Cecal Ligation and Puncture-induced Sepsis as a Model To Study Autophagy in Mice
Summary
Experimental sepsis can be induced in mice using the cecal ligation and puncture (CLP) method. Current protocols to assess autophagy in vivo in the context of CLP-induced sepsis are presented here: A protocol for measuring autophagy using (GFP)-LC3 mice, and a protocol for measuring autophagosome formation by electron microscopy.
Abstract
Experimental sepsis can be induced in mice using the cecal ligation and puncture (CLP) method, which causes polymicrobial sepsis. Here, a protocol is provided to induce sepsis of varying severity in mice using the CLP technique. Autophagy is a fundamental tissue response to stress and pathogen invasion. Two current protocols to assess autophagy in vivo in the context of experimental sepsis are also presented here. (I) Transgenic mice expressing green fluorescence protein (GFP)-LC3 fusion protein are subjected to CLP. Localized enhancement of GFP signal (puncta), as assayed either by immunohistochemical or confocal assays, can be used to detect enhanced autophagosome formation and, thus, altered activation of the autophagy pathway. (II) Enhanced autophagic vacuole (autophagosome) formation per unit tissue area (as a marker of autophagy stimulation) can be quantified using electron microscopy. The study of autophagic responses to sepsis is a critical component of understanding the mechanisms by which tissues respond to infection. Research findings in this area may ultimately contribute towards understanding the pathogenesis of sepsis, which represents a major problem in critical care medicine.
Introduction
Sepsis, a systemic inflammatory response to infection, represents a leading cause of death in critically-ill patients1. Intra-abdominal infections, often leading to polymicrobial sepsis, account for 20% of sepsis cases, which have substantial mortality of up to 60%2. Sepsis-associated mortality primarily results from multi-organ dysfunction with subsequent organ failure3,4. Additional investigation into the pathogenic mechanism of this disease is urgently needed to promote the development of novel and more effective therapies.
The cecal ligation and puncture (CLP) method is a commonly used procedure for modeling sepsis in vivo. As the cecum is full of bacteria, its puncture results in polymicrobial peritonitis, translocation of bacteria into the blood (bacteremia), septic shock, multi-organ dysfunction and, ultimately, death5. It is generally accepted that CLP reflects clinical reality more accurately than previous techniques, such as injection of endotoxin or even purified bacteria into rodents, Thus, CLP is considered the gold standard (albeit not without limitations)6 for the experimental induction and, hence, the investigation of the pathogenesis of sepsis. In this monograph, we describe protocols designed to assess whether pathogenic mechanisms of sepsis include autophagy.
Autophagy, an evolutionarily conserved cellular process, facilitates the turnover of damaged proteins and organelles such as mitochondria and plays an important role in the clearance of intracellular pathogens including bacteria7,8. During autophagy, cytosolic proteins or organelles are sequestered into double membrane-bound vesicles called autophagosomes, which are subsequently delivered to the lysosomes for degradation9. A number of proteins have been identified as the mammalian homologues of autophagy-related genes (Atg), originally identified in yeast, which regulate the process of autophagy. The conversion of microtubule associated protein-1 light chain 3B (LC3B) (homologue of Atg8) from LC3B-I (free form) to LC3B-II (phosphatidylethanolamine-conjugated form) represents a major step in autophagosome formation9. Autophagic dysfunction is associated with aging and human diseases including cancer and neurodegenerative disorders10. Moreover, autophagy affects innate and adaptive immunity such as antigen presentation, lymphocyte development and cytokine secretion by immune cells8. Thus, it seems reasonable that autophagy might also play a role in the systemic inflammatory response to infection (i.e. in sepsis).
To date several methods have been described to assess the role of autophagy in tissue injury in vivo. These include the use of green fluorescence protein (GFP)-LC3 expressing mice and the quantification of autophagosomes in tissue by electron microscopy (these two methods are described in this monograph). Additional methods include the quantification of autophagic protein expression in tissue homogenates, and the analysis of autophagic flux (as described elsewhere)11-13. The goal of this review is to provide current protocols for assessing autophagy in vivo in the context of experimental sepsis.
