57BL/6 mice for testing the neuroprotective effects against alcohol-induced apoptosis. We have adopted a 2-hr window instead of overnight breeding in order to accurately estimate the embryonic day 0 (E0), as it can be revealed by detection of a sperm plug and vaginal smear 1-5. We have used a liquid diet with feeding tubes, as this is considered free access instead of delivery of alcohol through gavage route, which may induce stress to pregnant mice. On the other hand, microdissection can be challenging due to the softness of the fetal brain tissue, which might be encountered when dissecting fetal brains at early embryonic stages. Here, we show visualized techniques to deal with all the challenges involving microdissection. Furthermore, since the fetal brain sectioning can also be challenging, we have adopted a technique that involves embedding the fetal brains in gelatin using peel-away embedding molds. We have been successful in cutting the fetal brains in free floating sections at 50 μm thickness. This allows us to investigate any alterations of content protein in fetal brain sections, including the identification of cell death.
Protocol
1. Animals Breeding and Trophic Peptide Treatment
- Breed C57BL/mice by placing the female mice (~6 week old, average weight 20 g) into male home cages for 2 hr.
- Check for a sperm plug and/or vaginal smear immediately afterwards.
- If positive, designate this time point as embryonic day 0 (E0).
- On E7, weight-match pregnant females to control and treatment groups (3 groups) are assigned as described recently 4: 1) Alcohol (ethanol) liquid diet group (ALC); 2) pair-fed control group (PF); 3) Peptide treatment group, which should receive intraperitoneal (i.p.) injection of ADNF-9 peptide alongside alcohol exposure liquid diet (ALC/ADNF-9). The details of the preparation of ALC and PF diets and the procedure of i.p. injections can be found in our recent work 4.
- Measure daily alcohol liquid diet that can be provided as free access as the sole source of nutrients. PF liquid diet yoked individually to an ALC dam can be measured daily as well.
- The volume of liquid diet consumed during the previous 24 hr can be recorded from 30-ml graduated screw-cap tubes and freshly prepared diet should be provided daily from E7 to E13.
2. Dissection of Fetuses and Microdissection of Fetal Brains
- Sacrifice pregnant mice on E13 by deeply euthanizing the mice with a CO2 procedure followed by a cervical dislocation.
- Clean the abdominal site of incision with 70% ethanol.
- Make an abdominal incision using sterile scissors and forceps.
- Once the abdomen is open, dissect out the uterus by holding it with one forceps and using the other forceps to tear the mesometrium away from the uterus. Make sure to do this procedure slowly in order to avoid puncturing the embryos.
- Remove the embryos from the uterus and transfer them into a cell culture dish (60 mm x 15 mm) containing Hanks’ Balanced Salt solution (HBSS) in ice cold water.
- Dissect fetal brains from the whole embryos under stereomicroscope for microdissection.
- Using ultra-fine forceps for microdissection, pull off the skin of the embryos at the top part of the head to expose the skull.
- Once the skull is exposed and clearly visualized under the microdissection stereomicroscope, then peel off the skull starting from the posterior part of the brain at the spinal cord and brainstem areas.
- Be sure to peel off the skull piece by piece from posterior to anterior parts of the head.
- Once fetal brains are visually exposed, proceed with extracting them.
- Dissect the fetal brains from the base of the primordium olfactory bulb to the base of the metencephalon.
- Postfix all dissected fetal brains in 4% paraformaldehyde for 2-3 days. You can also freeze other fetal brains from each dam if you are testing them for Western blot or enzymatic assays.
Note: The procedures for animal uses were approved by the Institutional Animal Care and Use Committee of University of Toledo which are in accordance with the guidelines of the Institutional Animal Care and Use Committee at the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals.
3. Embedding Fetal Brains in Gelatin for Sectioning
- Prepare 10% of gelatin grade A.
- Fill a peel-away embedding mold halfway and wait until the gelatin solidifies.
- Place control and prenatally treated fetal brains side-by-side on top of the solidified gelatin. This is to keep consistent conditions of immunodetection of cell death between both the control and experimental groups.
