Oral Transmission of Listeria monocytogenes in Mice via Ingestion of Contaminated Food
Summary
This paper describes a novel method for oral infection of mice using Listeria monocytogenes-contaminated food. The protocol can readily be adapted for use with other food borne bacterial pathogens.
Abstract
L. monocytogenes are facultative intracellular bacterial pathogens that cause food borne infections in humans. Very little is known about the gastrointestinal phase of listeriosis due to the lack of a small animal model that closely mimics human disease. This paper describes a novel mouse model for oral transmission of L. monocytogenes. Using this model, mice fed L. monocytogenes-contaminated bread have a discrete phase of gastrointestinal infection, followed by varying degrees of systemic spread in susceptible (BALB/c/By/J) or resistant (C57BL/6) mouse strains. During the later stages of the infection, dissemination to the gall bladder and brain is observed. The food borne model of listeriosis is highly reproducible, does not require specialized skills, and can be used with a wide variety of bacterial isolates and laboratory mouse strains. As such, it is the ideal model to study both virulence strategies used by L. monocytogenes to promote intestinal colonization, as well as the host response to invasive food borne bacterial infection.
Introduction
Listeria monocytogenes are facultative intracellular bacterial pathogens that cause food borne infections in humans. The bacteria are resistant to many of the processes used to protect our food supply, such as drying, salting, or refrigeration 1,2 and infections are typically linked to processed, “ready-to-eat” foods that are not heated prior to consumption. In several previous outbreaks, the source of L. monocytogenes-contaminated food was identified, and the restricted group of exposed individuals was monitored closely 3-6. In those examples, clinical disease in otherwise healthy individuals varied from a mild, self-limiting gastroenteritis to more severe intestinal and systemic infections that required hospitalization. L. monocytogenes infections in immune compromised individuals, including both neonates and the elderly, have been associated with a high mortality rate (25-30%), even with antibiotic treatment 7,8. Little is known about the infectious dose for L. monocytogenes, or the host factors that govern susceptibility to infection after oral transmission, primarily due to the lack of a small animal model that recapitulates this wide range of infection outcomes.
The most widely used model of listeriosis is intravenous (i.v.) inoculation of mice. The i.v. model is highly reproducible, and has been extremely useful for studying both naïve and memory T cell responses during infection 9,10. The drawback of the i.v. model is that it completely bypasses the intestinal phase of infection. After food borne transmission, the gut mucosa provides a barrier that presumably slows and limits the number of bacteria that can continually disseminate to peripheral tissues. In contrast, the entire inoculum can be found in the spleen and liver within minutes of i.v. administration, and this large bolus of organisms may overwhelm innate immune defenses in these tissues. Oral infection of mice by gavage is less commonly used, because large doses (109-1011 CFU) are typically required to achieve intestinal colonization 10. Also, intragastric (i.g.) inoculation with a feeding needle does not generate a reproducible period of gastrointestinal infection prior to systemic spread. Some labs have reported that L. monocytogenes reach the spleen and liver within 4-12 hr post infection (hpi), while others showed no systemic spread until 48 hpi 11-15. This lab-to-lab variation may be a consequence of the invasive nature of i.g. inoculation, which can result in minor trauma to the lining of the esophagus, and promote direct bloodstream invasion of the bacteria.
We recently developed a novel mouse model of oral L. monocytogenes infection that closely mimics all phases of human disease 16. Infection occurs when the mice ingest pieces of contaminated food, a process that is non-traumatic, and does not require specialized skills by laboratory investigators. For a discrete period of time (36-48 hr), L. monocytogenes reproducibly colonize only the gastrointestinal tract, thus allowing investigation of the mechanisms used by pathogenic Listeria to translocate across the gut mucosa and disseminate to peripheral tissues. Importantly, the model can be used to study differences in host innate resistance to infection. In a previous study, we showed that BALB/c/By/J mice were highly susceptible to food borne listeriosis, with exponential replication of L. monocytogenes occurring in the gut, spleen, liver, and gall bladder 16. In contrast, C57BL/6 mice were resistant to food borne infection, with only transient colonization of L. monocytogenes occurring in each of these tissues. An additional feature of the food borne model that closely mimics human disease is that natural dissemination to the brain occurred during the later stages of the infection (5-7 dpi).
