Live Imaging of Nicotine Induced Calcium Signaling and Neurotransmitter Release Along Ventral Hippocampal Axons
Summary
We developed a gene-chimeric preparation of ventral hippocampal – accumbens circuit in vitro that allows direct live imaging to analyze presynaptic mechanisms of nicotinic acetylcholine receptors (nAChRs) mediated synaptic transmission. This preparation also provides an informative approach to study the pre- and post-synaptic mechanisms of synaptic plasticity.
Abstract
Sustained enhancement of axonal signaling and increased neurotransmitter release by the activation of pre-synaptic nicotinic acetylcholine receptors (nAChRs) is an important mechanism for neuromodulation by acetylcholine (ACh). The difficulty with access to probing the signaling mechanisms within intact axons and at nerve terminals both in vitro and in vivo has limited progress in the study of the pre-synaptic components of synaptic plasticity. Here we introduce a gene-chimeric preparation of ventral hippocampal (vHipp)–accumbens (nAcc) circuit in vitro that allows direct live imaging to analyze both the pre- and post-synaptic components of transmission while selectively varying the genetic profile of the pre- vs post-synaptic neurons. We demonstrate that projections from vHipp microslices, as pre-synaptic axonal input, form multiple, reliable glutamatergic synapses with post-synaptic targets, the dispersed neurons from nAcc. The pre-synaptic localization of various subtypes of nAChRs are detected and the pre-synaptic nicotinic signaling mediated synaptic transmission are monitored by concurrent electrophysiological recording and live cell imaging. This preparation also provides an informative approach to study the pre- and post-synaptic mechanisms of glutamatergic synaptic plasticity in vitro.
Introduction
Cholinergic modulation of circuit excitability contributes to fundamental aspects of cognition, and altered cholinergic modulation is a feature of neurodegenerative and neuropsychiatric disorders including Alzheimer’s disease, Parkinson’s disease, schizophrenia and addiction1-4. An established mechanism of cholinergic facilitation of synaptic transmission in the CNS is via direct activation of nAChRs localized at pre-synaptic sites. Activation of these pre-synaptic receptors leads to increased intracellular Ca2+ ([Ca2+]i) in pre-synaptic terminals – both directly, due to the relatively high calcium conductance of certain nAChR subtypes, and indirectly, via intracellular signaling cascades5, thereby enhancing neurotransmitter release. In fact, the activation of pre-synaptic nAChRs has been linked with changes in release of a wide variety of neurotransmitters including glutamate, GABA, ACh, and dopamine6-10. Although this process has been studied indirectly using electrophysiological methods at various synapses, optical reporters of [Ca2+]i and synaptic vesicle recycling allow more direct and temporally precise measurement of pre-synaptic phenomena.
Pre-synaptic localization of nAChRs has been demonstrated convincingly with direct immuno-gold labeling of nAChRs at the electron microscopic (EM) level11,12. Several other techniques have also been used to address the nAChR localization indirectly, including detecting locations of nAChRs subunit- fluorescent protein chimeras in cultured neurons13,14, electrophysiological recording of nAChR currents in synaptic terminals15,16, monitoring nicotine induced changes in [Ca2+]i in synaptic nerve terminals by live cell imaging17, and indirect monitoring of neurotransmitter release at the synaptic terminal by live cell imaging techniques with fluorescent indicators, including exocytosis of synaptic vesicles viewed by styryl amphipathic FM dyes (FM1-43 and FM4-64) and/or synapto-pHluorin and by specific fluorescent neurotransmitter reporters, such as CNiFERs for ACh and iGluSnFr for glutamate18-20. Overall, these current approaches for identifying pre-synaptic localization of nAChRs are complicated, and require special systems and techniques to allow reliable identification and physiological monitoring of pre-synaptic activity.
