An Orthotopic Mouse Model of Anaplastic Thyroid Carcinoma
Summary
Generation of an orthotopic mouse model of anaplastic thyroid carcinoma is described here. This technique employs surgical placement of human anaplastic thyroid cancer cells into the thyroid of immunodeficient mice, thus creating a more clinically relevant setting to study disease progression as well as screen innovative therapeutic interventions.
Abstract
Several types of animal models of human thyroid carcinomas have been established, including subcutaneous xenograft and orthotopic implantation of cancer cells into immunodeficient mice. Subcutaneous xenograft models have been valuable for preclinical screening and evaluation of new therapeutic treatments. There are a number of advantages to using a subcutaneous model; 1) rapid, 2) reproducible, and 3) tumor establishment, growth, and response to therapeutic agents may be monitored by visual inspection. However, substantial evidence has shed light on the short-comings of subcutaneous xenograft models1-3. For instance, medicinal treatments demonstrating curative properties in subcutaneous xenograft models often have no notable impact on the human disease. The microenvironment of the site of xenographic transplantation or injection lies at the heart of this dissimilarity.
Orthotopic tumor xenograft models provide a more biologically relevant context in which to study the disease. The advantages of implanting diseased cells or tissue into their anatomical origin equivalent within a host animal includes a suitable site for tumor-host interactions, development of disease-related metastases and the ability to examine site-specific influence on investigational therapeutic remedies. Therefore, orthotopic xenograft models harbor far more clinical value because they closely reproduce human disease. For these reasons, a number of groups have taken advantage of an orthotopic thyroid cancer model as a research tool4-7.
Here, we describe an approach that establishes an orthotopic model for the study of anaplastic thyroid carcinoma (ATC), which is highly invasive, resists treatment, and is virtually fatal in all diagnosed patients. Cultured ATC cells are prepared as a dissociated cellular suspension in a solution containing a basement membrane matrix. A small volume is slowly injected into the right thyroid gland. Overall appearance and health of the mice are monitored to ensure minimal post-operative complications and to gauge pathological penetrance of the cancer. Mice are sacrificed at 4 weeks, and tissue is collected for histological analysis. Animals may be taken at later time-points to examine more advance progression of the disease. Production of this orthotopic mouse model establishes a platform that accomplishes two objectives: 1) further our understanding of ATC pathology, and 2) screen current and future therapeutic agents for efficacy in combating ATC.
Protocol
1. Cell Preparation
Notes: 1) complete RPMI 1640 based culture media recipe (500 ml) includes 435 ml RPMI 1640, 50 ml heat inactivated FBS, 5 ml non-essential amino acids, 5 ml sodium pyruvate, 5 ml antibiotic-antimycotic; 2) place matrigel at 4 °C one day prior to cell harvest.
- Human ATC cell line THJ-11T8 are grown in 6 well culture plates and harvested once 80-90% confluent.
- Pellet cells by centrifugation at 200 x g for 4 min at room temperature.
- Aspirate media and resuspend cells in RPMI 1640 media [2 ml/6 well plate].
- Take a small volume of the cell suspension, add it to one volume of 0.4% trypan blue dye and determine cell density using TC10 Automated Cell Counter.
- Calculate volume of cell suspension needed to get 5 x 105 cells/mouse times the number mice injecting.
Note: To ensure adequate cell suspension for injection, prepare cells as though you are injecting double the number of mice (e.g. if injecting 5 mice, then prepare cell suspension as though you were injecting 10 mice).
- Transfer needed volume of cell suspension into a 15 ml conical tube.
- Pellet cells by centrifugation at 200 x g for 4 min at room temperature.
- Aspirate media and resuspend cells in complete RPMI 1640 media.
- Transfer cells to a 1.6 ml tube, add 1 volume of matrigel and mix gently by slowly pipetting in and out.
Note: Each mouse will receive 10 μl of the cell suspension/matrigel cocktail. Since 5 x 105 cells suspended in a 10 μl injectable volume have been successfully used in previous ATC orthotopic studies9-10, we used those parameters in this report.
