Differentiation of the SH-SY5Y Human Neuroblastoma Cell Line
Summary
It is critical in neurobiology and neurovirology to have a reliable, replicable in vitro system that serves as a translational model for what occurs in vivo in human neurons. This protocol describes how to culture and differentiate SH-SY5Y human neuroblastoma cells into viable neurons for use in in vitro applications.
Abstract
Having appropriate in vivo and in vitro systems that provide translational models for human disease is an integral aspect of research in neurobiology and the neurosciences. Traditional in vitro experimental models used in neurobiology include primary neuronal cultures from rats and mice, neuroblastoma cell lines including rat B35 and mouse Neuro-2A cells, rat PC12 cells, and short-term slice cultures. While many researchers rely on these models, they lack a human component and observed experimental effects could be exclusive to the respective species and may not occur identically in humans. Additionally, although these cells are neurons, they may have unstable karyotypes, making their use problematic for studies of gene expression and reproducible studies of cell signaling. It is therefore important to develop more consistent models of human neurological disease.
The following procedure describes an easy-to-follow, reproducible method to obtain homogenous and viable human neuronal cultures, by differentiating the chromosomally stable human neuroblastoma cell line, SH-SY5Y. This method integrates several previously described methods1-4 and is based on sequential removal of serum from media. The timeline includes gradual serum-starvation, with introduction of extracellular matrix proteins and neurotrophic factors. This allows neurons to differentiate, while epithelial cells are selected against, resulting in a homogeneous neuronal culture. Representative results demonstrate the successful differentiation of SH-SY5Y neuroblastoma cells from an initial epithelial-like cell phenotype into a more expansive and branched neuronal phenotype. This protocol offers a reliable way to generate homogeneous populations of neuronal cultures that can be used for subsequent biochemical and molecular analyses, which provides researchers with a more accurate translational model of human infection and disease.
Introduction
The ability to use in vitro model systems has greatly enhanced the fields of neurobiology and the neurosciences. Cells in culture provide an efficient platform to characterize protein functionality and molecular mechanisms underlying specific phenomena, to understand the pathology of disease and infection, and to perform preliminary drug testing assessments. In neurobiology, the major types of cell culture models include primary neuronal cultures derived from rats and mice, and neuroblastoma cell lines such as rat B35 cells5, Neuro-2A mouse cells6, and rat PC12 cells7. Although use of such cell lines has advanced the field significantly, there are several confounding factors associated with handling non-human cells and tissue. These include understanding species-specific differences in metabolic processes, phenotypes of disease manifestation, and pathogenesis when compared to humans. It is also important to note that there are significant differences between mouse and human gene expression and transcription factor signaling, highlighting the limitations of rodent models and the importance of understanding which pathways are conserved between rodents and humans8-11. Others have employed the use of human neuronal cell lines including the N-Tera-2 (NT2) human teratocarcinoma cell line and inducible pluripotent stem cells (iPSCs). These cell lines provide good models for in vitro human systems. However, differentiation of NT2 cells with retinoic acid (RA) results in the generation of a mixed population of neurons, astrocytes, and radial glial cells12, necessitating an additional purification step to obtain pure populations of neurons. Additionally, NT2 cells demonstrate a highly variable karyotype13, with greater than 60 chromosomes in 72% of cells. iPSCs demonstrate variability in differentiation between different cell lines and vary in differentiation efficiency14. It is therefore desirable to have a consistent and reproducible human neuronal cell model to complement these alternatives.
SH-SY5Y neuroblast-like cells are a subclone of the parental neuroblastoma cell line SK-N-SH. The parental cell line was generated in 1970 from a bone marrow biopsy that contains both neuroblast-like and epithelial-like cells15. SH-SY5Y cells have a stable karyotype consisting of 47 chromosomes, and can be differentiated from a neuroblast-like state into mature human neurons through a variety of different mechanisms including the use of RA, phorbol esters, and specific neurotrophins such as brain-derived neurotrophic factor (BDNF). Prior evidence suggests that the use of different methods can select for specific neuron subtypes such as adrenergic, cholinergic, and dopaminergic neurons16,17. This latter aspect makes SH-SY5Y cells useful for a multitude of neurobiology experiments.
Several studies have noted important differences between SH-SY5Y cells in their undifferentiated and differentiated states. When SH-SY5Y cells are undifferentiated, they rapidly proliferate and appear to be non-polarized, with very few, short processes. They often grow in clumps and express markers indicative of immature neurons18,19. When differentiated, these cells extend long, branched processes, decrease in proliferation, and in some cases polarize2,18. Fully differentiated SH-SY5Y cells have been previously demonstrated to express a variety of different markers of mature neurons including growth-associated protein (GAP-43), neuronal nuclei (NeuN), synaptophysin (SYN), synaptic vesicle protein II (SV2), neuron specific enolase (NSE) and microtubule associated protein (MAP)2,16,17,20, and to lack expression of glial markers such as glial fibrillary acidic protein (GFAP)4. In further support that differentiated SH-SY5Y cells represent a homogeneous neuronal population, removal of BDNF results in cellular apoptosis4. This suggests that survival of differentiated SH-SY5Y cells is dependent on trophic factors, similar to mature neurons.
Use of SH-SY5Y cells has increased since the subclone was established in 19783. Some examples of their use include investigating Parkinson's disease17, Alzheimer's disease21, and the pathogenesis of viral infection including poliovirus22, enterovirus 71 (EV71)23,24, varicella-zoster virus (VZV)1, human cytomegalovirus25, and herpes simplex virus (HSV)2,26. It is important to note that several studies using SH-SY5Y cells have used these cells in their undifferentiated form, especially in the field of neurovirology27-36. The difference in the observed phenotype of undifferentiated versus differentiated SH-SY5Y cells raises the question of whether the observed progression of infection would be different in mature differentiated neurons. For example, differentiated SH-SY5Y cells have a higher efficiency of HSV-1 uptake versus undifferentiated, proliferating SH-SY5Y cells, which may be due to a lack of surface receptors that bind HSV and modulate entry on undifferentiated SH-SY5Y cells2. It is therefore critical that when designing an experiment focused on testing neurons in vitro, SH-SY5Y cells should be differentiated in order to obtain the most accurate results for translation and comparison to in vivo models.
