Culturing and Maintaining Clostridium difficile in an Anaerobic Environment
Summary
Clostridium difficile is a pathogenic bacterium that is a strict anaerobe and causes antibiotic associated diarrhea (AAD). Here, methods for isolating, culturing and maintaining C. difficile vegetative cells and spores are described. These techniques necessitate an anaerobic chamber, which requires regular maintenance to ensure proper conditions for optimal C. difficile cultivation.
Abstract
Clostridium difficile is a Gram-positive, anaerobic, sporogenic bacterium that is primarily responsible for antibiotic associated diarrhea (AAD) and is a significant nosocomial pathogen. C. difficile is notoriously difficult to isolate and cultivate and is extremely sensitive to even low levels of oxygen in the environment. Here, methods for isolating C. difficile from fecal samples and subsequently culturing C. difficile for preparation of glycerol stocks for long-term storage are presented. Techniques for preparing and enumerating spore stocks in the laboratory for a variety of downstream applications including microscopy and animal studies are also described. These techniques necessitate an anaerobic chamber, which maintains a consistent anaerobic environment to ensure proper conditions for optimal C. difficile growth. We provide protocols for transferring materials in and out of the chamber without causing significant oxygen contamination along with suggestions for regular maintenance required to sustain the appropriate anaerobic environment for efficient and consistent C. difficile cultivation.
Introduction
Clostridium difficile is a Gram-positive, spore-forming bacterium that is an obligate anaerobe and a potentially fatal gastrointestinal pathogen of humans and animals. Initially described in 1935 as a commensal organism found in fecal samples from newborns1, C. difficile was later demonstrated to be the causative agent of pseudomembranous colitis associated with antibiotic treatment2. C. difficile infections (CDI) are typically preceded by antibiotic treatment which results in the disruption of the normal colonic flora, creating a niche for C. difficile to thrive2. C. difficile is transmitted as a dormant spore via the fecal-oral route and subsequently germinates within the gastrointestinal tract, producing vegetative cells capable of generating several toxins and causing severe disease and colitis3. CDI are often refractory to conventional treatments and these infections are frequently recurrent4. As a result, CDI are responsible for up to $4.8 billion in health care costs in the United States5-7.
C. difficile is very sensitive to even low levels of oxygen in the environment. For C. difficile to persist in the environment and be efficiently transmitted from host to host, the formation of a metabolically inactive spore is critical8. Because the laboratory maintenance and manipulation of C. difficile requires a controlled, anaerobic environment, these techniques necessitate the use of an anaerobic chamber. Use of anaerobic chambers has resulted in increased recovery and isolation of obligate anaerobes9-11, and has allowed a number of molecular techniques to be performed in an anaerobic atmosphere.
In addition to C. difficile, the anaerobic chamber use and maintenance described here are applicable to other obligate anaerobes such as other Clostridial species (e.g. C. perfringens), other gastrointestinal species (e.g. Bacteroides species12) and periodontal pathogens (e.g. Peptostreptococcus species13).
Protocol
Note: C. difficile is a human and animal pathogen that can cause gastrointestinal disease. Experiments involving C. difficile must be performed with appropriate biosafety precautions (BSL-2).
1. Anaerobic Chamber Use and Maintenance
C. difficile is a strict anaerobe and is extremely sensitive to even low concentrations of oxygen in the atmosphere. Therefore, a controlled, anaerobic environment is needed for its successful manipulation. The use of an anaerobic chamber (Figure 1A) provides the most stable environment and the ideal conditions for effective cultivation of C. difficile and other anaerobic bacteria14. Here, an atmosphere containing a gas mixture (5% CO2, 10% H2, 85% N2) can be stably maintained.
To introduce any items into the chamber without significant oxygen contamination, an airlock must be used (Figure 1B). This device functions as a gas interchange and works automatically, semi-manually or manually. The airlock has two doors: one providing access to the outside of the airlock and the other providing access to the interior of the anaerobic chamber. Depending on the model, the airlock may have an additional door, which can offer access to an adjoining anaerobic chamber, thus saving the cost of a separate airlock. Unless actively moving items in or out of the chamber, both doors should remain closed at all times to avoid oxygen contamination. The airlock possesses an on/off switch on the front and a panel containing four buttons. The gas lines and vacuum pump hose are connected in the rear of the airlock, near the panel where the switches for full manual operation of the unit are located. It is recommended that two purge cycles, using nitrogen gas (N2), are used before filling the interchange with gas mix (5% CO2, 10% H2, 85% N2) to reduce the amount of oxygen introduced into the chamber. It is also important to never leave either door open for longer than the amount time required to move items in and out of the interchange and to carefully plan experiments to reduce the frequency of moving items in and out of the chamber. The different operating procedures for vinyl anaerobic chambers are outlined below. Before following these protocols, ensure that these are compatible with the manufacturer’s instructions provided with the chamber.
