A Murine Model of Group B Streptococcus Vaginal Colonization
Summary
The purpose of this protocol is to imitate human group B Streptococcus (GBS) vaginal colonization in a murine model. This method may be used to investigate host immune responses and bacterial factors contributing to GBS vaginal persistence, as well as to test therapeutic strategies.
Abstract
Streptococcus agalactiae (group B Streptococcus, GBS), is a Gram-positive, asymptomatic colonizer of the human gastrointestinal tract and vaginal tract of 10 – 30% of adults. In immune-compromised individuals, including neonates, pregnant women, and the elderly, GBS may switch to an invasive pathogen causing sepsis, arthritis, pneumonia, and meningitis. Because GBS is a leading bacterial pathogen of neonates, current prophylaxis is comprised of late gestation screening for GBS vaginal colonization and subsequent peripartum antibiotic treatment of GBS-positive mothers. Heavy GBS vaginal burden is a risk factor for both neonatal disease and colonization. Unfortunately, little is known about the host and bacterial factors that promote or permit GBS vaginal colonization. This protocol describes a technique for establishing persistent GBS vaginal colonization using a single β-estradiol pre-treatment and daily sampling to determine bacterial load. It further details methods to administer additional therapies or reagents of interest and to collect vaginal lavage fluid and reproductive tract tissues. This mouse model will further the understanding of the GBS-host interaction within the vaginal environment, which will lead to potential therapeutic targets to control maternal vaginal colonization during pregnancy and to prevent transmission to the vulnerable newborn. It will also be of interest to increase our understanding of general bacterial-host interactions in the female vaginal tract.
Introduction
Streptococcus agalactiae, group B Streptococcus (GBS), is an encapsulated, Gram-positive bacterium which is frequently isolated from the gut and genitourinary tract of healthy adults. In the 1970s, GBS emerged as the leading agent of infectious neonatal mortality, with over 7,000 cases of neonatal disease annually1. Early-onset GBS disease (EOD) occurs in the first hours or days of life, arises as pneumonia or respiratory distress, and often develops into sepsis, whereas late-onset disease (LOD) ensues after several months and presents with bacteremia, which frequently advances to meningitis2. As of 2002, the Centers for Disease Control and Prevention recommends universal screening for GBS vaginal colonization in late gestation and intrapartum antibiotic prophylaxis (IAP) to GBS-positive mothers1. Despite the reduction of early-onset disease to approximately 1,000 cases in the United States annually due to IAP, GBS remains the leading cause of early-onset neonatal sepsis, and late-onset occurrence remains unaffected1. Whether in utero, during labor, or even in late-onset cases, neonatal exposure to GBS requires survival, transversal through a number of host environments and barriers, immune evasion, and, in the case of meningitis, crossing of the highly regulated blood-brain barrier2. Upstream of these virulent interactions within the neonate is the initial colonization of the maternal vaginal tract. Maternal GBS vaginal colonization rates range from 8-18% in developed and developing countries, with an estimated average rate of 12.7%3,4. GBS colonization of the vaginal tract during pregnancy may be constant, intermittent, or transient in nature among individual women5. Interestingly, a maternal age > 36 years is associated with persistent colonization6. Numerous biological and socio-economical risk factors for GBS vaginal colonization have been identified. Biological factors include gastrointestinal GBS colonization and absence of Lactobacillus within the gut. However, ethnicity, obesity, hygiene, and sexual activity have also been associated with GBS vaginal carriage7.
Although notorious for causing neonatal infections, GBS also causes a variety of maternal infections both peripartum and postpartum. GBS carriage is increased in women presenting with vaginitis8 and, in some cases, may even be the disease entity9. Additionally, GBS ascension of the reproductive tract during pregnancy may result in intra-amniotic infection or chorioamnionitis10. Moreover, in up to 3.5% of pregnancies, GBS disseminates to the urinary bladder to cause a urinary tract infection or asymptomatic bacteriuria11. GBS bacteriuria during pregnancy is associated with an increased risk of intrapartum fever, chorioamnionitis, preterm delivery, and premature rupture of membranes12. Taken together, the presence of GBS within the vaginal tract is linked to infections of multiple host tissues, and the ability to eliminate GBS from this niche is imperative for both maternal and neonatal health.