Protocol
1. Preparation of Selective Agar Media (BHI/L+G) to Inhibit Intestinal Microbiota
- Weigh out 26 g Brain Heart Infusion Agar (Difco), 7.5 g LiCl and 5.0 g glycine and place in a 1 liter flask. Add 500 ml of deionized water.
- Heat on a magnetic stirrer until the agar boils, stirring continuously. Take the flask off the heat, let the bubbles settle briefly, and then bring back to a boil again.
- Autoclave for 30 min at 121 °C under 16-19 psi on a liquid cycle. Note: leave the stir bar in the flask.
- Equilibrate the media to 55 °C in a water bath or let cool at RT. Do not allow the solution to get cooler than 55 °C or crystals will form in the agar. The crystals do not inhibit L. monocytogenes growth or affect the selective properties of the media. However, the crystals will be similar in appearance to small colonies and will make it difficult to count bacterial CFU.
- Gently mix the agar for 1 min using a magnetic stirrer. Avoid the formation of air bubbles. Pour the agar into Petri dishes (~ 25 ml/plate).
Note: This media does not support the growth of all L. monocytogenes strains. For example, L. monocytogenes EGDe grows well on this agar, with colonies visible within 36-48 hr, but 10403s does not grow on this media.
2. Preparation of the Inoculum
- Cut sliced white bread (Kroger) into small (2-3 mm) cubes using sterile scissors ora sterile scalpel blade. Do not use the crusts. Store individual pieces in microcentrifuge tubes at -20 °C until the day of the infection.
- Using a sterile scalpel, cut small (0.5-1 cm) chunks of salted butter (Kroger) and place each in a sterile microcentrifuge tube. Each tube will contain enough butter to prepare 50- 60 bread pieces. Store at -20 °C until needed.
- Grow L. monocytogenes in BHI broth shaking at 30 °C until the culture reaches an OD600 of ~0.8-1.0. Prepare 500 μl aliquots in microcentrifuge tubes, taking care to vortex the sample frequently so all of the tubes contain the same number of bacteria. Store at -80 °C.
- To titer the bacterial aliquots, thaw a tube on ice, then add 500 μl to 9.5 ml BHI broth. Incubate for 1.5 hr standing at 30 °C. Plate serial dilutions on BHI agar. Expect a titer of ~ 1-5 x 108 CFU/ml. Note: If homogeneous aliquots are prepared, can expect each of the tubes to yield this titer when prepared in a similar manner with a variance of 2 to 4-fold.
- To infect mice, prepare an aliquot of L. monocytogenes as described in step 2.4. About 20 min before the L. monocytogenes culture is ready, thaw the bread pieces at RT. Melt an aliquot of butter at 55 °C and pre-warm a small volume of PBS at 55 °C. Prepare enough bread pieces so that there is at least one per mouse to be infected and at least one extra piece for determining the titer of the actual inoculum.
- Based on the predetermined titer of the bacterial aliquot, calculate the total volume of L. monocytogenes culture needed to prepare the inocula for the required number of bread pieces. Pellet the bacteria by centrifuging at 14,000 x g for 10 min Aspirate all BHI broth and resuspend the bacteria in a small volume of pre-warmed PBS (2 μl/bread piece).
- Vortex the melted butter, add to bacteria (using a total volume equal to 3 μl/bread piece) and mix thoroughly. Note: Do not attempt to prepare more than 10-15 bread pieces at one time or the butter will solidify.
- Working quickly, pipette 5 μl of the bacterial suspension onto a single bread piece in a microcentrifuge tube. The solution should be completely absorbed by the bread piece.
- To determine the actual titer of the inoculum, add 1 ml sterile PBS to one of the bread pieces. Vortex vigorously for 1 min. Prepare serial dilutions and plate both BHI and BHI/L+G agar.