- Place cell suspension on ice until ready to use.
2. Mouse Preparation
- Clean the surgical zone, which includes the dissecting scope and surrounding area, by wiping all surfaces with 70% ethanol.
- Turn on heating lamps/pads where animals will be recovering from surgery.
- Administer 0.1 ml ketamine/xylazine (drug cocktail consisting of ketamine 9 mg/ml and xylazine 1mg/ml) per 10 g body weight intraperitoneally using a 1 ml, 27G ½” Tuberculin syringe.
- Prep mouse for surgery: 1) shave neck region of mouse (from jaw line to top of sternum and out to arms), 2) scrub surgical area three times with chorhexadine soaked gauze squares, 3) final scrub done with betadine soaked gauze, 4) gently apply eye ointment to prevent eyes from drying.
- Check pedal reflex to ensure mouse is adequately sedated.
- Place animal in dorsal recumbency on a disposable sterile field barrier under the dissecting scope and secure the animal in place with cloth tape.
- Scrubs hands and fingernails then put on sterile surgical gloves.
- In a sterile fashion, open surgery pack and arrange instruments.
- Place sterile drape over animal, leaving only surgical site exposed.
3. Surgical Access to Thyroid and Cell Injection
- Using a sterile, disposable scalpel, make a 1-to-1.5 cm longitudinal incision along the midline of the throat.
Note: Deviation from the midline complicates deeper cutting to access the trachea. Fairly precise midline incision allows you to tease and cut through the membranous tissue holding left and right salivary glands together and reduces chance of nicking or severing large arteries.
- Make a second incision into the strap muscles surrounding the trachea then pull the right side of the incised muscle to the side to expose the right thyroid gland.
- Hemostats can be used at this point to hold the muscle layer apart for easy access to the thyroid gland (Figure 1).
Note: Alternatively, if procedure performed by two surgical staff, the muscle layer may simply be held back with forceps while a ready injectable syringe is handed to the surgeon by the assisting staff.
- Slowly inject 10 μl of cell suspension into the right thyroid gland using a 31G 5/16″ insulin syringe then gently remove needle.
4. Closure of Surgical Site and Mouse Recovery
- Suture muscle layer with 6.0 suturing material using either a continuous or interrupted suturing style.
- Stitch the skin in the same fashion.
- Apply a layer of triple antibiotic ointment directly over incision site.
- Allow animal to recover in dorsal recumbency on warm heating pad. Once animal can achieve sternal recumbency without assistance, it can be placed back with other mice.
5. Post-operative Monitoring and Tissue Collection
- Check the incision site and overall health of the animal daily for a week following surgery, then check once a week. If the sutures are still intact after 14 days, they are removed.
Note: Animals exhibiting signs of declining health (e.g. substantial weight loss, scruffy hair, labored breathing) should be euthanized and tissues collected.
- Once mice have reached a predetermined post-operative time-point, administer ketamine/xylazine as describe above to sedate.
- Perfuse animal intracardially and harvest thyroid/trachea and other tissues of interest (not described in detail here).
Representative Results
We detected 19 invasive thyroid tumors from a total of 20 mice injected with ATC cells after 4 weeks. In the example shown in Figure 2A, mice receiving an orthotopic injection of 5X105 ATC cells grown under attached conditions, exhibit significant infiltration of cancer cells into the thyroid by 4 weeks post-injection. The nature of the invading ATC cells with a characteristic spindled cells and medium- to giant-sized cells with eosinophilic cytoplasm and large nuclei was verified by hematoxylin and eosin (H&E) staining. For comparison, the thyroid and trachea from a non-injected control animal is shown in Figure 2B. Follicles of the uninjected animal display a close, yet loose, association and have essentially a round morphology.
