A Method for Systematic Electrochemical and Electrophysiological Evaluation of Neural Recording Electrodes
Summary
Different electrode coatings affect neural recording performance through changes to electrochemical, chemical and mechanical properties. Comparison of electrodes in vitro is relatively simple, however comparison of in vivo response is typically complicated by variations in electrode/neuron distance and between animals. This article provides a robust method to compare neural recording electrodes.
Abstract
New materials and designs for neural implants are typically tested separately, with a demonstration of performance but without reference to other implant characteristics. This precludes a rational selection of a particular implant as optimal for a particular application and the development of new materials based on the most critical performance parameters. This article develops a protocol for in vitro and in vivo testing of neural recording electrodes. Recommended parameters for electrochemical and electrophysiological testing are documented with the key steps and potential issues discussed. This method eliminates or reduces the impact of many systematic errors present in simpler in vivo testing paradigms, especially variations in electrode/neuron distance and between animal models. The result is a strong correlation between the critical in vitro and in vivo responses, such as impedance and signal-to-noise ratio. This protocol can easily be adapted to test other electrode materials and designs. The in vitro techniques can be expanded to any other nondestructive method to determine further important performance indicators. The principles used for the surgical approach in the auditory pathway can also be modified to other neural regions or tissue.
Introduction
Neural implants are being used increasingly for research, controlling prosthetics and treatment of disorders such as Parkinson's disease, epilepsy, and sensory loss1,2. Measuring and/or controlling both the chemical and electrical composition of the brain is the basis for all neural implants. However, it is important to administer a treatment only when the neural tissue is in the aberrant state to reduce side effects3. For instance, deep brain stimulators for epilepsy treatment should only apply an electrical pulse to the brain during a seizure. Some side effects may be dystonia, loss of memory, disorientation, impaired cognitive function, induced hallucinations, depression or anti-depression3,4. In many devices, a closed loop system is therefore necessary to record electrical activity and to trigger stimulation when an abnormal state is detected. Recording electrodes are also used to control prosthetic devices. It is critical to record the target neural activity with the highest possible signal-to-noise ratio to achieve the most accurate triggering and device control. A large signal-to-noise ratio is also highly desirable for research applications, as more reliable data can be obtained, resulting in fewer required test subjects. This will also allow a greater understanding of the mechanisms and pathways involved in neural stimulation and recording.
After a neural implant has been placed into the brain, an immune response is triggered5,6. The time course of the response is generally divided into acute and chronic phases, each consisting of different biological processes7. The immune response can have dramatic effects on the performance of the implant, such as isolation of the electrodes from the target neurons by encapsulation in a glial scar or chemical degradation of the implant materials8. This can reduce the signal-to-noise ratio of a recording electrode and the power output of a stimulating electrode, and lead to electrode failure9. Careful choice of implant design and materials are necessary to prevent failure over the implant lifetime.
Many different materials and implant designs have been developed recently to improve the signal-to-noise ratio and implant stability for neural recording. Electrode materials have included platinum, iridium, tungsten, iridium oxide, tantalum oxide, graphene, carbon nanotubes, doped conducting polymers, and more recently hydrogels. Substrate materials tested also includes silicon, silicon oxide, silicon nitride, silk, Teflon, polyimide, and silicone. Various electrode modifications have also been investigated, using coatings such as laminin, neurotrophins, or self-assembled monolayers and treatments using electrochemical, plasma and optical techniques. Implant designs could be 1-, 2- or 3-dimensional with the electrodes generally at the tip of an insulating probe or along the edge of a shank for penetrating electrodes or in a 2-dimensional array for cortex surface implants. Regardless of electrode design or material, previous literature has typically demonstrated the performance of the new implant without reference to other implant constructs. This prevents a systematic evaluation of their properties.
This protocol provides a method for comparing different electrode materials via a range of analytical and electrophysiological techniques. It is based on a recently published article which compared 4 different doped conducting polymer coatings (polypyrrole (Ppy) and poly-3,4-ethylenedioxythiophene (PEDOT) doped with sulfate (SO4) or para-toluene sulfonate (pTS)) and 4 different coating thicknesses10. This article found one material, PEDOT-pTS with a 45 sec deposition time, had the highest signal-to-noise ratio and spike count with the smallest background noise and that these parameters were dependent on electrode impedance. PEDOT-pTS also displayed superior acute biostability compared to the other doped conducting polymers and bare iridium electrodes. The protocol allows the critical parameters controlling the signal-to-noise ratio and stability to be determined and used to further improve the performance of neural recording electrodes.