- Alternate procedure for high titer (>109 CFU) inocula. If the bacterial pellet is too large, it can be difficult to suspend in such a small volume, and the resulting material is very viscous, like a paste, and some of the inoculum may stick to the sides of the microcentrifuge tube. In this case, it is better to inoculate the bread in a sterile 60 mm culture dish, and to offer the entire dish (without the lid) to the mouse (see below). Any excess inoculum that is not absorbed by the bread can then be eaten directly by the mouse. In general, it takes longer for the mice to eat all of the bread and lick the dish clean than the standard protocol described below, so this is only the preferred method when dealing with very high titer inocula.
3. Infection of Mice
- Purchase female BALB/c/By/J (stock #0010026) and C57BL/6/J (stock # 000644) from The Jackson Laboratory (Bar Harbor, ME) and use at 6-9 weeks of age.
- Fast the mice one day (24 hr) prior to infection by removing the food and placing the mice on raised (1 inch) wire flooring (#3 mesh; Allentown) to prevent coprophagy. Leave only enough bedding in the cage to absorb urine, but not enough to elevate shed feces. Allow unrestricted access to water.
- Infect the mice at the onset of the dark cycle, working in a laminar flow hood outfitted with a red light bulb. Transfer a single mouse to an empty (no bedding) cage. Using a sterile forceps, transfer the contaminated bread piece to the bottom of the empty cage. Typically, the mouse will pick up the bread piece and consume it entirely within 2-10 min. If the mouse does not eat the bread right away, return the cage to rack and leave mouse undisturbed for 20-30 min. The majority of animals will eat the bread within this time frame, however, the mice can be left undisturbed, without access to other food or water for up to 2 hr.
- After infection, return the mouse to its original cage and replenish the mouse chow. Continue to house the mice on raised wire flooring for the duration of the experiment.
4. Monitoring the Level of Bacteria Shed in Feces
- Label and pre-weigh microcentrifuge tubes to be used for collection of feces.
- Working quickly after retrieving a cage of mice, place each mouse in a 500 ml plastic beaker. Typically the mice will expel 1-4 stool pellets within 5 min.
- Use a sterile forceps to transfer feces to appropriately labeled tubes.
- Weigh each tube and calculate the weight of the feces by subtracting the weight of the empty tube. Typical weights for fecal pellets range from 10-30 mg.
- Add PBS to each tube (200 μl/30 mg feces) and use a sterile toothpick to mash the fecal pellets.
- Vortex each tube for 30 sec, then prepare serial dilutions and plate on BHI/L+G agar. Count the number of colonies and calculate CFU/mg feces.
5. Processing of Infected Intestinal Tissues
- Euthanize the mice, aseptically harvest the stomach and intestines from each mouse as a single tissue and collect in empty 100 mm Petri dishes.
- Separate the small intestines, cecum, and colon and place in separate 60 mm dishes stored on ice. The small intestines can be further divided by length into three equal pieces approximating the duodenum, jejunum, and ileum to facilitate removal of the luminal contents. The tissue thirds can then be processed either together (for CFU per whole small intestines) or separately. Wet tissues with ~ 0.5 ml sterile PBS to keep pliable and prevent tears during handling.
- Fill a 10 ml syringe with 8 ml sterile PBS and attach a 25 g needle. Use sterile forceps to squeeze out the luminal contents into a waste beaker (or a sterile 50 ml tube if collecting the luminal bacteria). Flush 4 ml PBS through one end of the tissue, use the forceps to squeeze out the contents, and then flip the tissue over and repeat on the other end.
- To quantify the number of Listeria in the luminal contents, centrifuge the pooled flushes for 20 min at 12,000 x g, suspend the pellet in 0.5 – 1.0 ml sterile water.
- To quantify the total number of cell-associated amount Listeria, open each washed tissue by cutting longitudinally with sterile scalpel blade, and then make several lateral cuts to slice the tissue into smaller fragments. Note: this step is essential to ensure that the intestinal tissue does not wrap around the homogenizer blade. Transfer the intestinal pieces to a 15 ml centrifuge tube containing 2 ml sterile water.
- Sterilize a tissue homogenizer (Fisher PowerGen 1000) by placing the probe in 70% ethanol at 60% power for 30 sec. Wait for ethanol to air dry, or process in sterile water for 10 sec. Homogenize each tissue for 1 min. Repeat treatment with sterile water between each sample, and use 70% ethanol between sample groups.