Here we describe protocols and equipment for an in vitro co-culture system of a ventral hippocampal (vHipp) – nucleus accumbens (nAcc) circuit that provides direct access to identify and analyze both pre- and post-synaptic components of synaptic transmission. We show examples of pre-synaptic localization of nAChRs and the live cell imaging of nAChR mediated Ca2+ signaling and neurotransmitter release along vHipp axons. A natural (and straightforward) extension of the protocol presented here is the preparation of pre- and post-synaptic contacts comprised of neurons from different genotypes. In this manner the contribution of a particular gene product to the pre- and/or post-synaptic mechanisms of modulation can be assessed directly.
Protocol
All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 2012) and studies were approved by Institutional Animal Care and Use for Research Committees at Stony Brook University (#1618 and #1792).
1. vHipp-nAcc Synaptic Co-cultures
- Sacrifice mice (postnatal day 0 – 3, from wild-type (WT) or α7 nAChRs transgenic mouse line) with CO2. Decapitate each pup and save the tail in a 1.5 ml centrifuge tube for post facto genotyping. Perform the following steps in a sterilized hood.
- Remove the top of the skull with scissors and forceps to expose the whole brain. Starting at the juncture where the cerebral cortices part from each other caudally, obtain a coronal section that contains the posterior cortices including the vHipp5,21 under a dissecting microscope.
- Dissect vHipp tissue strips containing the CA1-subiculum region out according to a developing mouse brain atlas22 (Figure 1A). Transfer to a 35 mm culture dish containing cold culture media (4 °C), cut into small thin slices (around 100 µm × 100 µm microslices).
- Pre-incubate 12 mm poly-D-lysine/laminin-coated glass coverslips inside 24 well tissue culture plates with culture media (~ 500 μl) for at least 30 min in the incubator at 37 °C. Remove media before plating microslices.
- Plate with a fire-polished Pasteur pipette at the center of the coverslip with minimal amount of media (~ 50 μl, containing ~ 20 pieces of microslices) to facilitate attachment of the explants. Plate vHipp microslices originating from a single animal (either the +/+ or the -/- genotype of the transgenic α7 line) on each coverslip.
- After the explants settle onto the coverslip, gently add additional media (~ 100 μl) by the wall so that the level of the media is high enough to completely cover the explants on the periphery, but not those at the center of the coverslip.
- After an O/N incubation in 37 °C, 5% CO2 incubator, disperse nAcc neurons from embryonic days 18 to postnatal day 1 WT mice and add to the explants.
- Dissect out nAcc tissues according to a developing mouse brain atlas20 (Figure 1B).
- Cut into small pieces (around 500 µm × 500 µm microslices) and transfer to a 15 ml tube.
- Treat with 0.25% trypsin (2 ml) for 15 min at 37 °C. Wash tissue chunks three times with cold washing media (5 ml, 4 °C) followed by one wash in cold culture media (5 ml, 4 °C). Allow suspension to rest for 5 min in each wash step, and then pour off supernatant carefully.
- Dissociate cells by gentle trituration in 2 ml of culture media with a lightly fire-polished Pasteur pipette.
- Transfer cell suspension to another 15 ml tube and centrifuge at 2,000 x rpm for 5 min. Remove the supernatant and resuspend the cells in culture media at 1 ml per 6 pups. Disperse cells by gently pipetting in media several times with a fire-polished Pasteur pipette.
- Add 0.25 ml of dispersed nAcc cells to each coverslip with vHipp explants.
- Maintain the cultures in a humidified 37 °C, 5% CO2 incubator. Place culture plates on dampened sterile gauze pads to mechanically stabilize and maintain humidity, facilitating synapse formation.
2. Immunocytochemistry
- Fix cultures (5 – 7 days in vitro) in 4% paraformaldehyde/4% sucrose/PBS (~500 μl per coverslip, 20 min, RT).
- Permeabilize cultures with 0.25% Triton X-100/ PBS (~500 μl per coverslip, 5 min, RT).
- Block cultures with 10% normal donkey serum in PBS (~500 μl per coverslip, 30 min, RT) prior to antibody staining. Incubate with primary antibodies O/N at 4 °C. Use the following primary antibodies: anti- nAChRs (α4-ECD 1:500; anti-α5 subunits, 1:500), anti-vesicular glutamate transporter 1 (1:250), anti-GAD65 (1:100).