The development of a reliable method to generate human neuronal cultures is imperative to allow researchers to perform translational experiments that accurately model the human nervous system. The protocol presented here is a procedure that delineates best practices derived from previous methods1-4 to enrich for human neurons that are differentiated using retinoic acid.
Protocol
1. General Considerations
- See the Table of Materials/Equipment for a list of necessary reagents. Perform all steps under strict aseptic conditions.
- Use heat-inactivated fetal bovine serum (hiFBS) for all media preparations that include FBS. To heat-inactivate, warm a 50 ml aliquot of FBS at 56 °C for 30 min, inverting every 10 min (see also Table 1).
Note: When FBS is used without heat-inactivation, the epithelial-like phenotype progresses more quickly throughout cultures of SH-SY5Y cells. - Prior to use, allow media to warm and equilibrate in an incubator to establish a proper pH balance before every step. For example, 50 ml of media takes approximately one hour to fully equilibrate (pH 7, 37 °C, 5% CO2).
Note: This protocol uses a two-step splitting procedure that requires partially differentiated SH-SY5Y cells to be trypsinized and re-plated. This is a stressful process for these exceptionally fragile cells. Therefore, it is important to incubate the cells in trypsin for a minimal amount of time. This will allow for the preferential lift-off of neurons, leaving epithelial-like cells still attached to the dish. - Perform trituration of differentiated cells slowly with a 10 ml plastic pipet with the tip against the bottom of the conical tube containing the cells. Perform trituration at a slow speed, up and down no more than five times.
2. Passage of SH-SY5Y Maintenance Cultures
- Split maintenance cultures when cells have reached 70-80% confluency, and do not exceed 10 to 15 passages. Cultures typically need to be passaged every 3-5 days (assuming cultures are not diluted more than 5-fold during splitting).
- To passage cells from a T-75 flask, aspirate off media, then rinse with approximately 10 ml 1x PBS.
Note: We do not recommend splitting the SH-SY5Y maintenance cultures further than 1:5 during passaging because this can cause the cells to die due to low confluency. - Aspirate PBS, and then add 2.5 ml 0.05% Trypsin-EDTA (1x).
- Incubate in incubator 2-3 min and tip gently to release cells from the surface of the flask.
- Add 10 ml Basic Growth Media (see Table 2) and triturate 1-2 times.
- Spin down for 2 min at 1,000 x g, aspirate media, then resuspend pellet in 5 ml Basic Growth Media.
- Dilute cells from 1:3 to 1:5 in a total volume of 20 ml for normal plating in T-75 flask, or count and plate for differentiation (section 5).
3. Freezing SH-SY5Y Cells
- Freeze early passages of SH-SY5Y neuroblastoma cells in Basic Growth Media supplemented with 5% (v/v) DMSO.
- Initially, freeze aliquots at -80 °C for 24 hr, then transfer to liquid nitrogen for long-term storage.
Note: For reference, a confluent (75-85%) T-75 flask will yield five 1 ml aliquots of SH-SY5Y cells for freezing. Each of these aliquots should contain anywhere from 2-5 million cells total.
4. Thaw and Culture Undifferentiated SH-SY5Y Neuroblastoma Cells
- Prepare Basic Growth Media.
- Rapidly thaw frozen cells in a 37 °C water bath (approximately 2 min).
- Resuspend cells in 9 ml Basic Growth Media in a 15 ml conical tube, and then centrifuge for 2 min at 1,000 x g.
- Aspirate the supernatant while being careful not to disturb the pelleted cells, and gently resuspend cells in 10 ml Basic Growth Media.
- Plate cells onto a T-25 flask or 60 mm2 dish.
- The next day, replace media to remove dead cells.
5. Day 0: Plating Cells for Differentiation
- See Figure 1 for the differentiation schedule.
- Rinse undifferentiated cells with 1x PBS, aspirate, and then trypsinize using 1-2 ml warmed 1x 0.05% Trypsin-EDTA.
- When cells are in trypsin, incubate for approximately 3 min in an incubator.
- Quench the trypsin by adding 10 ml Basic Growth Media, rinse the sides of the flask or dish, and gently triturate 1-3 times. Transfer contents to a 15 ml conical tube.
- Centrifuge for 2 min at 1,000 x g, and aspirate the media while being careful not to disturb the pellet.
- Resuspend pellet in 5 ml Basic Growth Media and triturate 1-3 times.
- Count cells using a hemocytometer, then dilute using Basic Growth Media to 50,000 cells/ml.
- Plate 2 ml of cells per 35 mm2 dish for a total of 100,000 cells per dish and place back into incubator.
6. Day 1: Change Media (Differentiation Media #1)
- Aliquot 50 ml of Differentiation Media #1 (see Table 2)and incubate in a 37 °C water bath.
- When media is warmed, allow it to equilibrate in an incubator (37 °C, 5% CO2) for at least one hr to establish a proper pH balance prior to use.
- Add Retinoic Acid (RA) (see Table 1) to warmed and equilibrated media immediately before adding media to dishes.
Note: Retinoic acid is light sensitive and should be stored in dark bottles at 4 °C - Gently aspirate off old media and discard.
- Add 2 ml Differentiation Media #1 with RA per 35 mm2 dish and return to incubator.
7. Day 3: Change Media (Differentiation Media #1)
- Repeat Section 6 (steps 1-5)
8. Day 5: Change Media (Differentiation Media #1)
- Repeat Section 6 (steps 1-5)
9. Day 7: Split Cells 1:1
- Add RA to warmed and equilibrated Differentiation Media #1 immediately before adding media to dishes.
- Gently aspirate off old media and discard.
- Add 200 µl warmed 0.05% 1x Trypsin EDTA per 35 mm2 dish and warm in incubator approximately 2-3 min or until cells are visibly lifted from the plate as observed under a microscope.