Protocol
Note: The Institutional Animal Care and Use Committee at Brigham and Women's Hospital/ Harvard Medical School Area approved the following procedures.
1. Cecal Ligation and Puncture
Use mice of the same background (C57Bl/6), male, 8-10 weeks old. Female mice are more resistant than males against sepsis-induced lethality. Mice older than 8 weeks produce less variable results than younger mice in terms of survival after CLP. Approximately N=10 mice per compared group should be used for survival analysis. N=3-5 mice is adequate for autophagy assays.
- Use sterile small surgical instruments (namely scalpel, scissors and blunt anatomical forceps), which should be appropriate for rodent surgery.
- Prepare anesthesia mixture: To 7.95 ml of PBS add 150 μl of Xylazine (AnaSed injection 100 mg/ml) and 700 μl of Ketamine (Ketaset CIII, 100 mg/ml).
- To ensure aseptic conditions during the procedure, wear gloves, face mask and surgical gown.
- Weigh animals. Anesthetize them by injecting intraperitonally (i.p.) the mixture mentioned in step 3 in a dosage of μl (10x body weight in g) . For example, for a mouse weighing 22 g, administer 10×22= 220 μl of the anesthetic mixture. Thus, the final doses of Xylazine and Ketamine are 17 and 80 mg/kg body weight, respectively. Ensure that anesthesia is adequate; no flexion of extremity should be produced after toe pinch. Note: Use ophthalmic ointment on eyes to prevent dryness while under anesthesia.
- Place animals onto a clean work surface on their backs. Tails and hind paws are oriented towards the investigator. Disinfect the abdominal skin using alcohol (70% EtOH solution). Note: Surgical area should be completely shaved, to avoid contamination of the wound through contact with fur. Also, optimally, a circulating warm water pad should be used to maintain normothermia of anesthetized mice during the surgery.
- By using a scalpel, make a small longitudinal skin incision parallel and approximately 1 cm left to midline. Do not penetrate yet into the peritoneal cavity. Use small scissors to extend the initial incision.
- Identify abdominal muscles and dissect them to gain access into the peritoneal cavity.
- By using blunt anatomical forceps, identify the cecum and move it out of the peritoneal cavity. Be careful not to damage mesenterial blood vessels (this could lead to lethal hemorrhage).
- Ligate 60% of the cecum to achieve a mid-grade sepsis. Ligating equal to or more than 75% of the cecum generally results in high-grade sepsis, whereas ligating equal to or less than 25% of the cecum results in low-grade sepsis. Be careful not to ligate the ileocecal valve (this could block the intestinal continuity).
- By using a 21 G needle, perforate the cecum by one through-and-through puncture (two holes) near to the ligation. The direction of the perforation should be from the mesenteric to the anti-mesenteric side of the cecum. Be careful not to puncture mesenterial blood vessels.
- Remove the needle. Gently squeeze the ligated cecum to confirm patency through the two holes; only a small amount of feces should be extruded through them.
- Move the ligated and punctured cecum into the peritoneal cavity.
Note: For sham mice, skip steps 1.10-1.12. - Use silk surgical sutures 6-0 with cutting needle to close abdominal musculature. Use silk surgical sutures 6-0 with cutting needle to close abdominal skin.
- Inject 1 ml prewarmed (37 °C) normal saline i.p. to replace heat and hydration lost during the procedure. Place the mice under a heat lamp until their resuscitation. Note: Optimally, a safer warming device (such as a circulating warm water pad) can be used instead of a heat lamp.
- Postoperatively inject buprenorphin (0.05 mg/kg body weight s.c.) to achieve analgesia. Note: An animal should not be left unattended until it has regained sufficient consciousness to maintain sternal recumbency. An animal which has undergone a surgical treatment should not be returned to the company of other animals until fully recovered.
- Place animals back in their cages with unlimited access to food and water. Mice will be euthanized when they become moribund (indicated by clinical signs such as failure to move when touched or cyanosis). Check mice every 4 hr to avoid having them die prior to euthanasia.