Due to the small size of the thyroid and use of injectable delivery, unintentional outcomes may arise. Figure 3A illustrates the most commonly observed, which is development of a tumor mass outside of, but not encompassing, the thyroid. This is more than likely due to needle penetration through and beyond the thyroid when cells are expelled. Occasionally, mice presenting with no tumor growth, both grossly and histologically, are detected as shown in Figure 3B. Absence of detectable tumor development can be caused by expulsion of the cell suspension from the injection site and dissemination into neighboring tissues and cavities.
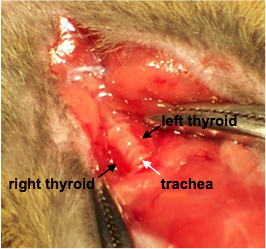
Figure 1. Exposing the thyroid. Labels indicate the right and left thyroid glands flanking the trachea.
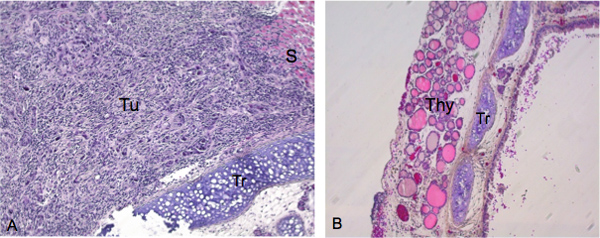
Figure 2. Histological examination of tumor growth and invasion after successful thyroid injection. Tissue specimens were sectioned along the coronal plane. A) Image taken at 200X of mouse exhibiting substantial tumor growth with characteristic spindled cells and medium- to giant- sized cells with eosinophilic cytoplasm and large nuclei in hematoxylin and eosin (H&E) stain. Tu indicates the primary lesion from which tumor cells invade the thyroid. B) Trachea and thyroid from control animal (image taken at 100X). Tu ,Tumors; S, smooth muscle; Tr, trachea; Thy, thyroid gland.
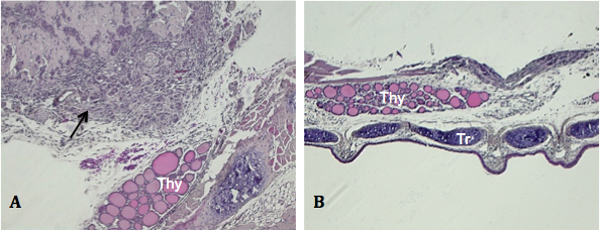
Figure 3. Unintentional outcomes of orthotopic injection. Tissue specimens were sectioned along the coronal plane. A) Peripheral tumor growth with no growth evident in the thyroid (black arrow, image taken at 100X). B) Lack of tumor detection upon gross (not shown) or histological examination (image taken at 50X). Thy, thyroid gland.
Discussion
In our model, animals demonstrated thyroid tumor metastasis and disease related cachexia and respiratory distress by 4 weeks post-injection. As with most other orthotopic models utilizing injectable delivery of a cell suspension, injection into surrounding tissues and post-injection leakage that spreads beyond the target are possible. With that being said, one recognizable potential unknown produced by this procedure is the effect of off target exposure to non-disease related metastases or complications. This is a general concern with most xenographic transplants, regardless of the disease being investigated. Nonetheless, unintentional consequences from metastasis may be significantly minimized by ensuring the entire bevel of the needle tip has penetrated the membrane snugly overlaying the thyroid gland and slowly expelling the cellular suspension. If leakage is observed during or after injection, those animals may be omitted from the study.
It is essential that the animals are perfused prior to harvesting the thyroid/trachea and other tissues. The benefits are two-fold, 1) red blood cells are purged and will not clutter histological specimens and 2) tissue morphology and integrity are preserved. Furthermore, resected thyroid/trachea that have either a tumorous mass attached or are entombed within it should be cut in a way that anatomical landmarks can be identified before being embedded for histological preparation. This will dramatically reduce specimen preparation time.