Until recently, the majority of work examining GBS interactions with the cervicovaginal tract was limited to in vitro cell models13-15. These in vitro experiments have revealed bacterial factors that are important for adherence, including surface proteins such a pili and serine-rich repeats17,18, as well as two-component regulatory systems15,19 and the global transcriptional response of the vaginal epithelium to GBS19. However, to fully elucidate the host-microbe interactions within the vaginal tract, a robust animal model is necessary. Early work demonstrated that GBS can be recovered from the vaginal tract of inoculated mice20,21 and rats22 in both pregnant and non-pregnant conditions. In 2005, short-term GBS vaginal colonization was modelled in mice to examine the efficacy of a phage lytic enzyme to treat vaginal GBS over a 24 hr period23. Several years later, a long-term GBS vaginal colonization mouse model was developed to study host and bacterial factors governing GBS persistence. This model has identified numerous GBS factors contributing to colonization, including surface appendages17,18 and GBS two-component systems19,24. This model has contributed to the identification of host response mechanisms19,25 and was used to test several therapeutic strategies, including immunomodulatory peptides26 and probiotics27. This protocol gives the necessary guidance to inoculate GBS into the mouse vaginal tract and to subsequently track colonization and collect samples for further analyses.
Protocol
All animal work was approved by the Office of Lab Animal Care at San Diego State University and conducted under accepted veterinary standards. Female mice, age 8 – 16 weeks, were used for the development of this method.
1. Preparation and Intraperitoneal Injection of β-estradiol
- Measure out β-estradiol (0.5 mg/mouse) on weigh paper while wearing appropriate personal protective equipment (PPE). CAUTION: β-estradiol can be absorbed through the skin and mucosal surfaces.
- Transfer β-estradiol to a 15-ml conical tube and vortex until all lumps are removed and the β-estradiol is a fine powder. Draw up sesame oil (100 µl/mouse) into a 10-ml syringe. Syringe-filter sesame oil into the 15-ml conical tube containing the β-estradiol using a 0.45-µm filter. Vortex the 15-ml conical tube until the β-estradiol is a homogenous suspension in the sesame oil.
- Draw up the β-estradiol suspension into a new 10-ml syringe. With an 18 G, 1-in. needle, aliquot 100 µl of the suspension into 1-ml tuberculin syringes. Prepare one syringe for each mouse. Place a new, sterile 26 G, ½-in. needle on each tuberculin syringe.
- Administer 0.5 mg of the β-estradiol suspended in 100 µl of sesame oil (5 mg/ml) to each mouse 24 hr prior to bacterial inoculation. Inject each mouse within the peritoneal cavity, in the lower abdominal quadrants, just to the right or left of the midline, as previously described28.
NOTE: No cleaning or clipping of the injection site is necessary.
2. Vaginal Inoculation with GBS
- One day prior to inoculation, grow a 5-ml overnight liquid culture of a GBS strain of interest, such as A909 (serotype Ia), in Todd Hewitt broth (THB) at 37 °C.
- Subculture the GBS overnight culture at a 1:10 volume into fresh THB and incubate at 37 °C. Grow the bacteria to mid-log phase (OD600 = 0.4-0.5). NOTE: This will typically take 2 – 3 hr, depending on the strain of GBS.
- Transfer the subculture to a sterile 15-ml conical tube and pellet bacteria at 3,000 × g for 5 min. Aspirate the supernatant. Resuspend the bacterial pellet in 200 µl of sterile phosphate-buffered saline (PBS).
- Using the resuspended pellet, bring 3 – 5 ml of PBS (1 ml per 10 mice) to exactly OD600 = 0.4 in a new 5-ml culture tube. This will be a concentration of ~ 1 × 108 colony forming units (CFU)/ml. Transfer to a new 15-ml conical tube and re-pellet the bacteria at 3,000 × g for 5 min. Aspirate the supernatant.