- Automatic Mode
This is a programmable and customizable mode and is performed entirely by the airlock. To program the airlock, refer to the manufacturer’s instructions. A recommended program for typical entry into the chamber involves two purge cycles using nitrogen gas followed by refilling the airlock with a gas mix that closely matches the atmosphere maintained within the chamber. During each vacuum cycle, the standard factory procedures suggest pulling a vacuum to 20 inHg and purging to 1 inHg.- Ensure that the inner airlock door is fully closed.
- Open the outer airlock door.
- Place items into airlock and close the outer airlock door. If introducing liquids, unscrew the caps halfway to ensure efficient gas exchange. Sealed packages and containers should be opened prior to beginning the cycle; refraining from doing so may warp and damage plasticware and containers. To maintain sterility, open the packages enough to only allow efficient gas exchange.
- Press the Start button.
- Wait until the airlock has cycled through the program and the display reads "Anaerobic."
- Open inner airlock door.
- Move items into the chamber.
- Close inner door.
- Semi-manual Mode
This mode may be used when moving items in or out of the chamber and is necessary when cycling the gas within the chamber is required.- Ensure that the inner airlock door is fully closed.
- Open the outer airlock door.
- Place items into airlock and close the outer airlock door. If introducing liquids, unscrew the caps halfway to ensure efficient gas exchange. Sealed packages, such as inoculating loops and 96-well plates, must be opened prior to beginning the cycle.
- Press Menu.
- Press Start. The airlock will display the function of each button and will not respond until this is completed:
- Up arrow: activates the vacuum pump.
- Down arrow: initiates the nitrogen (purge) gas flow.
- Start: initiates the gas mix flow.
- Menu: return to the default display.
- Press "Up," removing gas from the airlock, until the display reads 20 inHg.
- Press "Down," filling the airlock with nitrogen, until the display reads less than 1 inHg. Do not overshoot, as this will create positive pressure within the airlock, which may affect its integrity.
- Repeat steps 6 and 7 to perform an additional purge cycle.
- Press "Up" until the display reads 20 inHg.
- Press "Start," filling the interchange with gas mix, until the display reads less than 1 inHg.
- Open inner airlock door.
- Move items into the chamber.
- Close inner door.
- Manual Mode
This mode may be used whenever the automatic or semi-manual modes are not working or there is no power to the airlock. This mode does not function when the airlock is on; therefore, it is difficult to determine the pressure within the airlock. Because the vacuum pull and gas purge times is dependent upon vacuum and gas flow rates, respectively, the use of a pressure gauge within the interchange is required to monitor the pressure and reduce the risk of personal injury and damage to the airlock.- Ensure that the inner airlock door is fully closed.
- Open the outer airlock door.
- Place items, including the pressure gauge, into airlock and close the outer airlock door. If introducing liquids, unscrew the caps halfway to ensure efficient gas exchange. Sealed packages, such as inoculating loops and 96-well plates, must be opened prior to beginning the cycle.
- Locate the three switches on the back of the airlock labeled "Manual Control Switches." These are individually labeled as:
- To Vacuum
- Nitrogen
- Gas Mix
- Flip the To Vacuum toggle switch until the pressure gauge reads approximately 20 inHg.
- Flip the Nitrogen toggle switch until the pressure gauge reads approximately 1 inHg.
- Flip the To Vacuum toggle switch until the pressure gauge reads approximately 20 inHg.
- Flip the Nitrogen toggle switch until the pressure gauge reads approximately 1 inHg.
- Flip the To Vacuum toggle switch until the pressure gauge reads approximately 20 inHg.
- Flip the Gas Mix toggle switch until the pressure gauge reads approximately 1 inHg.
- Open inner airlock door.
- Move items into the chamber.
- Close inner door.
2. Culturing, Enumerating and Storing C. difficile from Stool Samples
This procedure is designed to recover C. difficile from fecal samples containing spores and subsequently maintain isolated colonies as vegetative cells or spores in long-term storage as glycerol stocks. Alternatively, this procedure may be used to enumerate the number of C. difficile present in stool samples (e.g. from animal studies). To selectively and differentially enrich for C. difficile, taurocholate-cefoxitin-cycloserine-fructose agar (TCCFA) is used to inhibit growth of normal fecal flora15-16. Cycloserine is bacteriostatic for Gram-negative bacteria, while cefoxitin more broadly inhibits growth of both Gram-negative and -positive bacteria, with the exception of C. difficile and most enter cocci strains. A pH indicator, neutral red, can be included in the medium, as the fermentation of fructose will result in a decrease in pH and a subsequent color change from red/orange to yellow. To efficiently recover spores of C. difficile as vegetative bacteria, the bile salt sodium taurocholate is used to induce germination17-18. Because C. difficile forms spores, alcohol or heat treatment of samples may be used to reduce or eliminate vegetative cells, limiting the growth of contaminating flora, which may increase the efficiency of C. difficile recovery19. As mentioned above, it is critical to pre-reduce all plates for at least 1-2 hr in the anaerobic chamber prior to use to ensure the removal of residual oxygen. Air drying the plates prior to use in the chamber can reduce condensation. Liquid medium may need up to 24 hr to reduce depending on the volume and the surface-to-air ratio of the container used.