In this report we describe local growth and invasion of advanced thyroid tumor following orthotopic injection of ATC cells. ATC metastasis may be investigated by analyzing histological preparations of other tissues using standard histological stains, such as hematoxylin and eosin, or by immunohistochemistry utilizing cell or pathology relevant markers. This system can be further developed by genetically modifying thyroid cancer cells with fluorescence reporters so that live imaging may be used to monitor metastasis and the efficacy of therapeutic treatments.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Drs. Wenjun Li and Daniel Kreisel (Washington University School of Medicine in St. Louis) for their assistance in surgery training. R.Y.L. is supported by the National Institutes of Health Grant R01 DK068057 and the President’s Research Fund of Saint Louis University.
Materials
| Name of Reagent/Material | Company | Catalogue Number | Comments |
| RPMI 1640 cell culture media | Mediatech | 10-041-CV | Media and additives used depend on cell line |
| TC10 Automated Cell Counter | Bio-Rad | 145-0001 | |
| Matrigel | Becton, Dickinson and Company | 354234 | |
| Graefe Forceps, Serrated; Slight Curve, 4″ | Roboz | RS-5135 | |
| Disposable scalpel, No. 15 | Feather | 2975#15 | |
| Olsen Hegar Needle Holder – 4 ½” delicate serrated | gSource | gS 21.5400 | |
| 6-0 nylon black monofilament suture | Surgical Specialties Corporation | 1279B | |
| Heating Pad, reusable, 8″ x 12″ | |||
| Cloth tape, 1″ X 10 yds | Medline | MIINON260101 | For restraining anesthetized mouse |
| Peanut Clipper/Trimmer | Wahl | 8655 | For removing hair from surgical site |
| ChlorHex-Q SCRUB 2% | Penn Veterinary Supply | VED1222 | |
| Betadine | Henry Schein Company | 6906727 | |
| Petrolatum ophthalmic ointment | Dechra Veterinary Products | 17033-211-38 | |
| 2 x 2 gauze, 12-ply | Butler Animal Health Supply | 6936 | |
| Sterile Field, Barrier, 18″ x 26″ | Busse | 696 | |
| Drapes (CSR Wrap) | Cardinal Health | AT 21 412 | |
| 27G ½” needle | Becton, Dickinson and Company | 309623 | |
| 31G 5/16″ needle | Becton, Dickinson and Company | 328438 | |
| Ketamine (9 mg/ml) / xylazine (1 mg/ml) solution | |||
| Triple antibiotic ointment |
Referencias
- Bibby, M. C. Orthotopic models of cancer for preclinical drug evaluation: advantages and disadvantages. Eur. J. Cancer. 40, 852-857 (2004).
- Hoffman, R. M. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest. New Drugs. 17, 343-359 (1999).
- Killion, J. J., Radinsky, R., Fidler, I. J. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev. 17, 279-284 (1998).
- Kim, S., et al. An orthotopic model of anaplastic thyroid carcinoma in athymic nude mice. Clin. Cancer Res. 11, 1713-1721 (2005).
- Nucera, C., et al. A novel orthotopic mouse model of human anaplastic thyroid carcinoma. Thyroid. 19, 1077-1084 (2009).
- Todaro, M., et al. Tumorigenic and metastatic activity of human thyroid cancer stem cells. Cancer Res. 70, 8874-8885 (2010).
- Tran Cao, H. S., et al. Real-time imaging of tumor progression in a fluorescent orthotopic mouse model of thyroid cancer. Anticancer Res. 30, 4415-4422 (2010).
- Marlow, L. A., et al. Detailed molecular fingerprinting of four new anaplastic thyroid carcinoma cell lines and their use for verification of RhoB as a molecular therapeutic target. J. Clin. Endocrinol. Metab. 95, 5338-5347 (2010).
- Nehs, M. A., et al. Late Intervention with anti-BRAF (V600E) therapy induces tumor regression in an orthotopic mouse model of human anaplastic thyroid cancer. Endocrinology. 153, 985-994 (2012).
- Nucera, C., et al. Targeting BRAFV600E with PLX4720 displays potent antimigratory and anti-invasive activity in preclinical models of human thyroid cancer. Oncologist. 16, 296-309 (2011).