- Wash primary antibodies out with PBS (3 times/~500 μl per coverslip, 5 min) and then incubate cultures in secondary antibodies conjugated to Alexa 488 (~500 μl per coverslip, 1:500) or Alexa 594 (~500 μl per coverslip, 1:500) for 1 hr at 37 °C.
- Incubate cultures in αBgTx conjugated to Alexa 594 (~500 μl per coverslip, 1:1,000 in culture media) for 45 min at 37 °C prior to fixation for labeling surface α7*nAChRs.
- Mount and seal coverslips.
- Capture images with a fluorescence microscope equipped with Plan-Apochromat objectives (20X with 0.8 NA or 63X oil with 1.4 NA) and a CCD camera.
3. FM1-43 Based Vesicular Fusion
- Maintain cultures (5 – 7 days in vitro) in an imaging chamber, mount the chamber on spinning disk confocal microscope. Continuously perfuse (1 ml/min) with HBS cocktail at RT.
- Stop perfusion and load cultures with 10 µM FM1-43 in 56 mM K+ ACSF (~500 μl) for 90 sec (staining), wash external dye away for 15 min with Ca2+ free HBS containing ADVASEP-7 (0.1 mM) to scavenge membrane-bound FM1-43.
- Stimulate the cultures with 56 mM K+ ACSF (~500 μl) without FM1-43 for 120 sec (destaining), reload cultures with FM1-43 using the same conditions, and wash again with Ca2+ free HBS containing ADVASEP-7 for 15 min.
- Acquire FM1-43 fluorescence images with spinning disk confocal microscope equipped with a Plan-Apochromat objective (60X water with 1.4 NA, excitation 488 nm, emission 530 nm) and capture images with a CCD camera.
- Collect baseline FM1-43 fluorescence images (every 2 s for 1 min) as pre-nicotine control; apply nicotine (1 µM) by rapid perfusion (2 ml/min) for 1 min, and then wash nicotine out with HBS cocktail. Keep capture time-lapse images before, during, and after nicotine application every 2 sec for 5 min.
- Quantify FM1-43 fluorescence intensity before (Fstaining) and after (Fdestaining) nicotine application at each synaptic bouton.
- Calculate total amount of releasable FM1-43 fluorescence along vHipp axons by quantifying the difference of FM1-43 fluorescence intensity before and after nicotine application (ΔF = Fstaining-Fdestaining). Analyze fraction of fluorescence intensity decrease after nicotine with the following equation: Fdecrease% = ΔF/Fstaining.
4. Calcium Imaging
- Rinse cultures (5 – 7 days in vitro) quickly with normal HBS, then load cultures with 5 µM Fluo-4 Ca2+ indicator (AM ester) and 0.02% Pluronic F-127 in HBS (2 ml) for 30 min at 37 °C and 5% CO2.
- Wash out Fluo-4 solution with HBS (3 times/5 min). Return cultures to incubator (37 °C / 5% CO2) for at least 30 min.
- Maintain cultures in the same imaging chamber and same conditions as described in FM1-43 based vesicular fusion section (see step 3.1).
- Collect Fluo-4 fluorescence images of axonal projections from the vHipp micro-slices with a Plan-Apochromat objective (60X water with 1.4 NA, excitation 488 nm, emission 530 nm) and a CCD camera.
- Collect baseline Fluo-4 fluorescence images (every 10 sec for 2 min) as pre-nicotine control, apply nicotine (1 µM) by rapid perfusion (2 ml/min) for 1 min, and then wash nicotine out with HBS cocktail. Keep capture time-lapse images before, during, and after nicotine application every 10 sec for 30 min.
- Save all frames of the raw fluo-4 fluorescence images, export as a series of TIFF format images for further analyzing.
- Collect the integrated intensity of fluo-4 fluorescence along vHipp axons before and after nicotine application.