Protocol
The protocol has been approved by the La Trobe University (09-28P) and RMIT University Animal Ethics committees (1315).
1. Electrode Preparation and Preliminary in vitro Testing
- Prepare electrode coating deposition solutions; for instance 10 mM 3,4-ethylenedioxythiophene (EDOT) and 0.1 M sodium para-toluene sulfonate (Na2pTS) to form poly-3,4-ethylenedioxythiophene-pTS (PEDOT-pTS).
- Connect the electrode array to a potentiostat.
- Carefully place the electrode array into the deposition solution and clamp into place.
- Place a platinum mesh counter electrode and Ag/AgCl reference electrode into the deposition solution and connect to a potentiostat.
- Using the potentiostat, deposit coatings onto the desired electrodes. Deposition conditions (potential, current and time) will vary depending on the desired coatings. For PEDOT-pTS coatings, an applied potential of 1 V for 15, 30, 45, or 60 sec has been used. Four electrodes on the array should be coated with the coating in a staggered configuration (Figure 1).
- Remove the electrode array from the deposition solution and gently rinse with deionized water.
- Repeat the coating procedure with other materials as desired.
- Prepare in vitro testing solution (0.3 M di-sodium phosphate (Na2HPO4) in deionized water).
- Connect the electrode array to a potentiostat.
- Carefully place the electrode array into the testing solution and clamp into place.
- Place a platinum mesh counter electrode and Ag/AgCl reference electrode into the testing solution and connect to a potentiostat.
- Using the potentiostat, perform sequential electrochemical impedance spectroscopy (EIS) (potential offset 0 V, amplitude 10 mV, frequency range 10-100,000 Hz) and cyclic voltammetry (1 cycle, potential range 0.8 to -0.8 V, scan rate 100 mV/sec) on all electrodes. Untested electrodes are kept at open circuit potential and a quiet time of 1 sec is used between each test. All 32 electrodes are in contact with the solution for the full testing session of 1 hr.
- Remove the electrode array from the testing solution and gently rinse with deionized water.
- Perform any other desired analyses such as optical microscopy.
- Store probes in a dry protective container to prevent damage and degradation of the electrode surfaces.
2. Electrode Implantation
- Weigh the rat.
- Inject urethane (20% w/v in distilled water, 1.3 g/kg i.p.) for nonrecovery anesthesia.
- Ensure anesthesia onset by testing for a toe pinch withdrawal reflex. If anesthesia is not sufficient, supplementary doses of urethane should be administered (0.3 g/kg i.p.).
- Apply eye lubricant, and then shave the head of the animal.
- Place the animal in the prone position on a homeothermic plate and insert a rectal probe (37.5 °C).
- Place one ear bar into the approximately expected final position within the stereotaxic frame, and then adjust the animal to position the ear bar in the external acoustic meatus.
- Align the second ear bar into the contralateral external acoustic meatus. Shift the animal in the ear bars to align with the tooth holder.
- Using rat-tooth forceps, open the animal's jaw, hook the upper incisors over the tooth holder and clamp the nose in place.
- Create an incision in the skin of the head, approximately 1 mm to the right of the midline and from 10 mm rostral to 10 mm caudal of lambda.
- Retract the skin and muscle laterally from the incision to expose the parietal and interparietal bones Using 20% hydrogen peroxide solution and a gauze pad, scrub the surface of the exposed bone.
- Drill a hole approximately 3 mm2 in the interparietal bone as close to lambda and the midline as possible and remove the bone plug. Using sterile saline, flush the hole to remove any bone dust or fragments which may damage the electrode.
- Using blunt-blunt scissors, dissect below the scruff of the neck and create a cavity. Wrap a Ag/AgCl wire in cotton wool, saturate it with saline and then insert the reference electrode into the cavity.
- Make an incision in the dura mater on the sagittal plane using the tip of a needle.
- Attach the electrode array to the electrode manipulator and adjust its position over the opening with a 19° rostro-caudal angle. Manually insert the electrode approximately 2 mm into the brain towards the inferior colliculus.
- Attach the speaker to the left hollow ear bar.
- Ensure the amplifier is turned on. Then verify animal anesthesia before sealing the recording chamber.