- Add liquid gelatin on top of the fetal brains to make a complete mold (make sure the gelatin is not too hot).
- Refrigerate the prepared gelatin mold that holds fetal brains for 15 min.
- Peel off the embedding mold plastic and then transfer the pre-made gelatin containing fetal brains into 4% paraformaldehyde for two days.
- Place and section fixed fetal brains in gelatin using vibratome sectioning machine (Leica VT 1000S) at 50 μm thickness for coronal sectioning. This thickness is tested in our studies because it provides better anatomical features. In addition, thicknesses of 25 μm and higher can also be tested with this technique involving embedded of fetal brains in 10% gelatin. Note that coronal sections were cut using vibratome at the basal ganglia eminence level on the forebrain
- Collect the fetal brain sections in phosphate-buffered saline (PBS) solution.
- Mount the fetal brain sections in Superfrost Plus slides in water.
- Dry the mounted sections for 15 min.
- Place the slides (mounted with the fetal brain sections) in PBS for next day testing of TUNEL reaction.
4. TUNEL Reaction for Detection of Cell Death or Apoptosis
(TUNEL: TdT-mediated dUTP Nick End Labeling; TdT: terminal deoxynucleotidyl transferase). This assay is aimed to determine cell death.
- Treat fetal brain sections mounted in Superfrost Plus slides with Proteinase K (20 μg/ml) for 5 min at 37 °C (300 μl of proteinase K mixed in 20 ml PBS). Slides are assembled in 5-Plend open maile).
- Rinse fetal brain sections (mounted in slides) with PBS three times for 5 min under Orbital shaker.
- Incubate the sections with 3% H2O2 in methanol for 10 min at room temperature (3 ml 30% H2O2 in 27 ml 100% methanol) (slides should not be in a shaker).
- Rinse sections with PBS three times for 5 min in orbital shaker.
- Incubate sections in permeabilization solution (0.1% TritonX-100 in 0.1% sodium citrate) for 2 min on ice (4 °C) (100 μl TritonX + 0.1 g sodium citrate + 99.9 ml distilled water).
- Rinse sections in PBS two times for 5 min in orbital shaker.
- Dry area around sections in the slides.
- Draw barrier on Superfrost Plus slides using PAP Pen. This is aimed to prevent any leaking of solution that is used to treat the sections for the detection of TUNEL-positive cells.
- Incubate the sections with TUNEL reaction mixture (50 μl from bottle 1 and 450 μl from bottle 2) for 1 hr at 37 °C, the control will be used by incubation in solution from bottle 2.
- Rinse with PBS three times 5 min under Orbit shaker.
- Dry area around tissue.
- Incubate sections with converter-POD for 30 min at 37 °C.
- Rinse the sections three times for 5 min with Tris HCl (pH 7.5, 0.05 M).
- Incubate the sections for 7 min in 0.05% 3′-3′-diaminobenzidine tetrahydrochloride (DAB) and 0.003% H2O2 (25 ml Tris HCl, pH7.5, 0.05 M + 25 μl 3% H202 + 500 μl DAB) under Oribtal shaker at 4 °C (in ice) and in dark (make sure to do this procedure in new 5-Plend open mailer).
- Rinse the sections with PBS three times 5 min under Orbital shaker.
- Keep the slides sections in PBS for no more than 24 hr in PBS, then process them for Nissl staining as detailed in the next section protocol.
Note: DAB is a carcinogen. Use gloves and clean all dishes used with the bleach once finished with all the experiments.
5. Nissl staining for Preparation of Fetal Brain Sections for Microscopic Observation
- Nissl staining procedure should be done in a biological safety hood.
- Rinse the slide sections with deionized water for 2 min.
- Incubate the sections in 70% ethanol for 2 min.
- Incubate the sections in 95% ethanol for 6 min.
- Incubate the sections in 70% ethanol for 2 min.
- Rinse the sections in deionized water for 2 min.
- Incubate the sections in Cresyl Violet Stain for 3 min.
- Rinse the sections in deionized water for 2 min.