- Resuspend the pellet in PBS at 1/10 the original volume. For example, if 3 ml of OD600 = 0.4 was pelleted, then resuspend it in 300 µl of PBS. NOTE: This is the final bacterial suspension (~ 1 × 109 CFU/ml) used for animal inoculation.
- Reserve 50 µl of this suspension for serial dilution and plating on THB agar to determine the exact inoculum.
- Inoculate each mouse with 10 µl of the final bacterial suspension so that 1 × 107 CFU is administered to each mouse.
- To inoculate, restrain the mouse manually by securing the loose skin at the scruff of the neck between the handler's thumb and index finger and then immobilizing the tail, as described previously28.
- Draw up 10 µl of the GBS prepared in step 2.6 into a 200-µl gel loading pipette tip. Insert the tip 5 to 10 mm into the vaginal lumen and dispense the 10 µl of inoculum.
NOTE: Gel loading tips are preferred over standard 200-µl tips to minimize the risk of organ trauma or injury, particularly in younger or smaller mice. - Immediately following inoculation, release the scruff of the neck and elevate the hind end of the mouse, lifting the mouse by the tail and walking the front paws on a hard surface for ~ 1 min.
- Visually inspect the vaginal opening for any backflow of inoculum. If backflow is observed, a fresh pipette tip may be used to manipulate or enlarge the vaginal opening, facilitating uptake of the backflow into the lumen. Additionally, backflow may be aspirated via pipette and re-inoculated.
NOTE: If administering topical agents, probiotic organisms, or proteins of interest, a volume up to 20 µl in a physiologic buffer may be given in the vaginal tract.
3. Swabbing the Vaginal Lumen to Quantify GBS Load
- Prepare one 1.5-ml micro-centrifuge tube per mouse by adding 100 µl of PBS. Just prior to swabbing, pre-wet the swab in PBS.
- Restrain the mouse as described in step 2.7.1 and insert the swab 10 mm into the vaginal lumen. Gently rotate the swab 4 times clockwise and 4 times counter clockwise, applying slight pressure to the vaginal wall.
- Transfer the swab to the 1.5-ml microcentrifuge tube with 100 µl of PBS. Prior to plating, vortex the microcentrifuge tube for ~15 sec to release the bacteria from the swab. Serially dilute each sample in PBS and plate 20 µl of dilutions 1:10 through 1:10,000 on differential medium agar plates prepared per the manufacturer's instructions. Incubate the plates at 37 °C for 24 hr. GBS colonies will appear either bright pink or mauve in color. Other endogenous flora will be inhibited, or will appear as blue, white, or grey colonies.
4. Collecting Vaginal Lavage Fluid
- Restrain the mouse as described in step 2.7.1. Using a 200-µl gel loading pipette tip, pipet 20 µl of PBS into the vaginal lumen. Gently pipet the entire volume up and down 4 times within the lumen, and then withdraw the entire volume in the same pipette tip. NOTE: If the lavage fluid is thick with mucous, a standard 200 µl pipette tip can be used to collect the final lavage fluid.
- If saving lavage fluid for cytokine analysis or CFU quantification, dispense into a 0.7-ml microcentrifuge tube. If determining the stage of estrus, dispense at least 5 µl of lavage fluid onto a glass slide and observe the cells under 100X magnification on a light microscope. NOTE: For examples of estrous stages, see Figure 1.
5. Tissue Dissection and Homogenization
- For each mouse, prepare three 2-ml screw cap tubes (one for each tissue: vagina, cervix, and uterus). Fill each tube with ample (0.4 – 0.5 g) 1.0 mm zirconia beads to cover the conical-shaped bottom of the tube.
- Autoclave the prepared tubes prior to collecting tissue for 30 min at 121 °C, especially if quantifying bacterial load. Add 500 µl of sterile PBS to each tube. Weigh each tube after autoclaving and record for future reference to calculate recovered tissue weight.