Before beginning, the following items should be placed into the anaerobic chamber:
- Stool sample
- Sterile swabs
- Sterile inoculating loops
- 1x PBS (see Materials)
- TCCFA plates (see Materials)
- BHIS (Brain Heart Infusion medium with yeast extract) plates and broth (see Materials)
- 50% glycerol (sterilized)
- 10% L-cysteine (sterilized)
- Cryogenic storage vials
*Alternatively, isolated colonies can be spread onto BHIS agar plates, and subsequently scraped off and resuspended in BHIS liquid medium with 15% glycerol for long-term storage at -80°C. **It is important to note that Gram-staining is not an effective strategy to identify C. difficile directly from stool samples23; C. difficile must first be isolated from the other flora present in the stool.
- Resuspend the stool sample in 1x PBS (this may be performed in aerobic conditions), and ensure that the stool sample is fully resuspended in 1x PBS by vortexing. If enumerating C. difficile from the stool, weigh the stool sample prior to the addition of 1x PBS.
- Make serial dilutions of the resuspended stool sample in 1x PBS for appropriate isolation or enumeration of colony forming units (CFU) per gram of stool.
- Using aseptic technique, apply 100 μl of each serial dilution to TCCFA plates.
- Using a series of sterile inoculating loops, streak the applied culture for isolated colonies or evenly spread the applied culture on the surface of the TCCFA plate for enumeration of colonies.
- Incubate the plate anaerobically for 48 hr at 37 °C. It is possible to detect and identify C. difficile colonies within 24 hr based on the flat, irregular, ground-glass appearance of the colonies15, although more accurate identification and enumeration is achieved after 48 hr.
- Using sterile inoculating loops, subculture any colonies that appear to be C. difficile onto pre-reduced BHIS agar plates, supplemented with 0.03% L-cysteine20. Pick an individual colony, and streak onto the plate in quadrants, using a new sterile inoculating loop for each quadrant, to obtain isolated colonies. Alternatively, count the C. difficile colonies of each dilution (this may be performed in aerobic conditions), and calculate the number of colony forming units per gram of stool sample.
- Confirm C. difficile identification via Gram-staining. After Gram-staining, C. difficile will appear as purple rods and some cells may contain terminal endospores. Polymerase chain reaction (PCR) identification of the genes that encode toxin B may provide further confirmation of toxigenic strains of C. difficile21 while multilocus sequence typing (MLST) is successful for identification and accurate typing of unknown and nontoxigenic strains of C. difficile22.
- To maintain a stock of C. difficile at -80 °C, pick an individual, isolated colony from the BHIS agar plate using a sterile inoculating loop and resuspend the colony in 10 ml of pre-reduced BHIS liquid medium supplemented with 0.03% L-cysteine.*
- Incubate overnight anaerobically at 37 °C, or until the culture becomes turbid.
- Add 333 μl of 50% glycerol and 666 μl of the C. difficile culture to a 1.8 ml cryogenic tube to create a 15% glycerol stock of the isolated strain.
- Tightly cap the cryogenic tube, mix well and immediately remove the stock from the anaerobic chamber and place in a -80 °C freezer for long-term storage.
3. Culturing C. difficile from Frozen Glycerol Stocks
This procedure provides for recovery of C. difficile from glycerol stocks stored at -80 °C. Because repeated freeze-thaw cycles may kill vegetative cells, it is important to keep the glycerol stocks frozen at all times. We do not recommend the use of dry ice for transferring strains in and out of the chamber as the evaporation of dry ice can change the environment within the chamber. Instead, we recommend using frozen cooling racks to keep glycerol stocks frozen during transport. For various selective and differential purposes, three media are commonly used for culturing C. difficile. TCCFA, as discussed above, is selective for C. difficile and contains sodium taurocholate, a germinant. Brain heart infusion supplemented with yeast extract (BHIS) is a commonly used, enriched, non-selective medium which allows for the growth of a wide variety of organisms (Figure 2A)24. Frequently, L-cysteine is added to BHIS as a reducing agent20. Finally, the addition of blood to the medium (Figure 2B) allows for more efficient sporulation than on TCCFA and provides for detection of the unique greenish or chartreuse fluorescence exhibited by C. difficile under long-wave ultraviolet (UV) light15 (Figure 2C). When culturing C. difficile from spore stocks, it is critical to remember that sodium taurocholate must be added to the medium to ensure germination.
Before beginning, the following items should be placed into the anaerobic chamber:
- Frozen glycerol stock (in cooling rack)
- Sterile inoculating loops
- TCCFA or BHIS plates (see Materials)
- BHIS broth (see Materials)
- 50% glycerol (sterilized)
- Cryogenic storage vials
- Place the frozen glycerol stock of C. difficile in a cooling rack that has been stored at -80 °C prior to use to prevent thawing.