- Normalize integrated fluorescence intensity with the following equation: ΔF/F0 = (F-F0)/F0, where F0 is the background-corrected pre-nicotine integrated fluorescence intensity and F is the integrated fluorescence intensity along vHipp axons at each time point after nicotine application. Analyze and plot normalized integrated fluorescence intensity.
Representative Results
The preparation employed consists of gene chimeric co-cultures of vHipp–nAcc circuits in vitro. Projections emanating from vHipp microslices, as pre-synaptic axonal input, can make synaptic contacts with post-synaptic targets, the dispersed neurons from nAcc. Nicotine induced a sustained (≥ 30 min) facilitation of glutamatergic transmission from nAcc neurons innervated by vHipp axons21 and prolonged calcium signaling along vHipp axons5 via pre-synaptic α7*nAChRs.
Figure 1 demonstrates directly that the projections from the vHipp micro-slices are glutamatergic ( vGluT1 positive) and that contacts are made with dispersed GABAergic medium spiny neurons from nAcc (GAD positive, Figure 1C, D). Both α7* and non-α7*nAChRs (including α4 and α5 subunits) were found along the vHipp axons (Figure 1E-G) and specifically at sites where vHipp projections contact nAcc neurons. It should be noted that dispersed neurons from nAcc do not express α7*nAChRs (Figure 1H); i.e. the receptor clusters at contacts are strictly presynaptic (Figure 1I), also see Figure 1 in Reference 5.
Once the chimeric co-cultures have been established, live imaging of [Ca2+]i and synaptic vesicles fusion along vHipp axons with and/or without nicotine can be recorded for up to 30 min without any apparent damage to the neurons.
Representative images, time-lapse movies and quantifications for nicotine-induced fluo-4 [Ca2+]i signaling along vHipp axons are shown in Figure 2 and Supplemental Movie 1 and 2. We found that activation of vHipp nAChRs by nicotine induces sustained Ca2+ influx into pre-synaptic axons (Figure 2A, D). The sustained phase of nicotine induced increases in [Ca2+]i signaling along vHipp axons was blocked by the specific α7*nAChR antagonist αBgTx but not by the non-α7*nAChR antagonist DHβE (Figure 2A-C).
Direct visualization of activity-dependent FM1-43 dye endocytosis and exocytosis has been used to monitor indirectly presynaptic neurotransmitter release23, 24. Here, representative images and quantification for nicotine-induced vesicular fusion along vHipp axons visualized by FM1-43 are shown in Figure 3 (Figure 3A, C). The presence of pre-synaptic α7*nAChRs is required for maximal nicotine induced vesicle fusion and neurotransmitter release (Figure 3B, C).
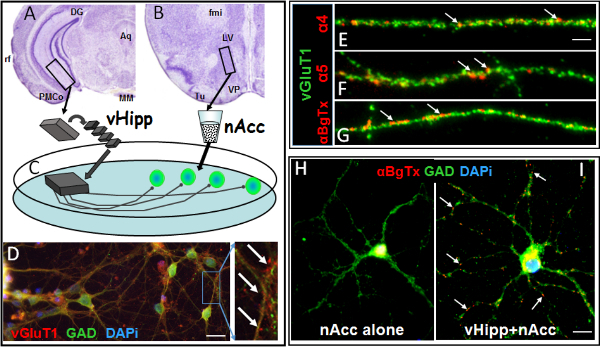
Figure 1. Synaptic co-culture of vHipp with nAcc allows examination of pre-synaptic localization of nAChRs. (A-C) Schematic cartoon of genotype-specific in vitro circuits (C) prepared by separate plating of ventral hippocampus/subiculum (A). slices from an individual WT or α7 -/- mouse and dispersed neurons from WT nucleus accumbens (B). Aq, aqueduct (Sylvius); DG, dentate gyrus; MM, medial mammillary nucleus; PMCo, posteromedial cortical amygdaloid nucleus; rf, rhinal fissure, fmi, forceps minor of the corpus callosum; LV, lateral ventricle; Tu, olfactory tubercle; VP, ventral pallidum. (D) vHipp microslices extend vGluT1 positive (red) axonal projections that contact GAD65 positive (green) nAcc neurons (white arrows are examples of those contact sites). Scale bar: 10 µm. (E-G) Representative micrographs of WT vHipp axons (staining with vGluT1, green) are shown for α4*nAChR (E), α5*nAChR (F) and surface α7*nAChR (G) staining in red clusters (white arrows). Scale bar: 5 μm. (H) There are no surface α7*nAChR clusters on dispersed GABAergic neurons (GAD65 positive) from nAcc alone. (I) Red “clusters” of surface α7*nAChR (white arrows) can be seen in those dispersed neurons co-cultured with vHipp microslices . Scale bar: 5 μm. Please click here to view a larger version of this figure.