- Sacrifice the mouse using approved institutional methods such as CO2 asphyxiation and cervical dislocation. Spray down the ventral abdomen with 70% EtOH. With sterile scissors, open the abdominopelvic cavity, lift the back skin and abdominal muscles, and displace the intestines so that the reproductive tract is exposed.
- Sterilize the scissors with 70% EtOH, wipe clean if necessary, and cut both uterine horns mid-length between the uterine body and ovaries. Using scissors and forceps, separate visceral fat, membranes, and the urinary bladder away from the reproductive tract, moving caudally.
- Sterilize scissors as described in step 5.4. Transversely cut the vagina as close to the vulva as possible to separate the reproductive tract from the body. Lift and remove the intact reproductive tract and place it in a sterile Petri dish.
- Using a new razor blade, separate the uterus from the cervix in one transverse cut. Sterilize the razor blade with 70% EtOH, and then separate the cervix from the vagina in one transverse cut. NOTE: There will be a minimal amount of uterine tissue still attached to the cervix. There will be a minimal amount of vaginal tissue still attached to the cervix.
- Using sterile forceps, transfer each of the tissues into their respective 2-ml tubes containing PBS and homogenizing beads. Clean the forceps with 70% EtOH in between handling each tissue.
- Weigh each 2-ml screw cap tube and subtract the original weight of tube to determine the tissue weight. Tissue weights typically vary between 20 – 100 mg. Tightly seal the screw cap tubes and homogenize the tissues for 1 min at maximum speed in a tissue homogenizer.
- To quantify bacterial load, serially dilute 25 µl of tissue homogenate and plate dilutions 1:10 through 1:10,000 on differential medium agar plates. Incubate the plates as described in step 3.3. To store samples for cytokine quantification, freeze tissue homogenates at -20 °C.
Representative Results
During the development of this model, multiple observations were made regarding factors that affect the duration of GBS vaginal colonization. To determine how estrous stage at inoculation impacts GBS bacterial persistence, mice were staged on the day of inoculation via vaginal lavage fluid. Figure 1 illustrates the four stages of the mouse estrous cycle, as determined by wet-mount vaginal lavage fluid, a well-established method29. Mice were divided into groups based on this initial stage, and GBS persistence was monitored over time via vaginal swabbing. Mice inoculated at the proestrus stage were colonized with GBS longer than any other stage of estrus, particularly those in diestrus at the time of inoculation (Figure 2). Based on these results, in the current model, mice are treated with β-estradiol one day prior to GBS inoculation to synchronize them into the proestrus stage.
Other murine models of reproductive tract infections have demonstrated an increased ability of the pathogenic organism to persist when mice are sustained in the estrus stage through exogenous estradiol treatment30,31. To determine if this phenomenon also occurred during vaginal colonization with GBS, GBS persistence was monitored during repeated β-estradiol treatment. Sustained estrus promoted GBS A909 (American Type Culture Collection, ATCC #BAA-1138) persistence in CD-1 mice, with 90% colonization 2 weeks post-inoculation (Figure 3A). In a typical experiment with one dose of β-estradiol prior to inoculation, only 40-50% of mice were colonized one week post-inoculation (Figure 4). The mean GBS CFU recovered from these mice mimics the percentage of colonization (Figure 3B). Although these results were obtained from independent experiments, these data demonstrate that maintaining continuous estrus promotes GBS vaginal persistence in the majority of CD-1 mice.
While conducting colonization experiments using different human GBS isolates, it was observed that strains varied in their ability to persist in CD-1 mice, ranging from several days to beyond a month. Of the strains tested, NCTC 10/84 had the shortest duration, A909 and COH1 persisted for one to two weeks, whereas strain CJB111 persisted in the majority of mice for two weeks (Figure 4) and even beyond a month (data not shown). To date, there has been no observed correlation of serotype and ability to persist in the mouse vaginal tract; however, significant differences between individual GBS strains have been reported25. The ability of GBS to colonize multiple inbred and outbred mouse lines was also examined. GBS strain A909 persisted in the vaginal tract for approximately one week in outbred CD-1 mice and inbred FVB mice (Figure 5). Alternatively, the majority of inbred BALB/c and C57BL/6 were colonized at one week (Figure 5) and remained colonized for a month or beyond (data not shown).