- Bring the frozen glycerol stock on dry ice into the anaerobic chamber.
- Using aseptic technique and a sterile inoculating loop, place a small amount of the stock onto the plate and streak across one quadrant of the plate.
- Rotate the plate 90° and, using a new sterile inoculating loop, continue to streak across the second quadrant.
- Repeat for the third and fourth quadrants to ensure the isolation of individual colonies.
- Immediately remove the frozen glycerol stock from the anaerobic chamber and return to the -80 °C.
- Incubate the plate anaerobically overnight at 37 °C. Individual isolated colonies should be observed after overnight growth.
4. Purifying Spores from C. difficile
As sporulation is required for survival in oxygen-rich environments and for efficient transmission of disease8, the preparation of spore stocks is often necessary for downstream applications, not limited to microscopy and animals studies. It is important to note that enumerating spores requires repetition to ensure reproducibility of counts. Pipetting up and down several times between dilutions also reduces loss since spores adhere to plastic well.
Sporulation of C. difficile is not as rapid or homogeneous as other sporogenic species. To optimize spore production and recovery, either sporulation medium (SMC)17,25 or 70:30 medium26 is recommended. Other media commonly used are BHIS, which requires 4-5 days of growth before efficient sporulation is seen27, and Clospore, a liquid medium that produces high titers of spores (107 – 108 spores per milliliter) after 72 hr of growth. Other protocols use ice-cold water rather than 1x PBS20; however, the use of an isotonic solution can reduce spores from sticking to each other and plastic surfaces. Alternatively, some investigators further purify their spores using a sucrose gradient to fully remove vegetative cells and debris29.
Before beginning, the following items should be placed into the anaerobic chamber:
- Sterile inoculating loops
- BHIS, SMC and/or 70:30 plates (see Materials)
- BHIS broth (see Materials)
- 1x PBS, filter-sterilized (see Materials)
- Culture strains from frozen glycerol stock onto pre-reduced BHIS plates and incubate anaerobically overnight at 37 °C.
- Restreak onto several pre-reduced SMC or 70:30 plates and incubate anaerobically at 37 °C for 24-48 hours. Spore formation can be followed via phase contrast microscopy. Spores will appear phase bright while vegetative and mother cells will appear phase dark. *As an alternative, spores can be purified from 70:30 liquid medium after 24-48 hours, which yields 105-106 spores per milliliter, depending on the strain used.
- Using a sterile inoculating loop, scrape plates and resuspend the cells in 10 ml sterile 1x PBS.
- Discard plates and remove spore suspension from the anaerobic chamber. Pellet the cells at 3,000 x g for 15 minutes. Wash the cells twice in 1x PBS, fully resuspending the cell pellet each time.
- Incubate overnight at 4 °C to aid in lysis of vegetative and mother cells.
- Incubate at 70 °C for 20 min to kill any residual vegetative cells.
- To determine colony forming units (CFU) per milliliter, serially dilute each spore preparation in 1x PBS and plate on BHIS + 0.1% sodium taurocholate. Incubate plates for a minimum of 24 hr before enumerating colonies.
- Spores can be stored in 1x PBS at either room temperature or 4°C for long-term storage. If stored at 4 °C, it may be useful to reheat the spore preparation at 55 °C for 15 min to restore efficient germination.
Representative Results
An example of C. difficile grown on BHIS and Columbia anaerobic sheep blood agar media can be seen in Figure 2. C. difficile forms irregular colonies that are flat and possess a ground-glass appearance which is evident on both media. Here, an erythromycin-sensitive clinical isolate of C. difficile, 630E30, is grown on BHIS agar, an enriched, non-selective medium, for 24 hr at 37 °C (Figure 2A). Colonies on Columbia anaerobic sheep blood agar appear similar to those grown on BHIS under white light (Figure 2B); however, the use of this medium also provides for detection of the greenish or chartreuse fluorescence exhibited by C. difficile under long-wave ultraviolet (UV) light15 (Figure 2C). C. difficile colonies on TCCFA agar look similar to growth on BHIS agar. Because of the presence of two antibiotics in TCCFA medium, a time period of 48 hr growth is necessary before enumerating colonies.