- Incubate the sections in 70% ethanol for 2 min.
- Incubate the sections in 95% ethanol + 3 ml glacial acetic acid for 3 min.
- Incubate the sections in 95% ethanol for 2 min.
- Incubate the sections in 100% ethanol for 2 min three times.
- Incubate the sections in Xylene for 10 min three times.
- Slides holding the fetal brain sections can be mounted using Permount and cover slip glass slides.
- Leave the mounted slides overnight prior to microscopic observation and analyses.
- Microscopic observation and photomicrographs can be performed under Leica upright microscope.
Representative Results
The experimental methods described here show that a 2-hr breeding window is essential to estimate accurate gestational stage. We have demonstrated the dissection of the embryos at the age of E13. We also demonstrated microdissection of fetal brains at E13. The procedure involves several stages (a-c), as described in Figure 1. Fetal brains are dissected from the base of the primordium olfactory bulb to the base of the metencephalon (Figure 1). Fetal brains are then fixed in 4% paraformaldehyde for immunochemical detection. After fixation, fetal brains are embedded in 10% gelatin and prepared for vibratome sectioning as described in Figure 2. Note that step-by-step embedding of fetal brains was performed at lower magnification. Fetal brains embedded can be visualized at higher magnification in Figure 1. Moreover, coronal fetal brain sections at the level of ganglionic eminence on the forebrain can be obtained using the vibratome and mounted in slides for immunohistochemical detection. This involves the number of TUNEL-positive cells (Figure 3). We show here that prenatal alcohol exposure induced increases in TUNEL-positive cells in ganglionic eminence (Figure 3b) compared to PF control group (Figure 3a). ADNF-9 administration significantly reduced alcohol-induced increase in TUNEL-positive cells (Figure 3c and 3d) compared to ALC group.
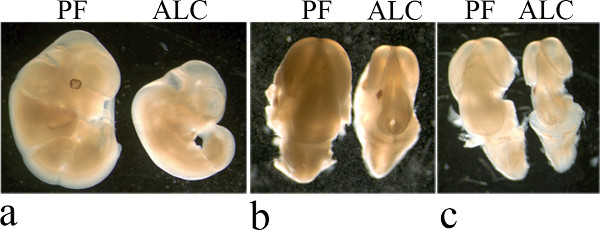
Figure 1. Microdissection of fetal brains at E13. Stages a-c show step-by-step procedures from embryos to dissected fetal brains. a) Dissected control (PF) and alcohol (ALC) exposed prenatally embryos. b) skin peeled off to expose the fetal brains of PF and ALC groups. c) Dissected fetal brains of PF and ALC groups.

Figure 2. Method for embedding fetal brains for sectioning. Stages (a-e) show step-by-step procedures from the preparation of the embedding of fetal brains to the setting of the gelatin block in the object for vibratome cutting. a) Peel-away embedding mold filled halfway with gelatin; b) Fetal brains from PF control and ALC groups are placed side-by-side in the mold and covered with gelatin; c) Peel-away embedding mold cut at four sides; d) fetal brains embedded in gelatin and ready to be fixed in paraformaldehyde; e) gelatin mold containing fetal brains are placed in the object for vibratome sectioning.