- Quench the trypsin by adding 2 ml Differentiation Media #1 with RA per 35 mm2 dish and use the media to rinse remaining neuronal cells off the plate. Then transfer contents to a 50 ml conical tube.
Note: During trypsinization steps, do not trypsinize too many dishes at once. This helps to ensure neuronal cultures are not incubated in trypsin for too long, which can be cytotoxic. - Combine contents from up to 10 dishes in the 50 ml conical tube and gently triturate slowly up and down no more than five times with a 10 ml plastic pipet.
- Aliquot 2 ml cell suspension into fresh 35 mm2 dishes and return to incubator.
10. Day 8: Change Media (Differentiation Media #2)
- Add RA (see Table 1) to warmed and equilibrated media immediately before adding media to dishes.
- Gently aspirate off old media and discard.
- Slowly add 2 mL Differentiation Media #2 (see Table 2) with RA per 35 mm2 dish and return to incubator. Do not allow neurons to be exposed to air for an extended period of time as they can dry out quickly.
11. Day 9: Prepare Extracellular Matrix (ECM) Coated Dishes
- Thaw one vial of ECM solution on ice and dilute 1:100 into cold DMEM.
- Dispense 2 ml of mixture into each 35 mm2 dish and ensure the entire base of the dish is covered.
- Place in an incubator (37 °C, 5% CO2) for 1 hr or overnight.
- Aspirate mixture and allow to air dry for approximately 1 hr in a hood. Store at room temperature for up to 2 months.
12. Day 10: Transfer Cells onto ECM Coated Plates 1:1
- Add RA (see Table 1) to warmed and equilibrated media immediately before adding media to dishes.
- Gently aspirate off media and discard.
- Add 200 µl warmed trypsin to each 35 mm2 dish and allow to incubate at room temperature for approximately 1-2 min or until neurons are visibly lifted from the dish as observed under a microscope.
Note: Execute this trypsinization step at room temperature so as not to over-incubate neurons with trypsin and cause damage. Neurons release from plates much faster than epithelial-like cells at this stage. - Quench the trypsin by adding 2 ml Differentiation Media #2 per 35 mm2 dish and use the media to rinse remaining neuronal cells off the plate. Then transfer contents to a 50 ml conical tube.
- Combine contents from up to 10 dishes in the 50 ml conical tube and gently triturate slowly up and down no more than five times with a 10 ml plastic pipet.
- Dispense 2 ml cell suspension into ECM-coated 35 mm2 dishes and return to incubator.
13. Day 11: Change Media (Differentiation Media #3)
- Add RA (see Table 1) to warmed and equilibrated media immediately before adding media to dishes.
- Gently aspirate off old media and discard.
- Slowly add 2 ml Differentiation Media #3 (see Table 2) with RA per 35 mm2 dish and return to incubator. Do not allow neurons to be exposed to air for an extended period of time.
14. Day 14: Change Media (Differentiation Media #3)
- Repeat Section 13 (steps 1-3)
15. Day 17: Last Media Change (Differentiation Media #3)
- Repeat Section 13 (steps 1-3)
16. Day 18: Neuronal Cultures Ready to Use
- Change media to fresh Differentiation Media #3 with RA every 3 days to maintain neuron health.
Note: Cells should be differentiated into neurons and exhibit a neuronal phenotype. Cultures are typically stable for up to 14 days following terminal differentiation, however duration of neuron viability is dependent on passage number of the undifferentiated cells at the start of differentiation. Higher passage numbers yield differentiated neurons with a shorter useful lifetime.
Representative Results
At present, there are many instances in the field of neurobiology and neurovirology where undifferentiated SH-SY5Y cells are being used as a functional model for human neurons27-36, and importantly, undifferentiated cells may lack phenotypes such as optimal viral uptake2 that are necessary for accurate interpretation. It is critical that when using SH-SY5Y cells or any other in vitro neuronal system, cells are appropriately differentiated into neurons, in order to obtain data that is the best possible representation of what may be occurring in neurons in vivo. The above protocol yields highly viable, homogenous, differentiated neuronal cultures within 18 days, that can be used for subsequent biochemical and imaging analyses. Undifferentiated SH-SY5Y neuroblastoma cells demonstrate a large, flat, epithelial-like phenotype with numerous short processes extending outward (Figure 2A), while differentiated cells possess several neuritic projections that connect to surrounding cells (Figure 2B). It is important that when differentiating SH-SY5Y cells, cells are incubated with trypsin for a minimal length of time to ensure that only neurons are released from the dish. This leaves behind the undifferentiated epithelial cells that would otherwise contaminate the differentiated neuronal cell population. The neuronal characteristics of fully differentiated SH-SY5Y cells are demonstrated by techniques such as immunofluorescence detection of classical neuronal markers (Figure 3).
SH-SY5Y cells demonstrate a variety of different phenotypes during the course of differentiation and it is important to be able to identify healthy neurons from those that are stressed. On differentiation day 1, prior to the introduction of Differentiation Media #1, cells have a flat, retracted phenotype with short, stubby processes (Figure 4A). Following 5 days of serum deprivation, SH-SY5Y cells begin to develop longer projections and demonstrate a more neuronal phenotype (Figure 4B). Passaging is a harsh process for SH-SY5Y cells, and on days immediately following passaging, cells appear unhealthy. This is evidenced by cell body clumping and the presence of fewer, shorter processes. (See before images: Figures 4C and 4E, and after images: Figures 4D and 4F). Approximately 48 hr following a split, cells appear to recover, and fully mature, differentiated neurons are obtained at Day 18 (Figure 2B). This is evidenced by a reduction in cell body clumping, and extension of numerous thin, branched neuritic processes that often connect to neighboring cells.