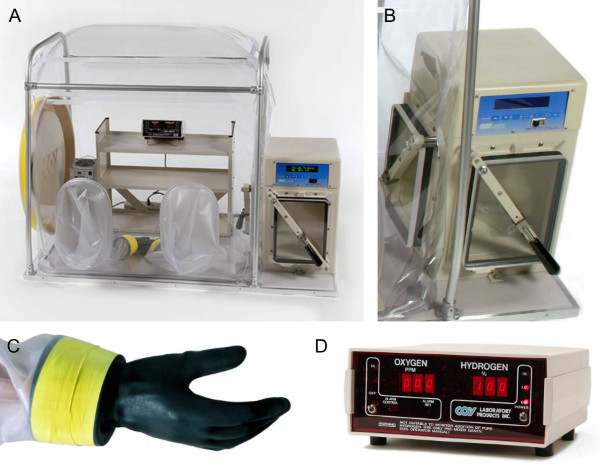
Figure 1. The Coy Laboratories Type C vinyl chamber and its components. (A) A Coy Laboratories Type C vinyl chamber which provides workspace for a single individual at one time (42 in. x 32 in.). It contains a catalyst fan box (back left corner) which circulates and heats the air, and holds the Stak-Pak containing the palladium catalyst required to reduce any oxygen contamination. (B) The airlock serves as an interchange and provides a mechanism for the transfer of materials in and out of the chamber while preventing significant oxygen contamination within the anaerobic environment. The airlock has two doors: one providing access to the outside of the airlock and the other providing access to the interior of the anaerobic chamber. The airlock is programmable and allows for customized cycles for entry into the chamber. It is operable in automatic, semi-manual and manual modes. (C) Attached, flexible latex gloves are provided which allow full range of motion and reach within the chamber. The gloves are secured to a specialized cuff attached to the vinyl sleeves with vinyl adhesive, permitting replacement of gloves without disrupting the anaerobic atmosphere of the chamber. Neoprene gloves are also available. (D) The Model 10 Gas Analyzer continuously monitors both oxygen and hydrogen levels providing an instantaneous readout of the atmosphere within the chamber. This unit allows for immediate alerts if a leak occurs, an incorrect gas mix is used or additional problems arise via audible alarms and a flashing LED light.

Figure 2. The appearance of C. difficile colonies on various media. The characteristic flat, irregular, ground-glass appearance is evident with an erythromycin-sensitive clinical isolate of C. difficile, 630E30, grown on BHIS agar for 24 hr (A) and Columbia anaerobic sheep blood agar for 48 hr under white light (B) and long wave ultraviolet light (C).
Discussion
The methods described here allow for simple and quick recovery of C. difficile from a variety of fecal samples, including humans, mice and hamsters, as well as the long-term storage of C. difficile as glycerol or spore stocks. C. difficile can be a difficult organism to cultivate, but careful maintenance of an anaerobic environment and the application of aseptic techniques can provide for robust growth and a reduction in contamination.
Anaerobic chambers: Considerations and Maintenance
There are two types of anaerobic chambers: rigid chambers or vinyl chambers. Rigid chambers are typically made of aluminum or a rigid polymer (e.g. Plexiglas or acrylic materials), allowing the use of caustic chemicals. Rigid chambers can be converted to a gloveless style and are less prone to punctures; however, leaks are difficult to detect and find and rigid chambers require some method to compensate for gas displacement during use. The polymer units often are equipped with a purge-only airlock, which may cost more to operate. For maintenance of stringent atmospheric conditions, cost, flexibility, laboratory space requirements and easy maintenance, vinyl anaerobic chambers are recommended (Coy Laboratory Products, Figure 1A). This anaerobic chamber is comprised of a vinyl glove box that is supported by a tubular aluminum structure and is connected to an airlock which functions as an interchange for the transfer of materials in and out of the chamber (Figure 1B), using latex or neoprene gloves attached to vinyl sleeves (Figure 1C). It is important to precisely follow manufacturer's instructions for proper set up of the anaerobic chamber. It may be temperature-controlled with one (or more, depending on the size of the chamber) heater box units which provide air circulation through a palladium catalyst. The palladium catalyst serves to reduce oxygen contamination introduced through the interchange by converting hydrogen and oxygen into water. Ideally, both oxygen and hydrogen levels are monitored using a gas analyzer (Figure 1D), displaying oxygen concentration in parts per million (ppm) and hydrogen concentration as a percentage.
Liquid and solid media should be pre-reduced in the anaerobic chamber before use. Petri dishes (100 mm in diameter) can be reduced for only two hours prior to use31; however, liquid media should be reduced overnight. Importantly, media should be cooled prior to transfer into the chamber to prevent liquid flashing during airlock evacuation. Plastic consumables, such as 96-well plates, should be pre-reduced overnight before use, as plastic is porous and can store oxygen molecules32. Depending on the application, some plastics must be pre-reduced for up to 48 hr before use33. Biohazard waste should be disposed of appropriately. A small biohazard bag may be kept inside the chamber, but should be replaced frequently.
A hydrogen concentration of at least 3% must be maintained inside the chamber, as this will ensure both good growth of C. difficile and efficient oxygen removal. If the hydrogen concentration drops below 3% or if the chamber has not been used for several days, a chamber cycle should be executed to restore the hydrogen concentration to desirable levels. Here, approximately a third of the gas in the chamber is vacuumed out (the vinyl box will deflate significantly) and replaced with fresh gas mix. Ensure that the airlock is anaerobic before beginning. Pumping too much gas into the chamber should be avoided, as this not only places unnecessary stress on the vinyl bag, but will also preclude the user from reaching into the back of the chamber. Always perform this procedure in a well-ventilated area. Take additional precautions if the chamber is kept in a small, poorly ventilated room. Open any doors to the room as the gas contents inside the chamber can asphyxiate the user.