2. Tissue Harvest and Fixation
- Prepare a 4% paraformaldehyde (PFA) solution: To 889 ml PBS add 111 ml of 37% PFA.
- Sacrifice mice using either CO2-induced narcosis or Ketamine/Xylazine solution in a high dosage.
- Immobilize the mice onto a clean work surface. Spray the abdominal and thoracic skin with 70% EtOH solution to disinfect it.
- Use a scalpel and small scissors to remove the abdominal and thoracic skin as well as the skin covering the tracheal area.
- Reveal trachea by removing its surrounding tissues (muscles and connective tissue).
- Place a suture (without cutting needle) around the trachea. Do not tighten it yet.
- Use a small peripheral venous catheter (20 G) to 'intubate' trachea. Be careful during the catheter insertion to avoid tracheal tear. Note that the largest diameter of trachea is just below the cricoid cartilage.
- Tighten the suture around the trachea/catheter. Remove the needle leaving only the plastic cannula of the catheter into the trachea. Needle served only as a guide-wire for inserting the plastic cannula.
- After having secured the plastic cannula into the trachea, cut the abdominal musculature, open the abdomen and reveal the diaphragm.
- Using a needle, puncture the diaphragm to produce pneumothorax (i.e. insertion of air into the pleural cavity). Lungs should then be collapsed.
- Using small scissors, remove the diaphragm, cut along the sternum (sternotomy), remove the rib cage and reveal the heart and the lungs.
- Through the plastic cannula inserted into the trachea, fix the lungs with the formaldehyde solution under 30 cm H2O pressure.
- Remove the lungs and place them into the 4% PFA for 24 hr and subsequently in 70% EtOH solution until processing.
- Identify and remove heart, liver and kidneys. In the same way, place them into the 4% PFA for 24 hr and subsequently in 70% EtOH solution until processing.
3. GFP Mice and Viewing of GFP Slides
- Perform CLP and sham surgery on GFP-LC3 mice as described in step 1, and postoperative procedures as described in step 2 of the protocol.
- Embed the tissues (namely lungs, heart, liver and kidneys) in paraffin and cut them in serial 5 μm sections.
- When ready for GFP staining, incubate at 60 °C for 30-60 min until paraffin melts and tissue appears translucent.
- Deparaffinize with standard graded series of xylene or xylene substitutes, 5 min xylene 1, xylene 2, and xylene 3 then 3 min incubations in graded series of EtOH: 100%, 95%, 75%, and then rinse in distilled water.
Note: After deparaffinization avoid tissue desiccation, which increases sample background. - Perform antigen retrieval using citric acid buffer (10 mM sodium citrate acid, 0.05% Tween 20, pH 6.0), by microwaving for 10-20 min. Be careful to replace buffer periodically to avoid tissue desiccation.
- Allow solution to cool for 20 min on the bench to complete antigen retrieval.
- Wash 2-3x with PBS. Remove excess buffer and transfer slides from rack to horizontal position in a slide box or other humidified chamber to prevent desiccation.
- Block 45-60 min at room temperature with 10% normal donkey serum (also 5% bovine serum albumin, or preferably serum from the animal in which the secondary antibody was generated) in PBS.
- Incubate samples in primary GFP antibody at 1:100 dilution in PBS overnight at 4 °C in humidified chamber. Gently apply small pieces of Parafilm over tissue with antibody to promote homogenous contact with the tissue and prevent evaporation.
Note: Remaining steps are performed at room temperature. - Wash 5x with PBS to remove excess primary antibody. Incubate with secondary antibody 1:500-1:1,000 in PBS for 1-2 hr at room temperature.
- Wash 5x with PBS to remove excess secondary antibody. If desired, counterstain the slides at this step.
- If using fluorescent secondary antibody, incubate 0.1% Sudan Black in 70% EtOH for 15-20 min to reduce autofluorescence. Wipe off excess Sudan Black from around tissue and wash 5x with PBS over 20 min.
- Mount sample with coverslip and store horizontally, until mounting solution has set.
4. Alternative Protocol: Direct Detection of GFP-LC3
- Cannulate and inflate lungs with a ~500 ml of 50/50 v/v OCT PBS.