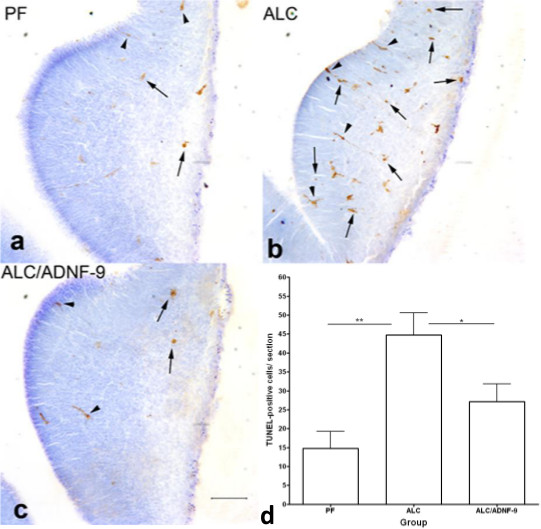
Figure 3. Effects of neurotrophic peptide, ADNF-9, against alcohol-induced apoptosis using TUNEL assay in ganglionic eminence at E13 stage. Prenatal alcohol exposure induced increases in cell death as indicated by arrowheads in ALC group (b) compared to PF group (a). ADNF-9 administration alongside prenatal alcohol exposure showed decrease in TUNEL-positive cells (c). Statistical analyses demonstrated that ADNF-9 administration prevented alcohol-induced increases in TUNEL-positive cells (d). Values are shown as means ± SEM. *p<0.05; **p<0.01 (Newman-Keul’s post hoc test). N= 4 for each group. Scale bar= 100 μm. Reprinted from publication (4), with permission from Elsevier (license# 2904310752995).
Discussion
The methodology and technology presented in this study demonstrate the effects of prenatal exposure to alcohol in fetal brains and the role of trophic peptides in the prevention of these effects. These may provide information on how to study other drugs of abuse or other toxic chemicals in fetal brains during different pregnancy stages.
In regards to the breeding paradigm, in some cases the detection of the sperm plug might not be observable. At this point, it is important to test the vaginal smear in a slide under microscopic observation, which can provide possible sperm positive detection.
It is stated in this study that the feeding involves free access of liquid diet containing PF control and alcohol. At least 30% of humidity and a temperature not exceeding 22-24 °C are required to monitor the quality of the diet during the 24-hr free access to the diet 1,2. It is also required that the diet be prepared fresh daily. The feeding tubes should be cleaned after each use.
There are difficulties in microdissection of fetal brains. These depend on the embryonic stage tested. For example, fetal brains dissected at age of E13 can be difficult to deal with compared to fetal brains dissected at E18. This is due in the fact that at E18, the fetal brains are well developed and the skull becomes easy to pull off compared to E13, when the skull is still soft and difficult to pull off. At this point, when dissecting fetal brains at E13, it is important to pull off the skull piece-by-piece as demonstrated in the video supplied with this manuscript.
In regards to embedding fetal brains in gelatin, we found that this is the best technique to obtain sections that are ready to be used for immunohistochemical analysis. In order to have better cutting of the fetal brain, it is important that the fixation in 4% paraformaldehyde of the gelatin block containing fetal brains is complete. Thus, this may take at least two days, and the gelatin block might be ready for cutting by the third day. The thickness of the sections is also a factor; 50 μm thickness is usually tested for immunochemical detection such as TUNEL assay 1,2,4. However, thicknesses of 25 μm and higher can also be tested.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This research project was supported by Award Number R21AA017735 (Y.S.) from the National Institutes on Alcohol Abuse and Alcoholism. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health. The author would also like to thank Charisse Montgomery for editing this manuscript.
Materials
| Name of the reagent/equipment | Company | Catalogue number | Comments (optional) |
| Superfrost Plus Slides | VWR | 48311-703 | |
| PAP PEN | Research Products Int. Corp. | 195506 | |
| 5-Plend Open Mailer | Heathrow Scientific, LLC | HS 15983G | |
| Peel away embedding molds | Electron Microscopy Sciences | 70182 | |
| In situ cell death detection kit, POD | Roche Diagnostics, Inc | 11684817910 | |
| Hanks’ Balanced Salt Solution | Invitrogen | 14170-120 | |
| Gelatin Type A | FisherScientific | G8-500 | |
| Stereomicroscope | W. NUHSBAUM, Inc | Leica M60, KL 200 LED | |
| Micro-Vibratome | Leica, Inc | Leica VT 1000S | |
| Moria Ultra Fine Forceps | Fine Science Tools | 11370-40 | 2 pairs |
| Graduate 30 ml feeding tubes | Dyets, Inc | 900012 | |
| Vitamin Mix | Bio-Serv. Inc. | F8031 | |
| Mineral Mix | Bio-Serv. Inc. | F8598 | |
| Maltose Dextrin | Bio-Serv. Inc. | 3650 | |
| Ethyl alcohol 190 Proof, 5 gallons | 111000190-SS05 | Pharmco-AAPER | |
| Upright microscope | W. NUHSBAUM, Inc | LEICA DM 4000 |
Referencias
- Sari, Y. Activity-dependent neuroprotective protein-derived peptide, NAP, preventing alcohol-induced apoptosis in fetal brain of C57BL/6 mouse. Neurociencias. 158 (4), 1426-1435 (2009).