- Prepare serial dilutions of each sample in sterile water and plate on BHI/L+G agar.
6. Processing of Infected Mesenteric Lymph Nodes
- Harvest lymph nodes aseptically, place in a sterile 60 mm dish on ice, and use sterile forceps to remove all attached fat. Expect to find 3-6 nodes per mouse.
- Prepare wire mesh screens by cutting 1.5-2 inch square pieces of stainless steel #80 mesh. Make a small cut in each corner, and fold down the four sides, creating a raised bed. Dip in 95% ethanol and then flame to sterilize, and place in a 60 mm Petri dish containing 0.75 ml sterile water. After each use, the screens can be recycled by soaking in 70% ethanol for 20 min, scrubbing with a brush to remove embedded tissues, boiling for 20-30 min in 2N NaOH, and then flushing extensively with water.
- Use the plunger from a sterile 3 ml syringe to mash the nodes through the mesh screen. Be sure to push down on the screen to the bottom of the dish so it makes contact with the water in the dish.
- Pass 0.75 ml sterile water through the screen and then pipette up and down several times to rinse the screen. Transfer cell suspension to a microcentrifuge tube.
- Vortex each sample vigorously for 30 sec to lyse the cells. Prepare serial dilutions and plate on either BHI/L+G agar.
7. Processing of Infected Spleens, Livers, & Gall Bladders
- Grasp spleen with sterile forceps and liberate by making two cuts, one at each end. Place in a 15 ml tube containing 2.5 ml sterile water. (Note: tubes with round bottoms may be easier to use with the homogenizer.)
- Locate the gall bladder (a small yellow fluid-filled sac attached to the liver) and snip carefully with sterile scissors to detach from liver without bursting. Transfer to a microcentrifuge tube containing 1 ml sterile water.
- Aseptically remove the liver, making sure to remove all lobes, and transfer to a 15 ml centrifuge tube containing 2.5 ml of sterile water.
- Homogenize spleens and livers as described above for 30 sec on 60% power. Forgall bladders, use sterile scissors to tear apart in the microcentrifuge tube, and then vortex vigorously for 30 sec.
- Prepare serial dilutions and plate each sample on either BHI or BHI/L+G agar.
8. Processing of Infected Brains
- Working from the back side of the mouse, cut the skin and tissue above the neck, and across both ears at a 45° angle. Use a sterile forceps to peel off the U-shaped flap of skin by pulling towards the nose and expose the skull and facial bones.
- Immobilize the skull by holding the bony ridge between the eyes with a forceps, and use scissors to make shallow cuts across the lateral edges of the skull. Be careful to cut only the bone and not the brain tissue. Next, cut the bony ridge between the eyes. Use a forceps to hold the top of the skull and pull backwards (toward the neck) to expose the brain.
- Gently lift the brain from its cavity using a sterile forceps and place in a 15 ml centrifuge tube containing 1.5 ml sterile water.
- Homogenize for 20 sec on 60% power as described above. Prepare serial dilutions and plate on either BHI or BHI/L+G agar.
9. Supplemental Procedure: Fractionation of Intestinal Tissues
9.1 Isolation of Bacteria in the Mucus Fraction
- For each intestinal tissue, prepare 3 tubes containing 3 ml of 6 mM N-acetylcysteine (NAC; Sigma #A-9165).
- Place the flushed and longitudinally cut tissue in the first tube for 1-2 min, vigorously swirling every 20-30 sec. Using sterile forceps, pick up the tissue and gently squeeze against the side of the tube to remove excess liquid.
- Repeat step 9.1.2 twice more using the remaining NAC tubes. Remove the intestinal tissue and set aside for further processing.
- Pool the NAC washes (total of 9 ml), and centrifuge for 20 min at 12,000 x g.
- Resuspend the pellet in sterile water, vortex for 30 sec, prepare serial dilutions and plate on BHI/L+G.