Additional maintenance includes regeneration of the palladium catalyst to ensure efficient oxygen removal within the chamber. As oxygen and hydrogen are reduced by the catalyst, water can accumulate on the surface of the palladium-covered aluminum pellets, reducing their efficiency over time. To overcome this, the palladium catalysts should be regenerated at least once a week by baking at least 230 °C for one hour. Additionally, as a result of both oxygen reduction and evaporation of water from plates and media, water accumulation is a common issue within anaerobic chambers. To ensure comfortable manipulation and increase the half-life of the equipment within the chamber, desiccant packs may be used, but should be dried out frequently following the same procedure used for the palladium catalysts. Alternatively, a dehumidifier may be used which circulates air through a metal block that condensates water and is collected in a container. The device prevents overflow of the water containers; however, dehumidifiers must be monitored and containers should be emptied when close to full. To clean the vinyl chamber, the use of a soft cloth and any commercially available cleaner recommended for polyvinyl chloride (PVC) is advised (e.g. Plastic Magic Cleaner, part no. 1600480, Coy Laboratories). To avoid scratching or damaging the vinyl chamber, do not use paper towels, Kim-wipes or products containing ketones or other compounds that will damage PVC.
Finally, C. difficile produces hydrogen sulfide gas (H2S), which is extremely reactive and corrosive. Hydrogen sulfide can be damaging to instrumentation, the palladium catalyst and any exposed metals. If possible, do not leave any instrumentation, such as homogenizers and spectrophotometers, in the chamber for long periods of times to avoid corrosion caused by hydrogen sulfide. Because of the production of hydrogen sulfide, items such as the palladium catalyst and gas analyzer will need to be periodically replaced as part of regular chamber maintenance. Alternatives for reducing hydrogen sulfide levels within the chamber, such as using activated charcoal, lead acetate, silver chloride or silver sulfate to absorb or chemically remove the hydrogen sulfide, have been described34 and may be utilized to slow down corrosion of equipment.
Additional Precautions
Never open one door to the airlock without ensuring that the other door is closed and locked to prevent oxygen contamination. Additionally, avoid use of sharp items within the chamber to reduce the risk of puncturing the vinyl.
It is important to note that hydrogen is a flammable gas in the presence of oxygen. Take care when introducing items into the chamber that high levels of oxygen contamination do not occur (greater than 999 ppm). It is important to use only premixed nonflammable anaerobic gas mix and closely monitor the gas analyzer within the chamber, especially when using a new gas tank. If both hydrogen and oxygen concentrations above 4% occur, ensure that the appropriate emergency procedures, which should be outlined in the laboratory's standard operating procedures (SOP), are followed.
Troubleshooting C. difficile growth
If poor growth of C. difficile cultures is observed in rich medium, this is most often due to oxygen contamination within the chamber. The addition of a reducing agent (e.g. L-cysteine or thioglycolate) to the medium may improve growth; however, the issue of oxygen contamination within the chamber would need to be addressed. Monitoring oxygen and hydrogen levels within the chamber using a gas analyzer can quickly alert the user to issues before a delay in progress and decrease in productivity occurs. If oxygen contamination occurs, quickly identifying the source with a gas leak detector (available from Coy Laboratories) is advised. To increase the chance in finding the location of a leak, a rag soaked in alcohol may be placed within the chamber since the gas leak detector can identify increased levels of hydrocarbons as well as increased levels of hydrogen. Once the source of the leak is found, it can be repaired using glue or silicone following the manufacturer's instructions.
A number of organisms readily grow in anaerobic environments, including other common Clostridial species (e.g. Clostridium perfringens) and the facultative anaerobe, Escherichia coli. Aseptic technique and other strategies can reduce the risk of contamination. Appropriate organization within the chamber, such as placing items within reach to lessen the potential for spills and prompt removal of accumulated biohazard waste, can reduce contamination risks. Additionally, paper towels dampened with a dilute bleach solution can be periodically brought into the chamber to wipe down surfaces and the latex or neoprene gloves. It is critical to not leave bleach or alcohol solutions inside the chamber for long periods of time as these can permeate the atmosphere and solid and liquid media, killing C. difficile, as well as damaging the vinyl34. Additionally, regular replacement of the latex or neoprene gloves can also reduce contamination risk. As mentioned above, if unsure if an organism is C. difficile or a contaminant, a test for the presence of the tdcB gene using PCR can quickly determine whether the organism is C. difficile21. Finally, if cultivating multiple organisms within the same anaerobic chamber, it is important to note that C. difficile can produce metabolic byproducts (e.g. hydrogen sulfide) that inhibit the growth of other anaerobic organisms and vice versa.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We would like to thank Coy Laboratories for kindly providing pictures of the anaerobic chamber. This work was supported by National Institutes of Health grant DK087763 (S.M. M.) and a STEP/HHMI Curriculum Development Fellowship (A.N. E.).