Using this protocol, GBS vaginal colonization in vivo was visualized by inoculating mice with a plasmid GFP-expressing GBS strain and collecting tissue for fluorescent microscopy. GFP-GBS was detected both adhering to murine vaginal epithelium (Figure 6A) and in close proximity to other native vaginal flora (Figure 6B). No GFP signal was detected in mice that had cleared the GFP-GBS at the time of tissue collection (data not shown).
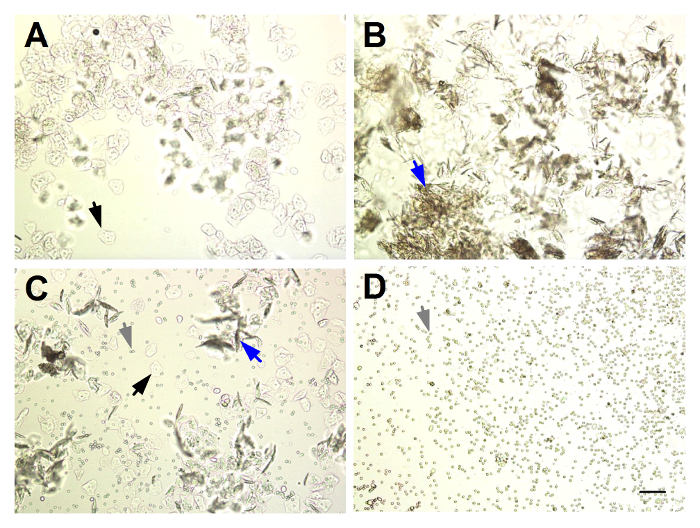
Figure 1: Identifying the Stage of Estrus from Unstained Murine Vaginal Lavage Fluid. (A) Proestrus: abundant nucleated squamous epithelial cells (black arrows). (B) Estrus: abundant cornified squamous epithelial cells (blue arrows). (C) Metestrus: mixture of nucleated and cornified squamous epithelial cells and predominantly leukocytes (grey arrows). (D) Diestrus: abundant leukocytes. Magnification = 100X, scale bar = 100 µm. Please click here to view a larger version of this figure.
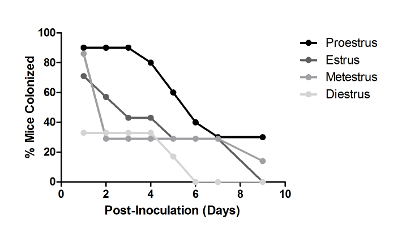
Figure 2: Estrous Stage Impacts Vaginal Persistence of GBS. Percent of CD-1 mice colonized with 1 × 107 CFU GBS A909 over time. Mice were grouped (n = 7 – 11/group) based on estrous stage at the time of GBS inoculation, as determined by vaginal lavage fluid. One independent experiment is shown. This figure has been modified from previously-published work and reprinted with permission19. Please click here to view a larger version of this figure.
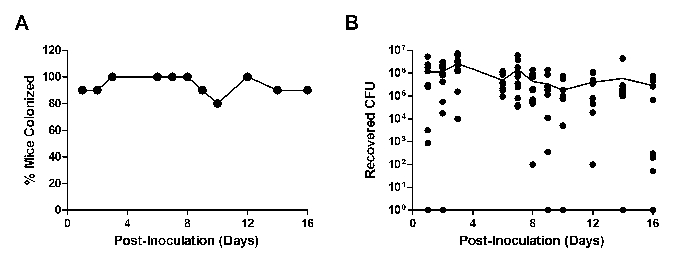
Figure 3: Continued Treatment with β-estradiol Promotes GBS Vaginal Persistence. Percent colonized (A) or recovered CFU (B) of CD-1 mice (n = 10) inoculated with 1 × 107 CFU GBS A909 and maintained on β-estradiol treatment. Mice were injected with β-estradiol one day prior to GBS inoculation and on days 1, 3, and 5 post-inoculation. One independent experiment is shown. The line in (B) represents mean recovered CFU. Please click here to view a larger version of this figure.