9.2 Isolation of Bacteria in the Epithelial Cell (EC) Fraction
- Cut each tissue into small pieces with sterile scissors and place in a 50 ml tube containing 5 ml of RPMI (Invitrogen #21870) supplemented with 5% FBS (RP-5), 5 mM EDTA, and 1 mM DTT. Incubate shaking at 37 °C for 20 min.
- Transfer the intestinal pieces to a fresh tube containing 5 ml RP5/EDTA/DTT and incubate shaking at 37 °C for 20 min. Save the media from the first tube and set aside at 37 °C. Repeat this process once more for a total of three 20 min incubations in RP5/EDTA/DTT. If proceeding to the lamina propria isolation step, transfer the remaining intestinal pieces into empty 50 ml tube.
- Combine the three RP5/EDTA/DTT washes (total volume of 15 ml) and centrifuge at low speed (1,200 x g) to pellet the cells.
- To quantify extracellular bacteria, collect the supernatant and centrifuge for 20 min at 12,000 x g. Resuspend the pellet in 0.5 – 1.0 ml sterile water and plate serial dilutions on BHI/L+G agar.
- To enumerate intracellular bacteria, resuspend the EC pellet in 5 ml of RP-5 containing 25 μg/ml gentamicin. Incubate the single cell suspension for 30 min at 37 °C plus 7% CO2 to kill any remaining extracellular L. monocytogenes. Wash the cells twice in PBS, suspend in 0.5 ml sterile water, and vortex for 30 sec to lyse the cells. Prepare serial dilutions in water and plate on BHI/L+G.
Note: The cells collected at this stage will also contain cells from the underlying Peyer’s Patches. If desired, the visible Peyer’s Patches can be removed from the intestinal tissues prior to flushing and cutting longitudinally.
9.3 Isolation of Bacteria in the Lamina Propria (LP) Fraction
- Rinse the excess DTT/EDTA from the intestinal pieces by adding 25 ml sterile PBS to the tube. Shake vigorously, transfer into a fresh tube and repeat twice.
- Transfer the intestinal pieces to a 50 ml tube containing 4 ml of digestion solution consisting of RP-5 supplemented with 1 mg/ml type IV collagenase and 40 μg/ml DNAse I. Incubate shaking at 37 °C for 40 min.
- Transfer the undigested pieces into a fresh tube containing 4 ml digestion solution and repeat once or twice until the tissue pieces are completely digested. Save each of the digestion solutions, which contain liberated LP cells, at 37 °C.
- Centrifuge the pooled digestion solutions at low speed (1,200 x g) to pellet the cells. Process the supernatant and cell pellet separately as described above to enumerate extracellular and intracellular bacteria, respectively.
Representative Results
L. monocytogenes colonies will be visible on BHI/L+G plates after 36-48 hr incubation at 37 °C. The colonies have a smooth, dome-shaped creamy white appearance (Figure 1A). Growth will be inhibited for the majority of the intestinal microbiota, but it is common to see some colonies that are not L. monocytogenes, particularly when plating small intestine or colon directly without significant dilution (Figure 1B). Suspect colonies can be confirmed by plating on ChromAgarTM Listeria plates. L. monocytogenes appear as blue colonies surrounded by a white halo on these plates (Figure 1C). The limit of detection for L. monocytogenes in each tissue is dependent on the volume of water used to homogenize the tissue. The sample volumes shown in Table 1 represent the minimal volume needed to effectively process each tissue. Homogenates of spleen, gall bladder, brain, or fractionated intestinal tissue can be stored at 4 °C for 1-2 days and re-plated if necessary. Colon, small intestine or liver homogenates can inhibit some L. monocytogenes growth unless sample is diluted sufficiently (Figure 1D), and these homogenates do not yield similar CFU counts if re-plated after storage at 4 °C.
We previously showed that BALB/c/By/J (BALB) mice were significantly more susceptible to food borne listeriosis than C57BL/6 (B6) mice 16. An inoculum of 107 CFU is sufficient to establish intestinal infection in BALB mice, but at least 108 CFU is needed to colonize the gastrointestinal tract of B6 mice (Figure 2). As shown in Figure 2, the bacterial load in the intestines, spleen, liver and gall bladder will be proportional to the challenge dose given to the mice. Preliminary studies suggest that 5 x 109 CFU is the approximate LD50 for BALB/c/By/J mice using this model 16.