- Embed in OCT by putting a small amount of OCT in the bottom of a cryomold.
- Carefully place the dissected lung complex in the mold.
- Slowly freeze over methylbutane chilled using dry ice.
- Add more OCT until the tissue is completely covered and frozen solid. Keep on dry ice and store at -80 °C.
- Using a cryomicrotome, cut 5-10 μm thick sections protecting the sections from light. Store sections and remaining tissue at -80 °C.
- To prepare slides for imaging, remove section from freezer and immediately apply a drop of PBS to prevent desiccation.
- Fix for 30 min with 4% PFA. Wash with PBS.
- Apply DAPI/Hoescht for 10 min. Wash 3x with PBS.
- Mount sample with coverslip and store horizontally, until mounting solution has set.
- Image using epifluorescence or confocal microscope. Store samples at 4 °C for up to one month.
5. Preparation of Tissue for Electron Microscopy
- Cut tissues into approximately 1 mm cubes for fixing.
- Fix the tissue in 2% formaldehyde, 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.4.
- Wash with 0.1 M sodium cacodylate buffer for 1-2 hr.
Note: Fixed tissue can be stored in 0.1 M sodium cacodylate at 4 °C. - Post fix in 1% osmium tetroxide in 0.2 M sodium cacodylate buffer for 1 hr.
- Rinse in 0.2 M sodium cacodylate buffer for 10 min 3x.
- Dehydrate in 70% EtOH, 20 min 2x. Dehydrate in 90% EtOH, 10 min 2x. Dehydrate in 100% EtOH, 20 min 2x.
- Incubate with propylene oxide (epoxy propane) for 10 min 2x.
- Incubate with propylene oxide/epoxy resin mixture (50:50) for 1 hr. The epoxy resin mixture is: 24 g Agar 100 resin, 13 g dodecenylsuccinic anhydride, 13 g methylnadic anhydride and 1 ml N-benzyldimethylamine. Mix thoroughly in a disposable beaker using a wooden spatula.
- Incubate with Epoxy resin overnight in uncapped vials (this allows any remaining propylene oxide to evaporate).
- Embed in labeled capsules with freshly prepared resin. Polymerize at 60 °C for 48 hr.
- Photograph tissue sections using a transmission electron microscope at 80 or 60 kV onto electron microscope film. Print the images onto photographic paper or store as digital media.
- Analyze autophagosome formation in intact cells containing nucleus. Low power fields (e.g. 2,000-4,000X) can be used to identify nucleated cells. Typically 6,800-10,000X images are used for this autophagosome quantification. Count autophagosomes per unit area from 15-30 fields for appropriate statistical representation. Autophagosomes have double membrane structure, where late stage autophogosomes resembled filled vacuoles.
Representative Results
Bacteremia is present in mice as early as 6 hr after CLP-induced sepsis14. Clinical signs of sepsis (including chills, tachypnoea and impaired motor activity) appear approximately 12 hr after the procedure. Mice subjected to CLP begin to die at around 18 hr after induction of peritonitis. The more severe is the sepsis the more increased is the lethality15. In detail, high-grade sepsis causes 100% mortality within 2-3 days, while mid-grade sepsis results in around 60% mortality at 7 days after CLP (beyond this time limit, no deaths are expected) (Figure 1). In contrast, mortality of sham mice should be zero
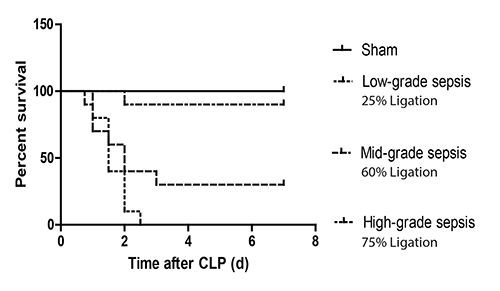
Figure 1. Survival curves after CLP of different severity in mice. C57Bl/6 mice were subjected to sham surgery or CLP-induced sepsis of different severity by adjusting the ligation keeping constant the number of perforations to the cecum (i.e. 1 'through-and-through' puncture (two holes) with a 21 G needle). Ligation of 60% of the cecum results in a mid-grade sepsis, which leads to around 60% mortality at 7 days after CLP. Ligation of ≥75% of the cecum results in high-grade sepsis and, subsequently, in 100% mortality within 2-3 days after CLP; while, ligation of ≤25% of the cecum leads in low-grade sepsis and, thus, in nearly zero mortality. Typical survival outcomes are shown.