Materials
| Name of Reagent/Material | Company | Catalog Number | Comments |
| Proteose Peptone no. 2 | BD | 212120 | |
| Na2HPO4 | Fisher | S373 | |
| KH2PO4 | Fisher | BP362 | |
| NaCl | Fisher | S27 | |
| MgSO4 (anhydrous) | Fisher | M65 | |
| ᴅ-Fructose | Fisher | L96 | |
| Sodium taurocholate | Sigma | T4009 | |
| ᴅ-cycloserine | Sigma | C6880 | |
| Cefoxitin | Fluka | C4786 | |
| Brain heart infusion medium | BD | 237300 | |
| Proteose Peptone | BD | 211684 | |
| (NH4)2SO4 | Sigma | A5132 | |
| Tris base | Fisher | BP152 | |
| Agar | BD | 214010 | |
| L-cysteine | Sigma | C7755 | |
| BactoPeptone | BD | 211684 | |
| Columbian sheep blood agar | Fisher | L21928 | |
| NaCl | Fisher | S27 | |
| KCl | Fisher | P217 | |
| Glycerol | Fisher | BP2291 | |
| Sterile inoculating loops | Fisher | 22363596 | |
| Sterile swabs | Fisher | 1495990 | |
| Coy Vinyl Anaerobic Chamber and Accessories | Coy Laboratory Products, Inc | Customer Specified | These items are custom ordered per laboratory needs |
| Materials | |||
| TCCFA agar Proteose peptone no. 2 (Difco) 40 g Na2HPO4 5 g KH2PO4 1 g NaCl 2 g MgSO4 (anhydrous) 0.1 g Fructose 6 g Agar 20 g Bring to 1 L with deionized water and autoclave at 121 °C for 20 min to sterilize. After autoclaving, add: 10 ml of 10% (w/v) sodium taurocholate, filter-sterilized (dissolve in water; final concentration, 0.1%) 25 ml of 10 mg/ml ᴅ-cycloserine, filter-sterilized (dissolve in water; final concentration, 250 μg/ml) 1.6 ml of 10 mg/ml cefoxitin, filter-sterilized (dissolve in water; final concentration, 16 μg/ml) BHIS Medium Brain heart infusion 37 g Yeast extract 5 g For plates, add 15 g agar. Bring to 1 L with deionized water and autoclave at 121 °C for 20 min to sterilize. Optional (add after autoclaving): 3 ml of 10% (w/v) L-cysteine (dissolve in water; final concentration, 0.03%) 10 ml of 10% (w/v) sodium taurocholate (dissolve in water; final concentration, 0.1%) SMC Sporulation Medium BactoPeptone 90 g Protease peptone 5 g (NH4)2SO4 1 g Tris base 1.5 g Agar 15 g Bring to 1 L with deionized water and autoclave at 121 °C for 20 min to sterilize. Optional (add after autoclaving): 3 ml of 10% (w/v) L-cysteine (dissolve in water; final concentration, 0.03%) 70:30 Medium BactoPeptone 63 g Protease peptone 3.5 g Brain heart infusion 11.1 g Yeast extract 1.5 g (NH4)2SO4 0.7 g Tris base 1.06 g For plates, add 15 g agar. Bring to 1 L with deionized water and autoclave at 121 °C for 20 min to sterilize. After autoclaving, add 3 ml of 10% (w/v) L-cysteine (final concentration, 0.03%). Blood agar The use of premade Columbia anaerobic sheep blood agar plates (Fisher Scientific, L21928)35 is recommended. 1X Phosphate buffered saline (PBS) NaCl 8.01 g KCl 0.2 g Na2HPO4 1.44 g KH2PO4 0.27 g Bring to 1 L with deionized water and adjust pH to 7.4 with HCl. Filter sterilize before use. |
Referencias
- Hall, I. C., O’Toole, E. Intestinal flora in new-borin infants – With a description of a new pathogenic anaerobe, Bacillus difficilis. Am. J. Dis. Child. 49, 390-402 (1935).
- Tedesco, F. J., Barton, R. W., Alpers, D. H. Clindamycin-Associated Colitis – Prospective Study. Ann. Intern. Med. 81, 429-433 (1974).
- Gerding, D. N. Clostridium difficile 30 years on: what has, or has not, changed and why. Int. J. Antimicrob. Agents. 33, 2-8 (2009).
- Gerding, D. N., Muto, C. A., Owens, R. C. Treatment of Clostridium difficile infection. Clin. Infect. Dis. 46, 32-42 (2008).
- Dubberke, E. R., Olsen, M. A. Burden of Clostridium difficile on the healthcare system. Clin. Infect. Dis. 55, 88-92 (2012).
- Bouza, E. Consequences of Clostridium difficile infection: understanding the healthcare burden. Clin. Microbiol. Infect. 18, 5-12 (2012).
- Peery, A. F., et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 143, 1171-1173 (2012).
- Deakin, L. J., et al. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect. Immun. 80, 2704-2711 (2012).