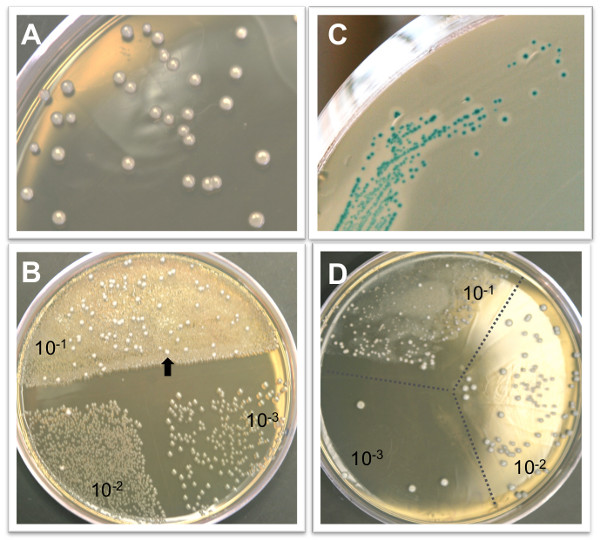
Figure 1. Representative colony growth on selective agar plates. A) L. monocytogenes colonies after 48 hr growth on BHI/L+G agar. B) BHI/L+G agar inhibits the growth of most intestinal microbiota, but some non-Listeria colonies (arrow) may be observed at low dilutions. The agar plate shown here contains 100 μl of a 10-1 dilution on half of the plate, and 50 μl each of the 10-2 and 10-3 dilutions of a colon homogenate. C) L. monocytogenes colonies can be confirmed by growth on CHROM agar Listeria plates. L. monocytogenes appear blue with a white halo surrounding the colony. D) It is not uncommon to see an inhibition of L. monocytogenes growth, resulting in colonies of varying sizes, in the lowest dilution of either intestinal or liver homogenates plates on BHI/L+G agar.
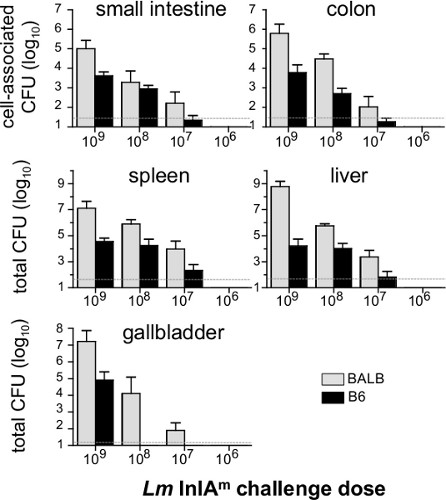
Figure 2. Dose response of food borne infection in BALB/c/By/J and C57BL/6 mice. Female BALB/c/By/J (BALB) and C57BL/6 (B6) mice were infected with 109(n=7), 108(n=8), 107(n=6) and 106(n=4) Lm InlAm and the number of CFU in each tissue was determined 5 dpi. Mean values +/- SD are shown. Pooled data from two different experiments were analyzed. Dashed lines indicate the limit of detection in each organ.
| Tissue | Sample volume (ml)a | Limit of detection (CFU) |
| Small intestine | 2.0 | 50 |
| Cecum | 2.0 | 50 |
| Colon | 2.0 | 50 |
| Mesenteric Lymph Nodes | 1.5 | 15 |
| Spleen | 2.5 | 50 |
| Liver | 2.5 | 50 |
| Gall bladder | 0.5 | 10 |
| Brain | 1.5 | 15 |
Table 1. Limit of detection for L. monocytogenes in tissue homogenates. a Average total sample volume consisting of sterile water plus the homogenized tissue.
Discussion
Inbred mice are not uniformly receptive to feeding at all times of the day, and their willingness to eat the contaminated bread will depend both on the strain type and the age of the mice 17. In our experience, 6-9 week old B6 mice are receptive to feeding at any time of day, but BALB mice will not consistently eat the bread piece unless it is offered during their dark cycle. The light cycle of the room used to house the animals can be altered so the dark phase coincides with the normal working day for laboratory personnel. However, the mice should be given at least two weeks to acclimate to the altered light cycle prior to infection. During the infection, only red lamps should be used in either the room itself or in the laminar flow hood.