Factors that are essential to obtaining reproducible and viable neuronal cultures include using heat-inactivated FBS, minimizing trypsin incubation time, and gentle trituration. Importantly, this protocol details the use of four different media formulations with various concentrations of hiFBS, thereby creating a gentle transition into a serum-starved state for the cells. When mature SH-SY5Y cells are healthy, they demonstrate numerous projections that connect to surrounding neurons (Figure 5A). It should be noted that a distinctive epithelial phenotype will overtake the neuronal cultures if they are mishandled during the differentiation process (Figure 5B). Additionally, if neurons begin to die, neurites will retract, cell bodies will begin to clump together and round up, and debris from neurite degeneration will start to accumulate at and around cell processes. This process is akin to what is demonstrated in Figures 5C and 5D. While Figure 5C shows a more natural progression of cell death following nutrient and environmental deprivation, Figure 5D shows differentiated SH-SY5Y neurons 4 hr following infection with an HSV-1 strain, KOS, at a multiplicity of infection (MOI) of 106 PFU. This protocol has been thoroughly optimized in the lab and yields reproducible, homogeneous populations of neurons, which are essential for downstream analyses and experimentation.
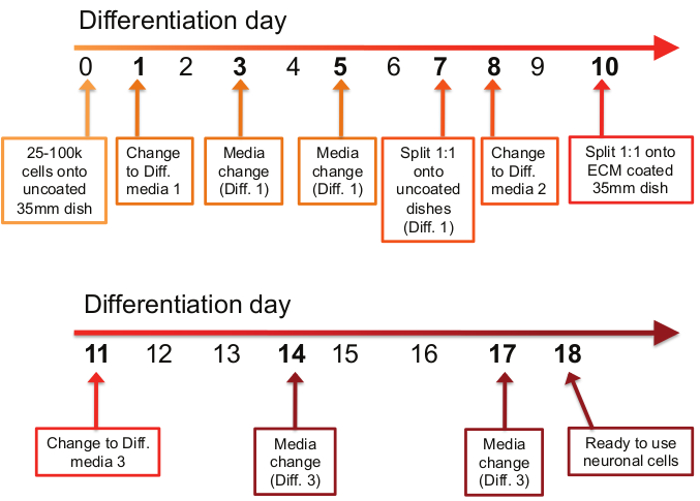
Figure 1: Timetable of differentiation procedure. The differentiation process consists of 11 steps spread out over the course of an 18 day period. On the first day of the differentiation protocol (day 0), between 25,000 and 100,000 cells are plated onto uncoated 35 mm dishes. On days 1, 3, and 5, old media is removed and Differentiation Media #1 is applied. On day 7, cells are split 1:1 onto uncoated 35mm dishes in Differentiation Media #1. On day 8, the media is changed to Differentiation Media #2, and on day 10, cells are again split 1:1, but this time onto ECM-coated 35 mm dishes in Differentiation Media #2. On days 11, 14, and 17, old media is removed and Differentiation Media #3 is applied. On day 18, differentiated neurons are ready to use for downstream applications.
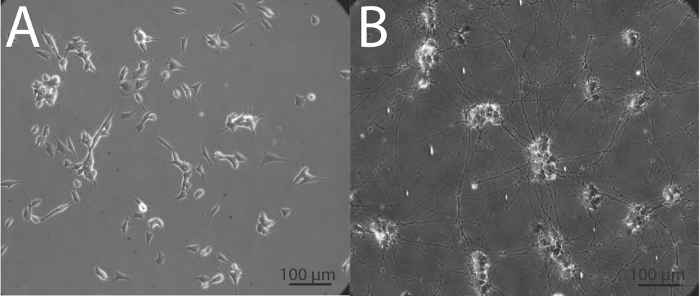
Figure 2: Morphological appearance of undifferentiated and differentiated SH-SY5Y cells. (A) Undifferentiated SH-SY5Y cells have a flat phenotype with few projections while (B) differentiated SH-SY5Y neurons demonstrate extensive and elongated neuritic projections. Images were obtained in phase at 20X magnification using an inverted epifluorescence microscope.
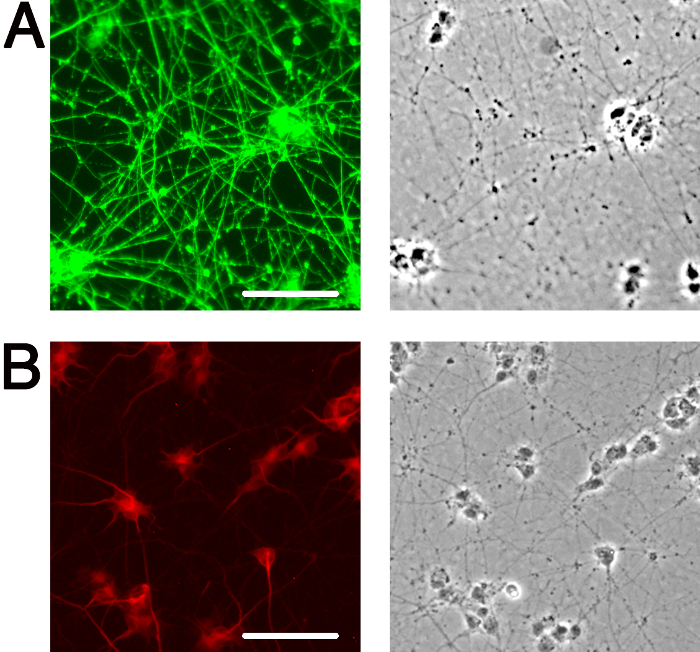
Figure 3: Markers of neuronal differentiation. Immunofluorescence illuminates neuronal features of fully differentiated SH-SY5Y cells. (A) Anti-SMI31 (green) stains the phosphorylated neurofilament H in the extensive network of neurites. (B) Anti-MAP2 (red) labels microtubule-associated protein 2, revealing the neuronal soma and proximal portion of neurites. Corresponding phase image for each immunofluorescence panel is shown to the right. Images were obtained at 10X magnification using an inverted epifluorescence microscope. Scale bar, 100 μm.