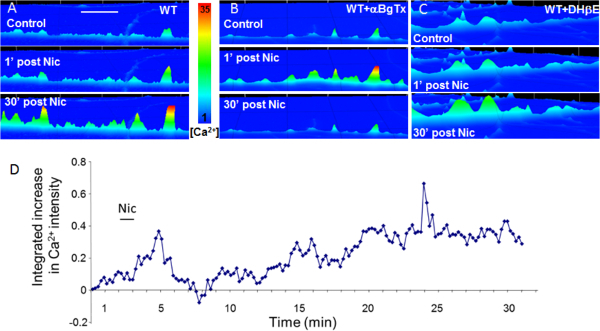
Figure 2. Synaptic co-culture of vHipp with nAcc allows examination of nicotine induced calcium signaling along vHipp axons. (A) Representative fluo-4 images of calcium bound fluo-4 in pseudo color scale along a WT vHipp axon before (Top), 1’ (Middle), and 30’ (Bottom) after nicotine application. The sustained phase (30’) of the nicotine-induced Ca2+ response is eliminated by addition of the α7*nAChR selective antagonist αBgTx (100 nM) (B) but not by the addition of the non-α7*nAChR selective antagonist DHβE (1 µM) (C). Scale bar: 5 µm. (D) Representative plot of normalized fluo-4 integrated fluorescence intensity from a live WT vHipp axon perfused with nicotine (1 µM) for 1 min. Please click here to view a larger version of this figure.
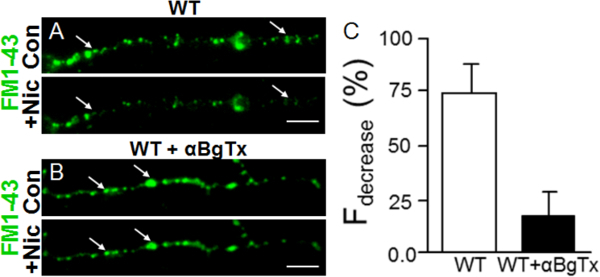
Figure 3. Synaptic co-culture of vHipp with nAcc allows examination of nicotine induced vesicular neurotransmitter release (with FM1-43) along vHipp axons. (A) Representative images of WT vHipp axons (loaded with FM1-43, green) before (top) and after (bottom) nicotine application. (B) Representative images of WT vHipp axons (loaded with FM1-43, pretreated with α7*nAChR selective antagonist αBgTx for 15 min) before (top) and after (bottom) nicotine application. Scale bar: 5 μm. (C) shows quantification of the changes in axonal FM1-43 fluorescence (Fdecrease) following nicotine treatment in the absence (WT) or presence (WT+αBgTx) of the α7*nAChR antagonist. Please click here to view a larger version of this figure.
Supplemental Movie 1: Time-lapse live imaging of basal Fluo-4/Ca2+ fluorescence along vHipp axons.
The time-lapse images of Fluo-4/Ca2+ fluorescence along vHipp axons were acquired with a 60x objective water lens every 10 sec for 30 min with a spinning disk confocal microscope. Acquired images were indicated on pseudo color scale and played back as a movie at 2 frames per s. This movie shows the baseline of Fluo-4/Ca2+ fluorescence activity along live WT vHipp axon perfused with HBS cocktail.