In developing this model, bread was chosen as the food source to transmit L. monocytogenes largely because bread is absorbent. Thus, it was easy to saturate small pieces with the bacterial inoculum and ensure that each mouse ingested the same dose. However, this model could readily be adapted to use other food sources. Indeed, this is the only mouse model that can be used to directly test the effect of food composition or storage conditions on bacterial infectivity. L. monocytogenes can easily adapt to growth in high salt, low pH, or cold temperatures 1,2,18, but it is not known if these growth conditions alter the ability of the bacteria to establish intestinal infection.
Approximately 10 min is needed to harvest all of the infected organs from a single mouse. The gall bladder is easily ruptured, so it is best to remove it first. The mesenteric lymph nodes are easiest to spot when the intestines have not been disturbed, so they should be removed next. The intestines can be harvested as a single tissue by cutting just below the stomach and just above the appendix, and then separated into duodenum, jejunum, ileum, cecum, and colon just prior to flushing and homogenizing. The spleen and liver are typically removed next, and the brain is harvested after retrieving organs from the peritoneal cavity, as it is necessary to turn the mouse over to access the skull. When working with multiple mice, all tissues should be stored on ice until processed. For most tissues, one agar plate was used to determine the total number of CFU present in the tissue. The plate was divided into thirds, and a 50-100 μl sample of three separate dilutions of the tissue homogenate was plated on each third.
The methods described here will identify the total number of L. monocytogenes in each mouse tissue, not just the intracellular organisms. The unique intracellular life style of L. monocytogenes is thought to be a key virulence determinant during infection 18,19. However, L. monocytogenes are facultative, not obligate, intracellular pathogens, and there are no definitive published reports to indicate the percentage of Listeria that actually reside within cells in vivo. Most previous studies using oral L. monocytogenes infection relied on gentamicin treatment of gut tissues to inhibit the growth of intestinal microbiota. Pre-treatment with gentamicin has the potential to eliminate extracellular L. monocytogenes, allowing recovery of only the intracellular bacteria, although it is not clear to what extent gentamicin is able to penetrate whole intestinal tissues in vitro. The supplemental protocol described here allows for determination of both extracellular and intracellular bacteria in the mucus layer, the epithelium, and the lamina propria compartment of infected intestinal tissues. In this procedure, gentamicin is used to treat single cell suspensions, ensuring that all recovered bacteria were inside cells within the tissue.
Three washes with NAC typically removed the majority of the mucus from intestinal tissues, and the pooled washes did not contain any eukaryotic cells (viable or dead), as determined by trypan blue staining. Additional washes did not yield visible mucus as determined by Diff-Quik staining of cytospin slide preparations. The cellularity of the EC and LP fractions can also be confirmed using Diff-Quik or Giemsa staining. The EC fraction should consist of primarily epithelial cells, which can readily be distinguished from the few intraepithelial lymphocytes present in the intestinal epithelium. The LP fraction is more diverse, containing a mixture of mononuclear cells that changes composition after infection when inflammatory monocytes infiltrate the tissue.
The food borne model of listeriosis can be used with a wide variety of L. monocytogenes isolates, including the mouse-adapted InlAm-expressing strain 15 described here, wild type EGDe, and deletion mutant derivatives of these strains 16. In a previous study, we showed that the level of intestinal colonization did not vary significantly among these strains, however, isolates that did not express a high affinity ligand for murine E-cadherin did have a slight defect in dissemination to the mesenteric lymph nodes and spleen 16. Likewise, any mouse strain can be used for infection, making this an attractive model system to study both bacterial pathogenesis and host responses to infection. Finally, although we have not yet tested this directly, we propose that the basic procedures used here should be widely applicable to other food borne bacterial pathogens such as Salmonella, Yersinia, Escherichia, Campylobacter and Citrobacter species.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants from the National Institutes of Health (AI079442 and AI091918) awarded to S.E.F.D.