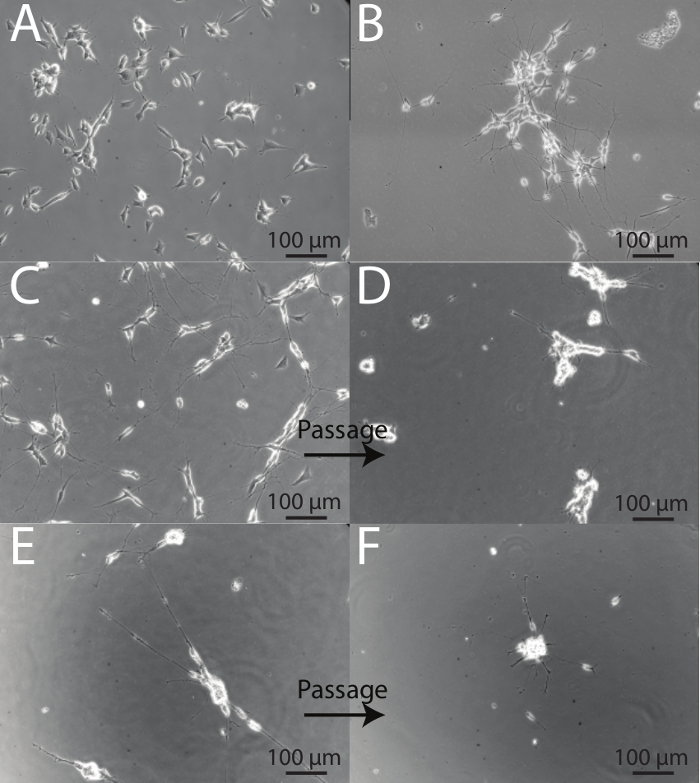
Figure 4: Intermediate steps of the differentiation protocol. (A) Day 1 of differentiation. Cells maintain a retracted phenotype with short projections. (B) Day 5 of differentiation. Cells have been exposed to 5 days of serum deprivation (Differentiation Media #1). The surviving cells begin to elongate and form longer processes that connect with neighboring cells. (C) Day 7 of differentiation prior to split. Cells demonstrate high numbers of long processes, with fewer numbers of cells demonstrating an epithelial-like phenotype. (D) Day 8 of differentiation, one day after the first passage. Following splitting, cell bodies form clumps and processes appear short as a result of the passaging procedure. (E) Day 10 of differentiation, prior to split onto ECM-coated plates. Cells demonstrate longer processes that make connections with nearby cells. Cell body clustering is also evident. (F) Day 11 of differentiation, one day following second split. Cells are stressed following the second passage and many neurons are ultimately lost. However, the remaining population is viable, homogenous, and neuronal in phenotype. Cell bodies produce larger clusters and processes begin to emit from the base of the clusters.
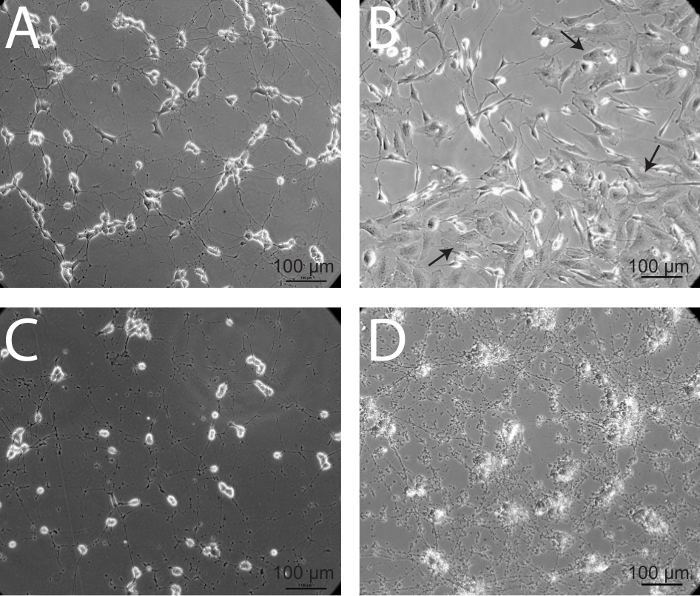
Figure 5: Epithelial overgrowth and neuronal death – two alternative outcomes of the differentiation process. (A) Healthy, mature SH-SY5Y neurons demonstrate diffuse axonal projections connecting to neighboring cells. (B) In some instances, cells with a more epithelial-like phenotype overtake maintenance cultures. This over-population of epithelial-like cells may be due to infrequent passaging of maintenance cultures. These cultures should be discarded, as the epithelial-like cells will continue to outnumber neurons. Arrows denote epithelial-like cells. (C) When SH-SY5Y cells are unhealthy and begin to die, cell bodies round up and processes degrade, generating a significant amount of debris. (D) Noticeable accumulation of cellular debris and retraction of neuronal processes is evident in mature, differentiated neurons, 4 hr following infection with a strain of herpes simplex virus 1 (HSV-1) KOS at a multiplicity of infection (MOI) of 106 PFU.
| Component | Details | Stock | Instructions | ||||
| 10 μM RA | All-trans retinoic acid | 5 mM | Resuspend 50 mg RA in 33.3 ml 95% EtOH. RA is sensitive to heat, light, and air. Keep in a dark bottle and store at 4 °C for up to 6 weeks. Use at 1:500 dilution and dilute into differentiation media immediately prior to use | ||||
| (300.44 g/mol) | |||||||
| 1x B-27 | B-27 Supplement | 50x | Thaw 1-10 ml bottle and aliquot remainder into single-use 1 ml aliquots and store at -80 °C. Store 10 ml bottles at -20 °C | ||||
| 20 mM KCl | Potassium Chloride | 1 M | Add 250 ml water to 18.6 g KCl and sterile filter. Store at room temperature | ||||
| (74.55 g/mol) | |||||||
| 2 mM db-cAMP | dibutyryl cyclic AMP | 1 M | Resuspend full bottle by adding 2.04 ml water to 1 g db-cAMP. Sensitive to light and moisture. Store in aliquots of 100 μl or 200 μl at either -20 °C or -80 °C | ||||
| (491.37 g/mol) | |||||||
| 50 ng/ml BDNF | Brain-derived neurotrophic factor (BDNF) | – | Centrifuge vial to get powder to bottom. Resuspend 10 μg vial in 1 ml Neurobasal + 1x B27 or 5 μg vial in 0.5 ml Neurobasal + 1x B27 to get 10 μg/ml. Use at 1:200 dilution. Store working aliquots at -80 °C (ex: 250 μl) | ||||
| hiFBS | Heat-inactivated Fetal Bovine Serum | – | Aliquot thawed FBS into 50 ml conical tubes. Heat at 56 °C in a water bath for 30 min. Remove and freeze working aliquots at -20 °C | ||||
Table 1: Stock solutions and components.