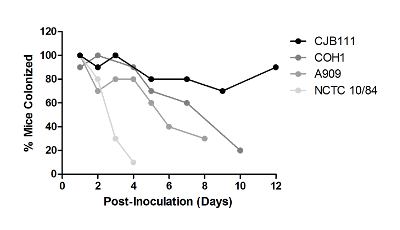
Figure 4: GBS Strains Differ in Their Ability to Persist in the Vaginal Tract. Outbred CD-1 mice (n = 10/group) were injected with a single dose of β-estradiol one day prior to GBS inoculation with 1 × 107 CFU of the given GBS strains. Experiments with each strain were carried out independently and were repeated at least three times; one representative result is shown. This figure has been modified from previously-published work and reprinted with permission25. Please click here to view a larger version of this figure.
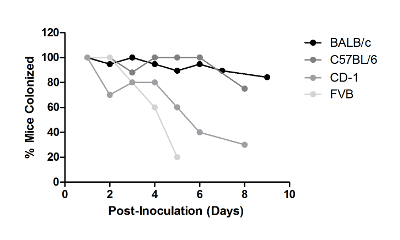
Figure 5: Mouse Strains Differ in Their Ability to Be Colonized with GBS in the Vaginal Tract. Mice from indicated background strains were injected with a single dose of β-estradiol one day prior to inoculation with 1 × 107 CFU of GBS A909. Experiments with each strain were carried out independently and were repeated at least twice; one representative result is shown. Please click here to view a larger version of this figure.
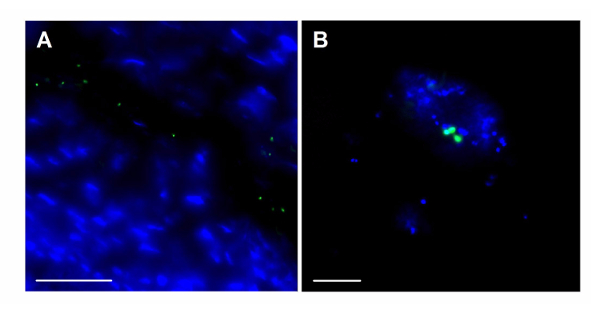
Figure 6: Fluorescent Imaging of GBS within the Mouse Vaginal Tract. Visualization of GFP- expressing GBS (green) along the vaginal epithelium at magnification = 630X, scale bar = 50 µm (A), and in close proximity to endogenous vaginal microbes at magnification = 1,000X, scale bar = 20 µm (B). Blue stain = DAPI. Please click here to view a larger version of this figure.
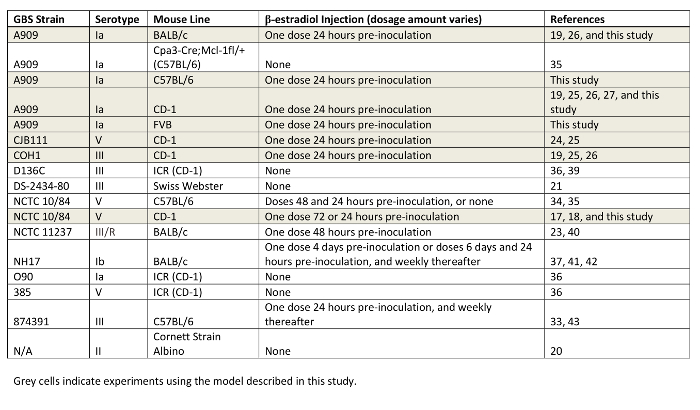
Table 1: Compilation of GBS Strains and Mouse Lines Utilized for Vaginal Colonization Studies. GBS background strains, mouse lines, and dosage of β-estradiol are indicated. Studies using the model described in this work are highlighted in grey.