3. In vivo Testing
- Deliver white noise bursts, (Gaussian distributed noise, 1-44 kHz; 10 msec rise-fall time) and monitor the activity on each electrode. The maximum rate at which bursts should be delivered is one burst every 200 msec.
- Using the motorized microdrive, slowly insert the electrode array until acoustically driven activity is recorded on the 3 most distal electrodes on each shank (the number and position of electrodes recording activity may vary with electrode placement or electrode design).
- Perform the acoustic stimulation protocol using 300 repetitions of 50 msec white noise bursts (Gaussian distributed noise, 1-44 kHz; 10 msec rise-fall time) with a 1 sec repetition rate at 70 dB, and record the multiunit activity at each electrode (24.4 kHz sampling rate).
- Slowly insert the electrode array another 200 μm into the IC to position each electrode in roughly the same position as the more distal electrode from the initial recording position.
- Repeat the acoustic stimulation and neural recording protocol.
- Continue inserting the electrode array in 200 μm steps and performing the acoustic stimulation and neural recording protocol until all electrodes have recorded acoustically driven activity from at least 3 positions (typically 8-12 electrode positions overall).
- Retract the electrode array in 200 μm steps and continue performing the acoustic stimulation and neural recording protocol until the initial electrode array position is achieved.
- Carefully retract the electrode array manually.
- Inject an overdose of sodium pentobarbitone (Lethobarb; 200 mg/kg i.p.) to euthanize the animal.
- Gently rinse the electrode array with distilled water. Then store probes in a dry protective container to prevent damage and degradation of the electrode surfaces.
4. Post-implantation in vitro Testing
- Gently rinse the electrode array with distilled water to remove any contamination.
- Connect the electrode array to a potentiostat.
- Carefully place the electrode array into the testing solution and clamp into place.
- Place a platinum mesh counter electrode and Ag/AgCl reference electrode into the testing solution and connect to the potentiostat.
- Using the potentiostat, perform sequential electrochemical impedance spectroscopy (EIS) (potential offset 0 V, amplitude 10 mV, frequency range 10-100,000 Hz) and cyclic voltammetry (1 cycle, potential range 0.8 to -0.8 V, scan rate 100 mV/sec) on all electrodes. Untested electrodes are kept at open circuit potential and a quiet time of 1 sec is used between each test. All 32 electrodes are in contact with the solution for the full testing session of 1 hr.
- Remove the electrode array from the testing solution and gently rinse with deionized water.
- Place the electrode array into an enzymatic cleaning solution for 24 hr.
- Remove the electrode array from the solution and rinse with distilled water.
- Connect the electrode array to a potentiostat.
- Carefully place the electrode array into the testing solution and clamp into place.
- Place a platinum mesh counter electrode and Ag/AgCl reference electrode into the testing solution and connect to the potentiostat.
- Using the potentiostat, perform sequential electrochemical impedance spectroscopy (EIS) (potential offset 0 V, amplitude 10 mV, frequency range 10-100,000 Hz) and cyclic voltammetry (1 cycle, potential range 0.8 to -0.8 V, scan rate 100 mV/sec) on all electrodes. Untested electrodes are kept at open circuit potential and a quiet time of 1 sec is used between each test. All 32 electrodes are in contact with the solution for the full testing session of 1 hr.
- Remove the electrode array from the testing solution and gently rinse with deionized water.
- Store probes in a dry protective container to prevent damage and degradation of the electrode surfaces.
Representative Results
A typical electrode array used for this experimental protocol is shown in Figure 1. There are 32 iridium electrodes on 4 shanks with 413 μm2 nominal geometric area and a 200 μm pitch. Every second electrode on the array has been coated with one of four different electrode coatings, labeled 1-4. The coating materials have been carefully chosen for their chemical, mechanical and electrochemical properties. As mentioned previously10, increased deposition times will increase the electrode area and coating thickness, while larger applied current or potential may also increase deposition rate, competing reactions may occur that affect the deposition process. The deposition protocol has been optimized previously for this particular conducting polymer to ensure a reproducible coating is achieved and so it is confined to the electrode (i.e. doesn't spread to an adjacent electrode)10.