For the purposes of the autophagy studies, we chose to implement a CLP model of mid-grade sepsis. Low-grade sepsis might be inadequate to induce autophagy, while high-grade sepsis may lead to early lethality of mice (before induction of autophagy).
For assaying autophagy, we have used transgenic mice expressing GFP-LC3 fusion protein subjected to the aforementioned sepsis protocols. For treatment procedures that result in stimulation of the autophagy pathway, it is anticipated that there will be localized enhancement of GFP signal (puncta), as assayed either by immunohistochemical or confocal assays, indicative of enhanced autophagosome formation16.
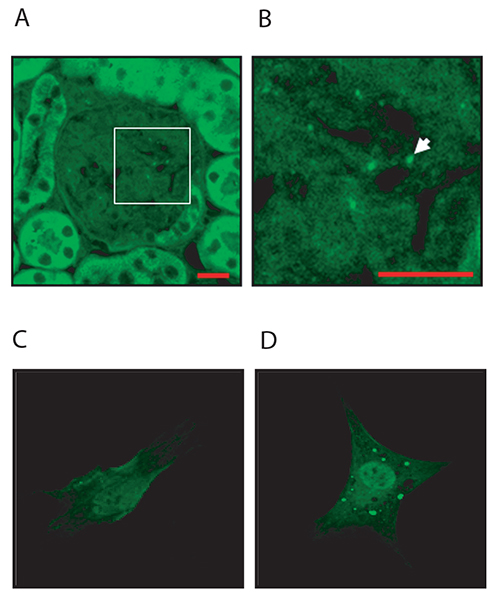
Figure 2. GFP imaging in vivo and in vitro. (A, B) Representative example of GFP imaging in mouse organs (e.g. kidney) obtained from GFP-LC3B mice. Image shows GFP-LC3 puncta in the glomeruli of kidney tissue obtained from untreated mice. Scale bars = 10 mm. (C, D) Additional data are provided to illustrate in vitro GFP-LC3 imaging. Primary mesangial cells were isolated from GFP-LC3 transgenic mice using a protocol for cell isolation from genetically-modified mice as previously described.17 The cells were incubated in the absence (C) or presence (D) of TGF-β1 (2 ng/ml) for 24 hr as previously described18. Treatment with TGF-β1 increased the abundance of autophagosomes (green puncta). Click here to view larger image.
This can be further tested by classical ultrastructural analysis using electron microscopy, as outlined in the protocol. It is anticipated that tissue sections obtained from septic mice or other tissue injury models would show enhanced autophagic vacuole (autophagosome) formation per unit tissue area.
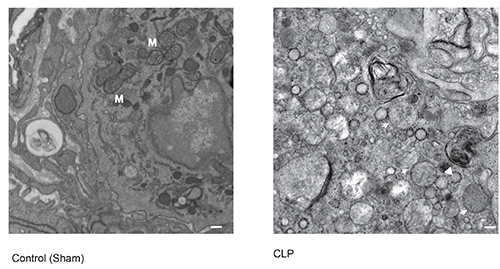
Figure 3. Electron microscopy of autophagosome formation. A representative micrograph of early and late-stage autophagosome formation in lung tissue taken from C57Bl/6 mice subjected to mid-grade CLP (60% ligation, 21 G, 2 holes). Εarly stage autophagosomes have a double-membrane structure (arrow), while late stage autophagosomes resemble a filled vacuole (arrowhead). M denotes mitochondria. Scale bar=1 μm. A representative control image is shown of lung tissue from C57Bl/6 mice subjected to sham operation. Click here to view larger image.