Supplemental Movie 2: Time-lapse live imaging of nicotine induced sustained Fluo-4/Ca2+ fluorescence along vHipp axons.
The time-lapse images of Fluo-4/Ca2+ fluorescence along vHipp axons were acquired with a 60x objective water lens every 10 sec for 30 min with a spinning disk confocal microscope. Acquired images were indicated on pseudo color scale and played back as a movie at 2 frames per sec. This movie shows nicotine (1 µM, perfused in at time point 43, washed out with HBS cocktail at time point 49) induced Fluo-4/Ca2+ fluorescence activity along live WT vHipp axon. Nicotine application increased the fluo-4/Ca2+ fluorescence intensity along vHipp axons.
Discussion
The co-culture preparation described re-capitulates ventral hippocampal–accumbens circuits in vitro. This preparation permits relatively straightforward and reliable examination of the spatial and temporal profiles by which activation of pre-synaptic nAChRs elicit enhanced glutamatergic transmission5, 21.
Co-cultures are defined as the growth of different specific cell types in one dish which can provide physiological conditions in vitro to demonstrate in vivo-like function. Conventional neuron-neuron co-cultures have been introduced to neuroscience research for studying the synaptic interactions between different cell types. However, it is hard to identify the pre- and post-sites of synapses in these co-cultures. This microslice-dissociated neuron co-culture protocol allows easy recognition of the pre-synaptic axons and post-synaptic targets. Using microslices from different brain regions of transgenic mouse line expressing different fluorescent proteins, this protocol can also be used to study axon-axon interactions.
The critical step of this microslice-dissociated neurons co-culture protocol is providing a stable environment to allow for the attachment of the explants, the outgrowth of the projections and the formation of synapses. Plating the microslices in a minimal volume of media and maintaining the culture plates on dampened sterile gauze pads are the two key steps for microslice attachment and synapse formation. The main limitation of this co-culture protocol is that not all dispersed nAcc neurons form functional synapses with vHipp axons.
By altering the genotype of the pre- and post- synaptic components, this preparation provides an informative approach to study the pre- and post-synaptic mechanisms of synaptic plasticity. Three distinct features of this preparation make it ideal for these studies. Brain regions that are normally connected in vivo via fiber paths that cannot be maintained, or readily identified in acute slices, can be combined in this in vitro preparation. Pre- and post-synaptic components come from different mice make allowing independent alteration of either the pre- or post-synaptic genotype. By plating pre- and post-synaptic components at different times, it is possible to selectively express exogenous genes in either the pre- or post-synaptic neurons.
Nicotine induced Ca2+ transients (in seconds to minutes range) by activation of nAChRs have been reported in cultured neurons, astrocytes and in several cell lines that express nAChRs25-27. However, most of these studies did not detect the long-term effects (over 10 min) of short time nicotine exposure largely due to photo damage during long-term imaging. By using the culture protocol described above and rapid spinning-disc confocal image collection, nicotine induced Ca2+ signaling, synaptic vesicle fusion and glutamate release can be recorded for up to 1 hr without any apparent damage to the neurons (Supplemental Movie 1, 2).
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We thank Yehui Qin and Mallory Myers for technical support. We also thank Dr. Sigismund Huck for providing us the anti-α4-ECD antibody. This work is supported by National Institutes of Health grant NS22061 to L. W. R.