After the electrode array has been modified, a series of optical and electrochemical analyzes should be undertaken. In this instance, cyclic voltammetry (Figure 2) and electrochemical impedance spectroscopy (Figure 3) have been utilized. This protocol uses cyclic voltammetry over a large potential range, beginning in the reductive scan direction. If the electrode charge density is required, the cyclic voltammetric data should be transformed to a current-time plot and either the reductive or oxidative regions integrated (Figure 2b). The impedance is obtained over a wide frequency range with a small amplitude at 0 V. The impedance data can be represented in a variety of formats including impedance (Figure 3a) or phase vs frequency (Figure 3b) or as a Nyquist plot (Figure 3c).
The electrode array should be manually inserted so the shank tips are just through the brain surface. White noise is used to drive multi-unit activity while the microdrive slowly inserts the array into the inferior colliculus (IC) in 200 μm steps. The electrode response should be monitored as the array is inserted, and once roughly the bottom 3 electrodes on each shank are displaying acoustically driven activity (Figure 4a), the white noise can be turned off. The in vivo acoustic stimulation protocol is then undertaken. Typical stream data from the electrode array will display a large increase in RMS in sync with the noise pulse over a stable baseline (Figure 5). It is important to minimize all external electrical and acoustic noise to reduce the baseline activity. On completion of the acoustic stimulation protocol, the electrode array is inserted and retracted in 200 μm steps. The acoustically driven activity represented as a peristimulus time histogram (PSTH) or raw data stream at different electrode array positions in the IC is shown in Figures 4 and 5.
After the full array insertion and retraction process, the electrodes are gently rinsed and retested with the in vitro protocol to determine the coating stability. Further details on the effects of protein fouling can be obtained from a previous article10.
The in vivo data can be comprehensively analyzed. One crucial parameter for neural recording is the signal-to-noise ratio (SNR)10. Two electrodes from the same array, one coated and one uncoated, were initially not in the IC (Figure 6a). After 400 μm insertion, the coated electrode displays an increase in SNR while the uncoated electrode requires 1,200 μm insertion. The SNR at both electrodes fluctuates at different positions in the IC, but does not degrade over time (position). This indicates that the neurons are still viable over the course of the experiment and that the electrode coatings are stable when using this protocol. The variation in SNR with position has been attributed to biological noise (different number and position of neurons in the vicinity of the electrodes)10.
Different sound pressure levels (SPL) can be used for the acoustic stimulation so long as they are above acoustic threshold and do not deafen the animal. The SNR varies with SPL and must therefore be consistent (Figure 6b). A high SPL is recommended for generating a larger multi-unit driven response, as a greater area of the IC will be stimulated, making electrode placement easier and reducing the biological noise while also providing a greater number of electrode positions for statistical analysis.
Tables and Figures:

Figure 1. Optical micrograph of a conducting polymer modified electrode array. The labels (1-4) represent four different coatings, enabling a statistical analysis of each coating within a single experiment. One uncoated electrode is also labeled. In this example, 1-4 are 15, 30, 45, and 60 sec deposition times of PEDOT-pTS. Click here to view larger image.
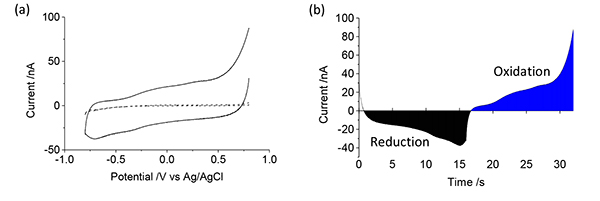
Figure 2. Cyclic voltammogram of a conducting polymer coated electrode (solid line) displayed versus (a) potential and (b) time with oxidation and reduction charge shaded for electrode charge density measurements. An uncoated electrode (dashed line) is shown for comparison. Click here to view larger image.
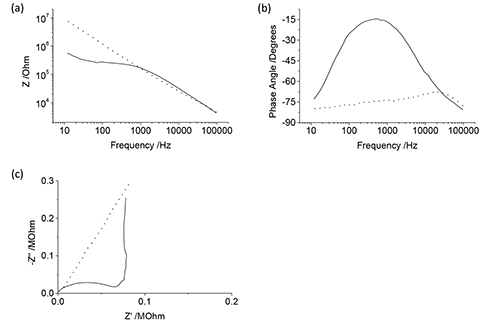
Figure 3. (a) Impedance, (b) phase and (c) high frequency range of a Nyquist plot for representative uncoated (dashed) and conducting polymer coated (solid) electrodes. Click here to view larger image.