Discussion
The major advantage of CLP is that it allows researchers to investigate sepsis of different severities (i.e. from low- to mid- and high-grade). Severity of induced sepsis is affected by the length of cecum ligated (which the most important determinant), the size of needle used for puncture and the number of holes performed15. In addition, the mouse strain and gender can impact on the severity of sepsis; several strains are more susceptible than others and males are generally more susceptible than females19,20. The above factors taken together determine the CLP-induced mortality.
Based on our experience, there is a difference between investigators with regard to achieved CLP-induced mortality. For example, Rittirsch et al. used 50% ligation, 21 G, 1 'through-and-through' puncture (two holes) to achieve a 60% mortality15; an investigator in our research group needed to use a slightly more severe model (60% ligation, 21 G, 1 'through-and-through' puncture) to obtain the same mortality; whereas, another investigator in our research group used a more severe model (near 100% ligation, 19 G, 1 'through-and-through' puncture) to achieve a much lower mortality (only 25%). With the above considerations in mind, one could argue that the characterization of severity of CLP (i.e. low-, mid- or high-grade sepsis) should be done retrospectively (i.e. by looking at the resulting mortality) rather than prospectively (i.e. by applying certain conditions, for example 50% ligation). When an operator achieves a mortality of around 60%, then, this is mid-grade sepsis, no matter which conditions (such as length of cecum ligated and number of holes performed) are implemented. Thus, it seems reasonable that the operator try different conditions to identify those under which 60% of mice die. Then, the operator has to implement exactly the same conditions in both groups being compared.
By implementing exactly the same conditions (e.g. the length of cecum ligated, size of needle used for puncture, number of holes performed, the mouse strain, and gender), it is anticipated that consistent and reproducible results will be produced15. However, even after performance of CLP in a standardized manner (taking into account the above determinants), variability may occur. It has been recognized by experts that 'even when identical insults are given to identically aged groups of animals - even from the same litter - a variable individual effect can be seen'6. Thus, in attempt to reduce variability, it seems reasonable that the operator perform CLP in both compared groups on the same day.
The analysis of autophagy, a dynamic cellular process, is complex. The protocols outlined in this monograph provide the basis for estimating autophagy activation in vivo, based on biochemical or morphological analysis of autophagosome formation. In principle, the protocols provided in this chapter can be applied to the analysis of autophagy in any organ tissue in mice subjected to CLP or alternative models of sepsis. While the liver is generally accepted to represent a primary target of CLP, this procedure may also cause injury to lung or kidney tissue, which is worthy of further study. The effect of CLP on autophagy of hepatocytes has been relatively well studied compared to its effects on autophagy of kidney or lung; and some relevant evidence has been recently published21,22.
It should be noted that these are static measures of autophagy, as increased autophagosome numbers may also reflect autophagy dysfunction through blockage of lysosomal function and end-stage processing. In this regard, these assays should be complemented with biochemical assays for autophagic substrate turnover (e.g. flux) as recently described and adapted for in vivo analyses11. These assays can also be complemented by standard Western immunoblot analysis for the expression of key autophagy proteins in tissue as recently described12,13. Thus, it is recommended that a combination of these tests be implemented to gain an accurate estimation of the status of autophagy in injured tissue16.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIH grants P01 HL108801, R01-HL60234, R01-HL55330, R01-HL079904, to A. M. K. Choi. S. Ryter received salary support from the Lovelace Respiratory Research Institute.