| Basic Growth Media | ||
| Component | Volume for 500 ml | Dilution |
| EMEM | 415 ml EMEM | |
| 15% hiFBS | 75 ml hiFBS | |
| 1x Pen/Strep | 5 ml Pen/Strep | 1:100 |
| 2 mM Glutamine | 5 ml Glutamine | 1:100 |
| *Keep for 6 weeks maximum | ||
| Differentiation Media #1 | ||
| Component | Volume for 50 ml | Dilution |
| EMEM | 48 ml EMEM | |
| 2.5% hiFBS | 1.3 ml hiFBS | |
| 1x Pen/Strep | 500 μl Pen/Strep | 1:100 |
| 2 mM Glutamine | 500 μl Glutamine | 1:100 |
| 10 μM RA | 100 μl RA (5mM stock) | 1:500 |
| *Keep for 2 weeks maximum and add RA immediately prior to use | ||
| *Do not keep extra media once RA is added – RA is unstable | ||
| Differentiation Media #2 | ||
| Component | Volume for 50 ml | Dilution |
| EMEM | 49 ml EMEM | |
| 1% hiFBS | 500 μl hiFBS | |
| 1x Pen/Strep | 500 μl Pen/Strep | 1:100 |
| 2 mM Glutamine | 500 μl Glutamine | 1:100 |
| 10 μM RA | 100 μl RA (5mM stock) | 1:500 |
| *Keep for 2 weeks maximum and add RA immediately prior to use | ||
| *Do not keep extra media once RA is added – RA is unstable | ||
| Differentiation Media #3 | ||
| Component | Volume for 50 ml | Dilution |
| Neurobasal | 47 ml Neurobasal | |
| 1x B-27 | 1 ml B-27 (50X stock) | 1:50 |
| 20 mM KCl | 1 ml KCl (1M stock) | 1:50 |
| 1x Pen/Strep | 500 μl Pen/Strep | 1:100 |
| 2 mM GlutamaxI | 500 μl GlutamaxI (100x stock) | 1:100 |
| 50 ng/ml BDNF | 250 μl BDNF stock (10 μg/ml) | 1:200 |
| 2 mM dibutyryl cyclic AMP (db-cAMP) | 100 μl db-cAMP (1M stock) | 1:500 |
| 10 μM RA | 100 μl RA (5 mM stock) | 1:500 |
| *Keep for 2 weeks maximum and add RA immediately prior to use | ||
| *Do not keep extra media once RA is added – RA is unstable | ||
Table 2: Media recipes.
Discussion
The above protocol provides a straightforward and reproducible method to generate homogenous and viable human neuronal cultures. This protocol utilizes techniques and practices that integrate several previously published methods1-4 and aims to delineate the best practices of each. Differentiation of SH-SY5Y cells relies on gradual serum deprivation; the addition of retinoic acid, neurotrophic factors and extracellular matrix proteins; and serial splitting to select for differentiated mature adherent neurons. This cell line begins as a heterogeneous population of adherent and suspended cells. This protocol aims to preserve both populations by withholding PBS washes before passaging or media change, but some loss of suspended cells is necessary to allow removal of the dead epithelial cells. The presented method produces a homogeneous population of differentiated human SH-SY5Y neurons for further experimentation.
Although other agents can be used to guide the differentiation of neurons into a cholinergic or adrenergic phenotype16,17, the use of RA to differentiate SH-SY5Y cells has been used previously to produce neurons with a dopaminergic phenotype37,38. Addition of RA has been shown to induce cellular differentiation through a number of mechanisms, including arresting cell cycle progression out of G0/G1, increasing expression of the cyclin-dependent kinase (CDK) inhibitors p21 and p27Kip1 and the anti-apoptotic proteins Bcl-2 and Bcl-xL, and enhancing PI3K/AKT activity which plays a role in neurite development and differentiation39.
It is imperative throughout the execution of this protocol to use gentle and sterile handling techniques, as SH-SY5Y cells are very sensitive to sudden change. It is because of this sensitivity that the gradual serum deprivation protocol is preferred over protocols that require making rapid changes in media composition. When splitting cells on days 7 and 10, it is important to minimize the amount of time that the partially differentiated neurons spend in trypsin. This reduces the probability of damaging neurons and promotes the preferential release of neurons versus epithelial cells, which take longer to detach. This helps to establish a more homogeneous neuronal population of cells and to minimize contamination with the proliferating and undifferentiated epithelial cells. It is also important to note reagents used for the culture and differentiation of SH-SY5Y cells should be replaced routinely and not be kept indefinitely. For example, Basic Growth Media can be kept up to 6 weeks while Differentiation Media should only be kept up to 2 weeks. This ensures reagents such as dbcAMP and BDNF are fresh and stable. Additionally, prepared RA should only be stored for up to 6 weeks and in the dark before a fresh batch should be made. We have observed greater consistency of neuronal outcomes with these precautions.