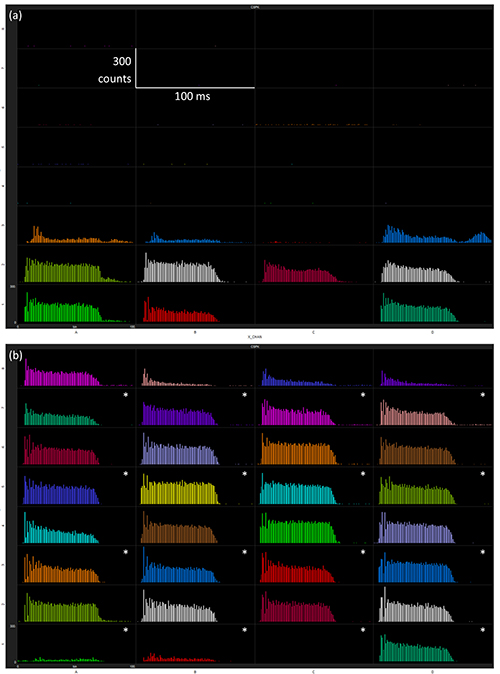
Figure 4. Peristimulus time histogram measured at each electrode, averaged over 300 repetitions at 70 dB white noise at two different depths in the IC; (a) 0 μm and (b) 800 μm insertion depths. Asterisks indicate the coated electrodes. Click here to view larger image.
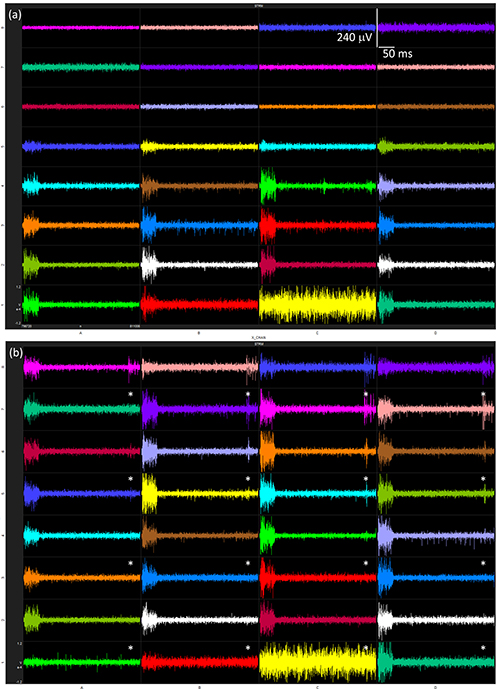
Figure 5. Streaming data measured at each electrode with 70 dB white noise bursts at two different depths in the IC; (a) 0 μm and (b) 800 μm insertion depths. Asterisks indicate the coated electrodes. Click here to view larger image.
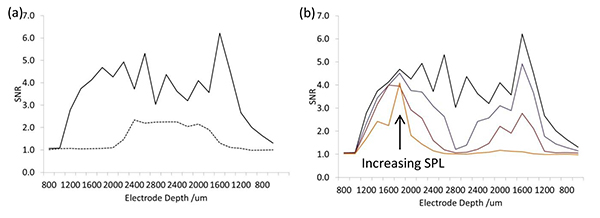
Figure 6. Signal to noise ratio during insertion and retraction of the electrode array into the IC. (a) 70 dB white noise at representative uncoated (dashed) and conducting polymer coated (solid) electrodes and (b) different sound pressure levels (40-70 dB) on a conducting polymer coated electrode. Click here to view larger image.
Discussion
This protocol provides a method for comparing neural recording electrode coatings within one animal. The electrode design used is ideal for implantation into a rat inferior colliculus (IC), with dimensions of a similar scale. Variations of this electrode such as more space between shanks would prevent all shanks being in the rat IC at the same time, while longer shanks and a larger pitch between electrodes increase the risk that the shank tips will come in contact with the base of the skull during insertion. Smaller electrode pitch increases the risk of the coating from one electrode contacting an adjacent electrode. The electrode area will affect the neural recording response, and must therefore be consistent across experiments. The area chosen is ideal for measuring multi-unit activity, resulting in more consistent data with variable electrode-neuron distances. Using 4 electrodes with the same coating allows statistical analysis from within the one animal and sufficient data can be obtained from 2 animals with 2 different electrode arrays (sample size 8 for each material). The electrode coatings have also been staggered on each shank to minimize error such as neuron death while inserting the electrode array over the course of the experiment or electric field effects from the change in shank width from tip to base. These types of error would give a different electrophysiological response at electrodes at the tip of the shank compared to those at the base. Inter-batch electrochemical and electrophysiological variations from the electrode arrays have been found; therefore it is recommended that a series of experiments are performed with electrode arrays from the same batch. A 3D electrode array could also be used to gather more data from the one animal, although care must be taken to ensure electrodes are consistently coated as mass transport may be different to the electrodes on the edge vs center shanks.