- Sari, Y., Chiba, T., Yamada, M., Rebec, G. V., Aiso, S. A novel peptide, colivelin, prevents alcohol-induced apoptosis in fetal brain of C57BL/6 mice: signaling pathway investigations. Neurociencias. 164 (4), 1653-1664 (2009).
- Sari, Y., Hammad, L. A., Saleh, M. M., Rebec, G. V., Mechref, Y. Alteration of selective neurotransmitters in fetal brains of prenatally alcohol-treated C57BL/6 mice: quantitative analysis using liquid chromatography/tandem mass spectrometry. Int. J. Dev. Neurosci. 28 (3), 263-269 (2010).
- Sari, Y., Weedman, J. M., Ge, S. Activity-dependent neurotrophic factor-derived peptide prevents alcohol-induced apoptosis, in part, through Bcl2 and c-Jun N-terminal kinase signaling pathways in fetal brain of C57BL/6 mouse. Neurociencias. 202, 465-473 (2012).
- Sari, Y., Zhang, M., Mechref, Y. Differential expression of proteins in fetal brains of alcohol-treated prenatally C57BL/6 mice: a proteomic investigation. Electrophoresis. 31, 1-14 (2010).
- Bassan, M., Zamostiano, R., Davidson, A., Pinhasov, A., Giladi, E., Perl, O., Bassan, H., Blat, C., Gibney, G., Glazner, G., Brenneman, D. E., Gozes, I. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J. Neurochem. 72 (3), 1283-1293 (1999).
- Brenneman, D. E., Hauser, J., Neale, E., Rubinraut, S., Fridkin, M., Davidson, A., Gozes, I. Activity-dependent neurotrophic factor: structure-activity relationships of femtomolar-acting peptides. J. Pharmacol. Exp. Ther. 285 (2), 619-627 (1998).
- Spong, C. Y., Abebe, D. T., Gozes, I., Brenneman, D. E., Hill, J. M. Prevention of fetal demise and growth restriction in a mouse model of fetal alcohol syndrome. J. Pharmacol. Exp. Ther. 297 (2), 774-779 (2001).
- Sari, Y., Gozes, I. Brain deficits associated with fetal alcohol exposure may be protected, in part, by peptides derived from activity-dependent neurotrophic factor and activity-dependent neuroprotective protein. Brain Res. Brain Res. Rev. 52 (1), 107-118 (2006).
- Brenneman, D. E., Gozes, I. A femtomolar-acting neuroprotective peptide. J. Clin. Invest. 97 (10), 2299-2307 (1996).
- Zamostiano, R., Pinhasov, A., Gelber, E., Steingart, R. A., Seroussi, E., Giladi, E., Bassan, M., Wollman, Y., Eyre, H. J., Mulley, J. C., Brenneman, D. E., Gozes, I. Cloning and characterization of the human activity-dependent neuroprotective protein. J. Biol. Chem. 276 (1), 708-714 (2001).
- Offen, D., Sherki, Y., Melamed, E., Fridkin, M., Brenneman, D. E., Gozes, I. Vasoactive intestinal peptide (VIP) prevents neurotoxicity in neuronal cultures: relevance to neuroprotection in Parkinson’s disease. Brain Res. 854 (1-2), 257-262 (2000).
- Steingart, R. A., Solomon, B., Brenneman, D. E., Fridkin, M., Gozes, I. VIP and peptides related to activity-dependent neurotrophic factor protect PC12 cells against oxidative stress. J. Mol. Neurosci. 15 (3), 137-145 (2000).