Once cells have been differentiated into mature neurons, they can be maintained for up to 2 weeks post-terminal differentiation and used for experimentation. Following terminal differentiation, media should be changed every 3-4 days (Differentiation Media #3). In contrast to differentiated neurons, maintenance flasks containing undifferentiated SH-SY5Y cell cultures may be kept over many weeks with regular passaging. It is important to passage maintenance cultures regularly, to prevent over-population of epithelial-like cells that are no longer capable of differentiating into neurons. When these cells outnumber those with neuro-potential, serum starvation will cause disproportionate amounts of cell death. Undifferentiated cultures can only be maintained for up to approximately 15 passages. Once the culture has exceeded around 15 passages, undifferentiated cells begin to die and have a rough, unhealthy appearance. Additionally, differentiation of high passage SH-SY5Y cells is more difficult as fewer cells will survive each split, and those that do often aggregate both cell bodies and axons, making subsequent analyses more difficult.
There is a distinct decrease in cell number during the differentiation process as many cells are lost or do not survive the splitting process. Therefore, at the beginning of any experiment involving differentiated SH-SY5Y cells, one should account for ~30-40% loss of neurons and ensure a proper number of cells is plated at the start to achieve the desired yield. While plating 100,000 cells produces plenty of healthy, mature SH-SY5Y neurons, fewer or larger numbers may be initially plated depending on experimental need.
It is clear that fully differentiated SH-SY5Y neurons provide a closer approximation of mature human neurons found in vivo than do their undifferentiated progenitor cell counterparts (Figure 2). These neurons will provide an advantageous model for future characterizations of their neurobiology, for the study of neurotropic viruses, or to screen for chemotherapeutic toxicity in neurons.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We are grateful for the contributions of Yolanda Tafuri in optimizing conditions for SH-SY5Y differentiation, and for the support of Dr. Lynn Enquist, in whose lab this work was initiated. Y. Tafuri contributed the images shown in Figure 3. This work was supported by the NIH-NIAID Virus Pathogens Resource (ViPR) Bioinformatics Resource Center (MLS and L. Enquist) and K22 AI095384 (MLS).
Materials
| B-27 | Invitrogen | 17504-044 | See Table 1 for preparation |
| Brain-Derived Neurotrophic Factor (BDNF) | Sigma | SRP3014 (10ug)/B3795 (5ug) | See Table 1 for preparation |
| dibutyryl cyclic AMP (db-cAMP) | Sigma | D0627 | See Table 1 for preparation |
| DMSO | ATCC | 4-X | – |
| Minimum Essential Medium Eagle (EMEM) | Sigma | M5650 | – |
| Fetal Bovine Serum (FBS) | Hyclone | SH30071.03 | See Table 1 for preparation |
| GlutamaxI | Life Technologies | 35050-061 | – |
| Glutamine | Hyclone | SH30034.01 | – |
| Potassium Chloride (KCl) | Fisher Scientific | BP366-1 | See Table 1 for preparation |
| MaxGel Extracellular Matrix (ECM) solution | Sigma | E0282 | See step 11 of the protocol |
| Neurobasal | Life Technologies | 21103-049 | – |
| Penicillin/Streptomycin (Pen/Strep) | Life Technologies | 15140-122 | – |
| Retinoic acid (RA) | Sigma | R2625 | Should be stored in the dark at 4° C because this reagent is light sensitive |
| SH-SY5Y Cells | ATCC | CRL-2266 | – |
| 0.5% Trypsin + EDTA | Life Technologies | 15400-054 | – |
| Falcon 35mm TC dishes | Falcon (A Corning Brand) | 353001 | – |
Referencias
- Christensen, J., Steain, M., Slobedman, B., Abendroth, A. Differentiated Neuroblastoma Cells Provide a Highly Efficient Model for Studies of Productive Varicella-Zoster Virus Infection of Neuronal Cells. Journal of Virology. 85 (16), 8436-8442 (2011).
- Gimenez-Cassina, A., Lim, F., Diaz-Nido, J. Differentiation of a human neuroblastoma into neuron-like cells increases their susceptibility to transduction by herpesviral vectors. Journal of Neuroscience Research. 84 (4), 755-767 (2006).
- Biedler, J. L., Roffler-Tarlov, S., Schachner, M., Freedman, L. S. Multiple Neurotransmitter Synthesis by Human Neuroblastoma Cell Lines and Clones. Investigación sobre el cáncer. 38 (11 Pt 1), 3751-3757 (1978).
- Encinas, M., Iglesias, M., et al. Sequential Treatment of SH-SY5Y Cells with Retinoic Acid and Brain-Derived Neurotrophic Factor Gives Rise to Fully Differentiated, Neurotrophic Factor-Dependent, Human Neuron-Like Cells. Journal of Neurochemistry. 75 (3), 991-1003 (2000).
- Otey, C. A., Boukhelifa, M., Maness, P. B35 neuroblastoma cells: an easily transfected, cultured cell model of central nervous system neurons. Methods in Cell Biology. 71, 287-304 (2003).
- LePage, K. T., Dickey, R. W., Gerwick, W. H., Jester, E. L., Murray, T. F. On the use of neuro-2a neuroblastoma cells versus intact neurons in primary culture for neurotoxicity studies. Critical Reviews in Neurobiology. 17 (1), 27-50 (2005).
- Shafer, T. J., Atchison, W. D. Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: a model for neurotoxicological studies. Neurotoxicology. 12 (3), 473-492 (1991).
- Yue, F., Cheng, Y., et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 515 (7527), 355-364 (2014).
- Cheng, Y., Ma, Z., et al. Principles of regulatory information conservation between mouse and conservation between mouse and human. Nature. 515 (7527), 371-375 (2014).
- Stergachis, A. B., Neph, S., et al. Conservation of trans-acting circuitry during mammalian regulatory evolution. Nature. 515 (7527), 365-370 (2014).
- Lin, S., Lin, Y., et al. Comparison of the transcriptional landscapes between human and mouse tissues. Proceedings of the National Academy of Sciences of the United States of America. 111 (48), 17224-17229 (2014).
- Coyle, D. E., Li, J., Baccei, M. Regional Differentiation of Retinoic Acid-Induced Human Pluripotent Embryonic Carcinoma Stem Cell Neurons. PLoS ONE. 6 (1), e16174 (2011).