Materials
| GFP-LC3 Transgenic Mice | Riken (Japan) | RBRC00806 | GFP-LC3#53 |
| Xylazine | Henry-Schein | 568-0606 | Xylazine HCl Injection Vet |
| Ketamine | Henry-Schein | 995-2949 | Ketaset Inj 100 mg/ml |
| EtOH | Fisher | A405-20 | Histology Grade |
| EtOH | Fisher | A407-1 | For Sterilizatiion |
| silk surgical sutures 6-0 | Owens & Minor | 2300-0078OG, 017624 | |
| buprenorphine-HCl | Henry-Schein | 614-5157 | Buprenex Ampules |
| paraformaldehyde (37%) solution | JT Baker | S898-09 | |
| xylenes | Fisher | X3P-1GAL | |
| anti-GFP monoclonal antibody | Life Technologies | G10362 | |
| Hoescht | Sigma | 944403 | |
| DAPI | Invitrogen | D1306 | |
| OCT | VWR scientific | 25608-930 | |
| Sudan Black | Santa Cruz | sc-203760 | |
| EM grade Glutaraldehyde 2.5% in sodium cacodylate | Electron microscopy Sciences | 15960 | |
| propylene oxide | Sigma | 240397 | |
| Agar 100 resin | Agar scientific | R1045 | |
| dodecenylsuccinic anhydride | Sigma | 46346 | |
| methylnadic anhydride | Sigma | 45359 | |
| N-benzyldimethylamine | Sigma | 185582 |
Referencias
- Hotchkiss, R. S., Karl, I. E. The pathology and treatment of sepsis. N. Engl. J. Med. 348, 138-150 (2003).
- Anaya, D. A., Nathens, A. B. Risk factors for severe sepsis in secondary peritonitis. Surg. Infect. 4, 355-362 (2003).
- Bone, R. C., Grodzin, C. J., Balk, R. A. Sepsis: A new hypothesis for pathogenesis of the disease process. Chest. 112, 235-243 (1997).
- Rivers, E., Med, s. h. o. c. k. .. N. .. E. n. g. l. .. J. .., et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med.. 345, 1368-1377 (2001).
- Deitch, E. A. Rodent models of intra-abdominal infection. Shock. 24 (Suppl 1), 19-23 (2005).
- Raven, K. Rodent models of sepsis found shockingly lacking. Nat. Med. 18, 998 (2012).
- Levine, B., Kroemer, G. Autophagy in the pathogenesis of disease. Cell. 132, 27-42 (2008).
- Virgin, H. W., Levine, B. Autophagy genes in immunity. Nat. Immunol. 10, 461-470 (2009).
- Mizushima, N., Komatsu, M. Autophagy: renovation of cells and tissues. Cell. 147, 728-741 (2011).
- Choi, A. M., Ryter, S. W., Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 368, 651-662 (2013).
- Haspel, J., et al. Characterization of macroautophagic flux in vivo using a leupeptin-based assay. Autophagy. 7, 629-642 (2011).
- Chen, Z. H., et al. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLos One. 3, 3313 (2008).
- Kim, H. P., Chen, Z. H., Choi, A. M., Ryter, S. W. Analyzing autophagy in clinical tissues of lung and vascular diseases. Meth. Enzymol. 453, 197-216 (2009).
- Flierl, M. A., et al. Adverse functions of IL-17A in experimental sepsis. FASEB J. 22, 2198-2205 (2008).
- Rittirsch, D., Huber-Lang, M. S., Flierl, M. A., Ward, P. A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4, 31-36 (2009).
- Mizushima, N., Yoshimori, T., Levine, B. Methods in mammalian autophagy research. Cell. 140, 313-326 (2010).
- Wang, L., Ma, R., Flavell, R. A., Choi, M. E. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for activation of p38α and p38δ MAPK isoforms by TGF-β1 in murine mesangial cells. J. Biol. Chem. 277, 47257-47262 (2002).
- Ding, Y., Kim, J. K., Kim, S. I., Na, H. J., Jun, S. Y., Lee, S. J., Choi, M. E. TGF-{beta}1 protects against mesangial cell apoptosis via induction of autophagy. J Biol Chem. 285, 37909-37919 (2010).
- Baker, C. C., Chaudry, I. H., Gaines, H. O., Baue, A. E. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 94, 331-335 (1983).
- Godshall, C. J., Scott, M. J., Peyton, J. C., Gardner, S. A., Cheadle, W. G. Genetic background determines susceptibility during murine septic peritonitis. J. Surg. Res. 102, 45-49 (2002).
- Carchman, E. H., Rao, J., Loughran, P. A., Rosengart, M. R., Zuckerbraun, B. S. Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology. 53, 2053-2062 (2011).
- Lo, S., et al. Lc3 over-expression improves survival and attenuates lung injury through increasing autophagosomal clearance in septic mice. Ann. Surg. 257, 352-363 (2013).