Materials
| 1, Culture Media (50 ml) | |||
| Neurobasal | GIBCO | 10888022 | 48 ml |
| B-27 Supplements | GIBCO | 0080085-SA | 1 ml |
| Penicillin-Streptomycin | GIBCO | 10908-010 | 0.5 ml |
| GlutaMAX Supplement | GIBCO | 35050-061 | 0.5 ml |
| Brain-derived neurotrophic factor (BDNF) | GIBCO | 15140-122 | 20 ng/ml |
| 2, washing media (HBSS, 100 ml) | |||
| HBSS, no calcium, no magnesium, no phenol red | GIBCO | 14175-095 | 99 ml |
| HEPES ( 1M) | GIBCO | 15630-130 | 1 ml |
| 3, HEPES buffered saline (HBS) pH=7.3 | |||
| NaCl | Sigma | S9888 | 135 mM |
| KCl | Sigma | P9333 | 5 mM |
| MgCl2 | Sigma | M8266 | 1 mM |
| CaCl2, | Sigma | C1016 | 2 mM |
| HEPES | Sigma | H3375 | 10 mM |
| Glucose | Sigma | G0350500 | 10 mM |
| 4, HBS Cocktail for live imaging pH=7.3 | |||
| NaCl | Sigma | S9888 | 135 mM |
| KCl | Sigma | P9333 | 5 mM |
| MgCl2 | Sigma | M8266 | 1 mM |
| CaCl2, | Sigma | C1016 | 2 mM |
| HEPES | Sigma | H3375 | 10 mM |
| Glucose | Sigma | G0350500 | 10 mM |
| tetrodotoxin | Tocris | 1078 | 2 µM |
| bicuculline | Tocris | 131 | 10 µM |
| D-AP-5 | Tocris | 105 | 50 µM |
| CNQX | Tocris | 1045 | 20 µM |
| LY341495 | Tocris | 1209 | 10 µM |
| 5, Calcium-free HBS pH=7.3 | |||
| NaCl | Sigma | S9888 | 135 mM |
| KCl | Sigma | P9333 | 5 mM |
| MgCl2 | Sigma | M8266 | 1 mM |
| HEPES | Sigma | H3375 | 10 mM |
| Glucose | Sigma | G0350500 | 10 mM |
| 6, 56 mM Potassium ACSF pH=7.4 | |||
| NaCl | Sigma | S9888 | 119 mM |
| KCl | Sigma | P9333 | 56 mM |
| MgSO4.7H | Sigma | M1880 | 1.3 mM |
| CaCl2 | Sigma | C1016 | 2.5 mM |
| NaH2PO4 | Sigma | S8282 | 1 mM |
| NaHCO3 | Sigma | S5761 | 26.2 mM |
| Glucose | Sigma | G0350500 | 10 mM |
Referencias
- Changeux, J. P., et al. Brain nicotinic receptors: structure and regulation, role in learning and reinforcement. Brain Res Brain Res Rev. 26 (2-3), 198-216 (1998).
- Levin, E. D. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 53 (4), 633-640 (2002).
- Dani, J. A., Bertrand, D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 47, 699-729 (2007).
- Mineur, Y. S., Picciotto, M. R. Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends Pharmacol Sci. 31 (12), 580-586 (2010).
- Zhong, C., Talmage, D. A., Role, L. W. Nicotine elicits prolonged calcium signaling along ventral hippocampal axons. PloS one. 8 (12), e82719 (2013).
- McGehee, D. S., Heath, M. J., Gelber, S., Devay, P., Role, L. W. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 269 (5231), 1692-1696 (1995).
- Gray, R., Rajan, A. S., Radcliffe, K. A., Yakehiro, M., Dani, J. A. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 383 (6602), 713-716 (1996).
- Dickinson, J. A., Kew, J. N., Wonnacott, S. Presynaptic alpha 7- and beta 2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol Pharmacol. 74 (2), 348-359 (2008).
- Zappettini, S., Grilli, M., Salamone, A., Fedele, E., Marchi, M. Pre-synaptic nicotinic receptors evoke endogenous glutamate and aspartate release from hippocampal synaptosomes by way of distinct coupling mechanisms. Br J Pharmacol. 161 (5), 1161-1171 (2010).
- Zappettini, S., et al. Presynaptic nicotinic alpha7 and non-alpha7 receptors stimulate endogenous GABA release from rat hippocampal synaptosomes through two mechanisms of action. PloS one. 6 (2), e16911 (2011).
- Fabian-Fine, R., et al. Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J Neurosci. 21 (20), 7993-8003 (2001).