- Mostert, M. M., van de Pol, M., et al. Fluorescence in situ hybridization-based approaches for detection of 12p overrepresentation, in particular i(12p), in cell lines of human testicular germ cell tumors of adults. Cancer Genetics and Cytogenetics. 87 (2), 95-102 (1996).
- Hu, B. -. Y., Weick, J. P., et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proceedings of the National Academy of Sciences of the United States of America. 107 (9), 4335-4340 (2010).
- Biedler, J. L., Helson, L., Spengler, B. A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Investigación sobre el cáncer. 33 (11), 2643-2652 (1973).
- Påhlman, S., Ruusala, A. I., Abrahamsson, L., Mattsson, M. E., Esscher, T. Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester-induced differentiation. Cell Differentiation. 14 (2), 135-144 (1984).
- Xie, H., Hu, L., Li, G. SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chinese Medical Journal. 123 (8), 1086-1092 (2010).
- Kovalevich, J., Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods in Molecular Biology. 1078, 9-21 (2013).
- Påhlman, S., Hoehner, J. C., et al. Differentiation and survival influences of growth factors in human neuroblastoma. European Journal of Cancer. 31 (4), 453-458 (1995).
- Cheung, Y. -. T., Lau, W. K. -. W., et al. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology. 30 (1), 127-135 (2009).
- Agholme, L., Lindström, T., Kågedal, K., Marcusson, J., Hallbeck, M. An in vitro model for neuroscience: differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. Journal of Alzheimer’s disease: JAD. 20 (4), 1069-1082 (2010).
- La Monica, N., Racaniello, V. R. Differences in replication of attenuated and neurovirulent polioviruses in human neuroblastoma cell line SH-SY5Y. Journal of Virology. 63 (5), 2357-2360 (1989).
- Cordey, S., Petty, T. J., et al. Identification of Site-Specific Adaptations Conferring Increased Neural Cell Tropism during Human Enterovirus 71 Infection. PLoS Pathog. 8 (7), e1002826 (2012).
- Xu, L. -. J., Jiang, T., et al. Global Transcriptomic Analysis of Human Neuroblastoma Cells in Response to Enterovirus Type 71 Infection. PLoS ONE. 8 (7), e65948 (2013).
- Luo, M. H., Fortunato, E. A. Long-term infection and shedding of human cytomegalovirus in T98G glioblastoma cells. Journal of Virology. 81 (19), 10424-10436 (2007).
- Sun, Z., Yang, H., Shi, Y., Wei, M., Xian, J., Hu, W. Establishment of a cell model system of herpes simplex virus type II latent infection and reactivation in SH-SY5Y cells. Wei Sheng Wu Xue Bao = Acta Microbiologica Sinica. 50 (1), 98-106 (2010).
- Yun, S. -. I., Song, B. -. H., et al. A molecularly cloned, live-attenuated japanese encephalitis vaccine SA14-14-2 virus: a conserved single amino acid in the ij Hairpin of the Viral E glycoprotein determines neurovirulence in mice. PLoS pathogens. 10 (7), e1004290 (2014).
- Garrity-Moses, M. E., Teng, Q., Liu, J., Tanase, D., Boulis, N. M. Neuroprotective adeno-associated virus Bcl-xL gene transfer in models of motor neuron disease. Muscle & Nerve. 32 (6), 734-744 (2005).
- Kalia, M., Khasa, R., Sharma, M., Nain, M., Vrati, S. Japanese Encephalitis Virus Infects Neuronal Cells through a Clathrin-Independent Endocytic Mechanism. Journal of Virology. 87 (1), 148-162 (2013).
- Haedicke, J., Brown, C., Naghavi, M. H. The brain-specific factor FEZ1 is a determinant of neuronal susceptibility to HIV-1 infection. Proceedings of the National Academy of Sciences. 106 (33), 14040-14045 (2009).
- Xu, K., Liu, X. -. N., et al. Replication-defective HSV-1 effectively targets trigeminal ganglion and inhibits viral pathopoiesis by mediating interferon gamma expression in SH-SY5Y cells. Journal of molecular neuroscience: MN. 53 (1), 78-86 (2014).
- Oh, J., Fraser, N. W. Temporal association of the herpes simplex virus genome with histone proteins during a lytic infection. Journal of Virology. 82 (7), 3530-3537 (2008).
- Stiles, K. M., Milne, R. S. B., Cohen, G. H., Eisenberg, R. J., Krummenacher, C. The herpes simplex virus receptor nectin-1 is down-regulated after trans-interaction with glycoprotein D. Virology. 373 (1), 98-111 (2008).
- Thomas, D. L., Lock, M., Zabolotny, J. M., Mohan, B. R., Fraser, N. W. The 2-kilobase intron of the herpes simplex virus type 1 latency-associated transcript has a half-life of approximately 24 hours in SY5Y and COS-1 cells. Journal of Virology. 76 (2), 532-540 (2002).
- Handler, C. G., Cohen, G. H., Eisenberg, R. J. Cross-linking of glycoprotein oligomers during herpes simplex virus type 1 entry. Journal of Virology. 70 (9), 6076-6082 (1996).
- Nicola, A. V., Hou, J., Major, E. O., Straus, S. E. Herpes Simplex Virus Type 1 Enters Human Epidermal Keratinocytes, but Not Neurons, via a pH-Dependent Endocytic Pathway. Journal of Virology. 79 (12), 7609-7616 (2005).
- Korecka, J. A., van Kesteren, R. E., et al. Phenotypic characterization of retinoic acid differentiated SH-SY5Y cells by transcriptional profiling. PloS One. 8 (5), e63862 (2013).
- Presgraves, S. P., Ahmed, T., Borwege, S., Joyce, J. N. Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotoxicity Research. 5 (8), 579-598 (2004).
- Qiao, J., Paul, P., et al. PI3K/AKT and ERK regulate retinoic acid-induced neuroblastoma cellular differentiation. Biochemical and Biophysical Research Communications. 424 (3), 421-426 (2012).