The in vitro experiments have been performed in a nondegassed buffer solution to better represent conditions in vivo. Although this is not critical, it should be consistent across experiments to prevent variations associated with oxygen reduction. The specific composition of the testing solution was based on recommendations from NeuroNexus (private communications) but variations are possible, such as addition of electrolyte or adjusting pH. Ultimately, a highly conductive, nonreactive solution is required to ensure the in vitro response is dominated by the electrode behavior, but it should be consistent between experiments. More details on performing electrochemical analysis should be obtained from suitable sources11. The electrode coating or cyclic voltammetric protocol when using iridium electrodes must be chosen carefully, as application of very positive potentials for long periods of time will form iridium oxide and alter the electrode properties. Alternatively, platinum electrodes can be used, eliminating the possibility of oxide formation. The scan rate and potential range is based on previous literature and must be consistent across experiments, although no correlations between charge density and neural recording response were seen10, further details on these parameters will be addressed in future publications. It is also important to keep the EIS parameters consistent, as large amplitudes, different offset potentials and electrochemical cell configurations will alter the impedance response.
The frequency range used for the EIS was discussed in the preceding article10. The electrode impedance for neural implants is routinely only measured at 1 kHz. This may result in a loss of significant information. For instance an uncoated and coated electrode may generate similar impedance values at 1 kHz (Figure 3a). However at lower frequencies, this coated electrode possesses significantly lower impedance. Similarly for the phase (Figure 3b), at 1 kHz the uncoated and coated electrodes have a very different phase, but at lower and higher frequencies they are similar. This difference in properties is very apparent on the Nyquist plot (Figure 3c) where the uncoated electrode is linear while the coated electrode possesses a semi-circle at high frequencies and a vertical response at low frequencies.
The central nucleus of the IC of a rat animal model was chosen as a suitable site for comparing recording electrodes due to its easy accessibility, relatively large size, and direct monaural innervation via the contralateral cochlear nucleus. The tonotopic arrangement allows easy initial positioning of the probe and delivery of pure tone frequencies can also be used to help with probe placement. During electrode array insertion into the IC, the neural activity to white noise is monitored. Depending on the angle and precise positioning of the electrode array, one lateral shank may register an acoustically driven response only at the most distal electrode while the contralateral shank displays activity on 3 or 4 electrodes. The specific pattern of activity on the electrode array is not critical, as only a series of recording responses on each electrode are required with different electrode-neuron distances. If activity is not seen on all 4 shanks, the electrode array may not be in the correct position. In this situation, the array should be fully retracted, its position relative to lambda and the midline adjusted slightly, and then reinserted. If multiple locations in the one animal have been unsuccessfully implanted, the ear bars should be checked for correct positioning. Inspection of the stream data can indicate problems with an electrode, for instance one electrode in Figure 5 only displays large noise compared to the other electrodes, this was traced to a faulty connector and explains the absence of response in the PSTH (Figure 4).
The surgery described in this protocol accesses the right inferior colliculus with the speaker in the left ear bar. This could easily be altered to put the speaker on the right ear bar and the electrode array into the left IC.
This protocol has been designed for use with electrode coatings on commercially available electrode arrays (NeuroNexus). This specific testing protocol may not be suitable for different electrode configurations. For instance, insertion of flexible polyimide substrate arrays and comparison with Utah style arrays may be difficult. The materials must also be compatible with these arrays, as certain materials or their coating methods may degrade the probes. Some potential issues are that a vacuum deposition method must ensure only the electrodes are coated; solvents used must not dissolve or etch the metal, silicon or wire bond encapsulant; and processing temperatures must not be too high. This protocol also doesn't test the chronic performance of the implant as demonstrated in Ludwig et al.12 Nevertheless, this protocol can be extended to include many other electrode configurations, material types and testing protocols. For instance other analytical techniques can be used for the in vitro testing. The enzymatic cleaner can be modified to other treatments to better understand the electrode fouling occurring during acute implantation. Other deposition methods can also be used to modify the electrodes. However, uncoated electrodes should always be included as a reference to the test electrodes.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge the support of the Australian Research Council through the Centre of Excellence for Electromaterials Science.