Materials
| Name of Reagent/Material | Company | Catalog Number | Comments |
| Brain Heart Infusion Agar | Difco | BD-241830 | |
| Lithium chloride | Sigma | L9650 | |
| Glycine | Omnipur | 4840 | |
| EDTA | Gibco | 15575-038 | |
| DTT | Sigma | D5545 | |
| Collagenase, type IV | Worthington | LS004089 | |
| DNAse I | Worthington | LS002007 | |
| Diff-Quik | Dade-Behring | B4132-1A | |
| PowerGen 1000 homogenizer | Fisher | 14-261-06 | |
| stainless steel type 304 mesh #80 | Small Parts, Inc. | CX-0080-C | |
| Cytospin | Statspin | M801-22 |
Referencias
- Becker, L. A., Evans, S. N., Hutkins, R. W., Benson, A. K. Role of sigmaB in adaptation of Listeria monocytogenes to growth at low temperature. Journal of Bacteriology. 182, 7083-7087 (2000).
- Neunlist, M. R., et al. Effect of salting and cold-smoking on the culturability, viability, and virulence of Listeria monocytogenes strain Scott A. Journal of Food Protection. 68, 85-91 (2005).
- Aureli, P., et al. An outbreak of febrile gastroenteritis associated with corn contaminated by Listeria monocytogenes. N. Engl. J. Med. 342, 1236-1241 (2000).
- Dalton, C. B., et al. An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N. Engl. J. Med. 336, 100-105 (1997).
- Frye, D. M., et al. An outbreak of febrile gastroenteritis associated with delicatessen meat contaminated with Listeria monocytogenes. Clin. Infect. Dis. 35, 943-949 (2002).
- Salamina, G., et al. A foodborne outbreak of gastroenteritis involving Listeria monocytogenes. Epidemiol. Infect. 117, 429-436 (1996).
- Mead, P. S., et al. Food-related illness and death in the United States. Emerging Infectious Diseases. 5, 607-625 (1999).
- Wing, E. J., Gregory, S. H. Listeria monocytogenes: Clinical and experimental update. The Journal of Infectious Disease. 185, S18-S24 (2002).
- Condotta, S. A., Richer, M. J., Badovinac, V. P., Harty, J. T. Probing CD8 T cell responses with Listeria monocytogenes infection. Advances in Immunology. 113, 51-80 (2012).
- Disson, O., et al. Modeling human listeriosis in natural and genetically engineered animals. Nat Protoc. 4, 799-810 (2009).
- Czuprynski, C. J., Faith, N. G., Steinberg, H. A/J mice are susceptible and C57BL/6 mice are resistant to Listeria monocytogenes infection by intragastric inoculation. Infect. Immun. 71, 682-689 (2003).
- Gajendran, N., et al. Regional IFNgamma expression is insufficient for efficacious control of food-borne bacterial pathogens at the gut epithelial barrier. Int. Immunol. 19, 1075-1081 (2007).
- Lecuit, M., et al. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science. 292, 1722-1725 (2001).
- Pron, B., et al. Comprehensive study of the intestinal stage of listeriosis in a rat ligated ileal loop system. Infect. Immun. 66, 747-755 (1998).
- Wollert, T., et al. Extending the host range of Listeria monocytogenes by rational protein design. Cell. 129, 891-902 (2007).
- Ghanem, B. o. u., N, E., et al. InlA promotes dissemination of Listeria monocytogenes to the mesenteric lymph nodes during food borne infection of mice. PLoS Pathogens. , (2012).
- Kowal, M., Buda-Lewandowska, D., Plytycz, B., Styrna, J. Day/night food consumption in mice is strain and age-dependent. Folia Biol. (Krakow). 50, 1-3 (2002).
- Freitag, N. E., Port, G. C., Miner, M. D. Listeria monocytogenes – from saprophyte to intracellular pathogen. Nature reviews. Microbiology. 7, 623-628 (2009).
- Camejo, A., et al. The arsenal of virulence factors deployed by Listeria monocytogenes to promote its cell infection cycle. Virulence. 2, 379-394 (2011).