- Jones, I. W., Barik, J., O’Neill, M. J., Wonnacott, S. Alpha bungarotoxin-1.4 nm gold: a novel conjugate for visualising the precise subcellular distribution of alpha 7* nicotinic acetylcholine receptors. J Neurosci Methods. 134 (1), 65-74 (2004).
- Nashmi, R., et al. Assembly of alpha4beta2 nicotinic acetylcholine receptors assessed with functional fluorescently labeled subunits: effects of localization, trafficking, and nicotine-induced upregulation in clonal mammalian cells and in cultured midbrain neurons. J Neurosci. 23 (37), 11554-11567 (2003).
- Drenan, R. M., et al. Subcellular trafficking, pentameric assembly, and subunit stoichiometry of neuronal nicotinic acetylcholine receptors containing fluorescently labeled alpha6 and beta3 subunits. Mol Pharmacol. 73 (1), 27-41 (2008).
- Wu, J., et al. Electrophysiological, pharmacological, and molecular evidence for alpha7-nicotinic acetylcholine receptors in rat midbrain dopamine neurons. J Pharmacol Exp Ther. 311 (1), 80-91 (2004).
- Parikh, V., Ji, J., Decker, M. W., Sarter, M. Prefrontal beta2 subunit-containing and alpha7 nicotinic acetylcholine receptors differentially control glutamatergic and cholinergic signaling. J Neurosci. 30 (9), 3518-3530 (2010).
- Nayak, S. V., Dougherty, J. J., McIntosh, J. M., Nichols, R. A. Ca(2+) changes induced by different presynaptic nicotinic receptors in separate populations of individual striatal nerve terminals. J Neurochem. 76 (6), 1860-1870 (2001).
- Richards, C. I., et al. Trafficking of alpha4* nicotinic receptors revealed by superecliptic phluorin: effects of a beta4 amyotrophic lateral sclerosis-associated mutation and chronic exposure to nicotine. J Biol Chem. 286 (36), 31241-31249 (2011).
- Colombo, S. F., Mazzo, F., Pistillo, F., Gotti, C. Biogenesis, trafficking and up-regulation of nicotinic ACh receptors. Biochem Pharmacol. 86 (8), 1063-1073 (2013).
- St John, P. A. Cellular trafficking of nicotinic acetylcholine receptors. Acta Pharmacol Sin. 30 (6), 656-662 (2009).
- Zhong, C., et al. Presynaptic type III neuregulin 1 is required for sustained enhancement of hippocampal transmission by nicotine and for axonal targeting of alpha7 nicotinic acetylcholine receptors. J Neurosci. 28 (37), 9111-9116 (2008).
- Jacobowitz, D. M., Abbott, L. C. . Chemoarchitectonic Atlas of the Developing Mouse Brain. , (1998).
- Betz, W. J., Mao, F., Bewick, G. S. Activity-dependent fluorescent staining and destaining of living vertebrate motor nerve terminals. J Neurosci. 12 (2), 363-375 (1992).
- Amaral, E., Guatimosim, S., Guatimosim, C. Using the fluorescent styryl dye FM1-43 to visualize synaptic vesicles exocytosis and endocytosis in motor nerve terminals. Methods Mol Biol. 689, 137-148 (2011).
- Garduno, J., et al. Presynaptic alpha4beta2 nicotinic acetylcholine receptors increase glutamate release and serotonin neuron excitability in the dorsal raphe nucleus. J Neurosci. 32 (43), 15148-15157 (2012).
- Guo, J. Z., Liu, Y., Sorenson, E. M., Chiappinelli, V. A. Synaptically released and exogenous ACh activates different nicotinic receptors to enhance evoked glutamatergic transmission in the lateral geniculate nucleus. J Neurophysiol. 94 (4), 2549-2560 (2005).
- Szabo, S. I., Zelles, T., Vizi, E. S., Lendvai, B. The effect of nicotine on spiking activity and Ca2+ dynamics of dendritic spines in rat CA1 pyramidal neurons. Hippocampus. 18 (4), 376-385 (2008).