Materials
| Programmable Attenuator | TDT | PA5 | Controls the amplitude of the acoustic signal across frequencies |
| Electrostatic speaker driver | TDT | ED1 | Drives the electrostatic speakers (EC1) |
| Coupled electrostatic speaker | TDT | EC1 | Delivers sound to the animal |
| Processing base station | TDT | RZ2 | Records neural activity from electrode array (using PZ2 preamplifier) |
| Preamplifier | TDT | PZ2-256 | 256-channel high impedance preamplifier |
| Multifunction Processor | TDT | RX6 | Used to generate acoustic stimuli |
| Multichannel electrode | NeuroNexus Technologies | A4 × 8–5mm-200-200-413 | 4-shank 32-channel electrode array |
| Potentiostat | CH Instruments | CHI660B | Deposits electrode coatings and performs cyclic voltammetry and EIS (used with CHI684) |
| Multiplexer | CH Instruments | CHI684 | Switches between electrodes on the potentiostat |
| di-sodium phosphate | Fluka | 71644 | Used in the test solution |
| 3,4-ethylenedioxythiophene (EDOT) | Sigma Aldrich | 483028 | An electrode coating material |
| para-toluene sulfonate (Na2pTS) | Sigma Aldrich | 152536 | An electrode coating material |
| Urethane | Sigma Aldrich | U2500 | Used to anaesthetise the animal |
| Silver/Silver chloride electrode | CH Instruments | CHI111 | Used for testing the electrode in vitro |
| Platinum electrode | CH Instruments | MW4130 | Used for testing the electrode in vitro |
| Motorized microdrive | Sutter Instruments | DR1000 | To control the electrode array position during surgery |
| Enzymatic cleaner | Advanced Medical Optics | Ultrazyme | Cleans the protein off the electrode array after implantation |
| Acoustic enclosure | TMC Ametek | 83-501 | Isolates the animal from acoustic and electrical noise |
| Stereotaxic frame | David Kopf Instruments | 1430 | Secures and positions the animal |
| Temperature controller | World Precision Instruments | ATC1000 | Controls the animal temperature |
| Bone drill | KaVo Dental | K5Plus | Used to perform the craniectomy |
| Aspirator | Flaem | Suction pro | Used to perform the craniectomy |
Referencias
- Oluigbo, C. O., Rezai, A. R. Addressing Neurological Disorders With Neuromodulation. IEEE Trans. Biomed. Eng. 58, 1907-1917 (2011).
- Shivdasani, M. N., Mauger, S. J., Rathbone, G. D., Paolini, A. G. Inferior Colliculus Responses to Multichannel Microstimulation of the Ventral Cochlear Nucleus: Implications for Auditory Brain Stem Implants. J. Neurophysiol. 99, 1-13 (2008).
- Perlmutter, J. S., Mink, J. W. Deep Brain Stimulation. Ann. Rev. Neurosci. 29, 229 (2006).
- Weaver, F. M., et al. Bilateral Deep Brain Stimulation vs Best Medical Therapy for Patients With Advanced Parkinson Disease. J. Am. Med. Assoc. 301, 63-73 (2009).
- Biran, R., Martin, D. C., Tresco, P. A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 195, 115-126 (2005).
- McConnell, G. C., et al. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J. Neural Eng. 6, (2009).
- Liu, X., et al. Stability of the interface between neural tissue and chronically implanted intracortical microelectrodes. IEEE Trans. Rehab. Eng. 7, 315-326 (1999).
- Rousche, P. J., Normann, R. A. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. J. Neurosci. Methods. 82, 1-15 (1998).
- Williams, J. C., Rennaker, R. L., Kipke, D. R. Long-term neural recording characteristics of wire microelectrode arrays implanted in cerebral cortex. Brain Res. Protoc. 4, 303-313 (1999).
- Harris, A. R., et al. Conducting polymer coated neural recording electrodes. J. Neural Eng. 10, (2013).
- Bard, A. J., Faulkner, L. R. . Electrochemical Methods. , (2001).
- Ludwig, K. A., Uram, J. D., Yang, J., Martin, D. C., Kipke, D. R. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J. Neural Eng. 3, 59 (2006).