- Drasar, B. S. Cultivation of anaerobic intestinal bacteria. J. Pathol. Bacteriol. 94, 417-427 (1967).
- Leach, P. A., Bullen, J. J., Grant, I. D. Anaerobic CO 2 cabinet for the cultivation of strict anerobes. Appl. Microbiol. 22, 824-827 (1971).
- Killgore, G. E., Starr, S. E., Del Bene, ., Whaley, V. E., N, D., Dowell, V. R. Comparison of three anaerobic systems for the isolation of anaerobic bacteria from clinical specimens. Am. J. Clin. Pathol. 59, 552-559 (1973).
- Bacic, M. K., Smith, C. J. Laboratory maintenance and cultivation of bacteroides species. Curr. Protoc. Microbiol. Chapter 13, Unit 13C 11 (2008).
- Doan, N., Contreras, A., Flynn, J., Morrison, J., Slots, J. Proficiencies of three anaerobic culture systems for recovering periodontal pathogenic bacteria. J. Clin. Microbiol. 37, 171-174 (1999).
- Socransky, S., Macdonald, J. B., Sawyer, S. The cultivation of Treponema microdentium as surface colonies. Arch. Oral. Biol. 1, 171-172 (1959).
- George, W. L., Sutter, V. L., Citron, D., Finegold, S. M. Selective and differential medium for isolation of Clostridium difficile. J. Clin. Microbiol. 9, 214-219 (1979).
- Wilson, K. H., Silva, J., Fekety, F. R. Suppression of Clostridium difficile by normal hamster cecal flora and prevention of antibiotic-associated cecitis. Infect. Immun. 34, 626-628 (1981).
- Wilson, K. H., Kennedy, M. J., Fekety, F. R. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. J. Clin. Microbiol. 15, 443-446 (1982).
- Bliss, D. Z., Johnson, S., Clabots, C. R., Savik, K., Gerding, D. N. Comparison of cycloserine-cefoxitin-fructose agar (CCFA) and taurocholate-CCFA for recovery of Clostridium difficile during surveillance of hospitalized patients. Diagn. Microbiol. Infect. Dis. 29, 1-4 (1997).
- Marler, L. M., et al. Comparison of five cultural procedures for isolation of Clostridium difficile from stools. J. Clin. Microbiol. 30, 514-516 (1992).
- Sorg, J. A., Dineen, S. S. Laboratory maintenance of Clostridium difficile. Curr. Protoc. Microbiol. Chapter 9, Unit 9A 1 (2009).
- Bouillaut, L., McBride, S. M., Sorg, J. A. Genetic manipulation of Clostridium difficile. Curr. Protoc. Microbiol. Chapter 9, Unit 9A 2 (2011).
- Lemee, L., Pons, J. L. Multilocus sequence typing for Clostridium difficile. Methods. Mol. Biol. 646, 77-90 (2010).
- Shanholtzer, C. J., Peterson, L. R., Olson, M. N., Gerding, D. N. Prospective study of gram-stained stool smears in diagnosis of Clostridium difficile colitis. J. Clin. Microbiol. 17, 906-908 (1983).
- Smith, C. J., Markowitz, S. M., Macrina, F. L. Transferable tetracycline resistance in Clostridium difficile. Antimicrob. Agents Chemother. 19, 997-1003 (1981).
- Permpoonpattana, P., et al. Surface layers of Clostridium difficile endospores. J. Bacteriol. 193, 6461-6470 (2011).
- Putnam, E. E., Nock, A. M., Lawley, T. D., Shen, A. SpoIVA and SipL are Clostridium difficile spore morphogenetic proteins. J. Bacteriol. , (2013).
- Burns, D. A., Minton, N. P. Sporulation studies in Clostridium difficile. J. Microbiol. Methods. 87, 133-138 (2011).
- Perez, J., Springthorpe, V. S., Sattar, S. A. Clospore: a liquid medium for producing high titers of semi-purified spores of Clostridium difficile. J. AOAC Int. 94, 618-626 (2011).
- Sorg, J. A., Sonenshein, A. L. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J. Bacteriol. 192, 4983-4990 (2010).
- O’Connor, J. R., et al. Construction and analysis of chromosomal Clostridium difficile mutants. Mol. Microbiol. 61, 1335-1351 (2006).
- Buggy, B. P., Wilson, K. H., Fekety, R. Comparison of methods for recovery of Clostridium difficile from an environmental surface. J. Clin. Microbiol. 18, 348-352 (1983).
- Koch, C. J., Kruuv, J. The release of oxygen from polystyrene Petri dishes. Br. J. Radiol. 45, 787-788 (1972).
- Ethapa, T., et al. Multiple factors modulate biofilm formation by the anaerobic pathogen Clostridium difficile. J. Bacteriol. , (2012).
- Speers, A. M., Cologgi, D. L., Reguera, G. Anaerobic cell culture. Curr. Protoc. Microbiol. Appendix 4, Appendix 4F (2009).
- Lyras, D., et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 458, 1176-1179 (2009).