Discussion
To further the advancement of the understanding of GBS interactions with the both the host and other microbes within the context of the host, an animal model is required. This work describes the technical aspects of establishing GBS vaginal colonization in mice. This protocol achieves > 90% colonization of mice without the use of anesthetics to inoculate bacteria or to collect swab samples, immune-suppressants to enable colonization, vaginal pre-washing, or additives to thicken the inoculum. Moreover, this model demonstrates robust reproducibility, with modest inter-experimental variability in both the length of GBS persistence and the bacterial burden. The representative results demonstrated in this study are the compilation of independent experiments and should be a reference for future experimental design; however, direct comparisons across GBS strains and mouse lines should be made with care.
This model mimics human colonization in that mucosal vaginal GBS colonization of mice appears to be restricted to the reproductive tract. Although ascension into the cervix and uterus has been observed with multiple GBS strains25, mice do not display signs of morbidity or mortality, even after months of colonization. Furthermore, depending on the GBS and mouse strains studied, mice display consistent or transient vaginal colonization, which is useful for studying bacterial factors and host immune responses, respectively. In this model, some mice display intermittent colonization; it is currently unknown whether mice become recolonized at later time points or if colonization falls below the limit of detection, typically 50 to 100 CFU, at certain time points. Of note, transmission between mice has not been observed when colonized and non-colonized mice are housed together over several weeks (data not shown).
This method uses a commercially-available selective and differential medium for GBS to quantify mouse bacterial burdens. GBS grows as bright pink or mauve colonies that are readily visible after 24 hr of incubation. This media has been shown to have higher sensitivity for detecting GBS compared to other media, including blood agar and Granada medium32, and has been used in combination with latex bead agglutination tests to confirm GBS clinical isolates18. In this study, bright pink or mauve colonies from non-GBS colonized mice have never been recovered. Some endogenous flora, typically Enterococcus species, will grow as blue colonies, and some colonies will appear white, grey, or very pale pink. Importantly, plates should be counted after 18 to 24 hr of incubation, as non-GBS colonies may incorporate the pink pigment if left in the incubator or on the benchtop for longer periods. We have observed, as has been reported previously32, that some S. pyogenes isolates will form pink colonies on CHROMagar StrepB, but these colonies are typically smaller than GBS colonies. S. pyogenes has not been isolated from the endogenous vaginal flora of mice in these studies, but this observation should be considered in future work.
The majority of results were obtained from the outbred CD-1 mouse line, which demonstrates robust innate immune responses within the first few days post-inoculation, with subsequent bacterial clearance in the majority of mice19. Others have also tested additional GBS strains and have observed longer persistence times33,34. Since the development of this model, other groups have begun to develop similar mouse models of GBS vaginal colonization to examine the impact of host immune responses33,35, preventative therapies36,37, and transmission to the fetus in utero34,38. These differences may be explained by a variety of factors, including genetic determinants that impact immune responses and the composition of native vaginal flora. We have compiled a list of the GBS strains and respective mouse lines that have been studied to date in GBS vaginal colonization studies (Table 1). The number of recent studies with animal models highlights the interest and necessity of these types of models within the field of GBS pathogenesis research.
Within the vaginal tract, mucosal immunity is tightly regulated by steroid hormones44, and even one dose of β-estradiol, as described in this model, perturbs the host immune response. Even so, there are robust innate immune responses within the first few days of GBS colonization19,25, suggesting that affected immune responses are largely intact. Models that involve repeated β-estradiol injections may prolong GBS vaginal persistence, as demonstrated in this study (Figure 3) and by others33. However, immune responses and reproductive tract physiology may be more confounded, making results difficult to interpret. Importantly, the differences described across different GBS isolates and mouse strains in this study may be altered during models of sustained estrus and should be investigated in future work. Of note, neither estrus stage nor GBS serotype impacted vaginal colonization in a rat model22. Additionally, the human acidic vaginal pH of 3.6 to 4.545 drastically differs from the more neutral murine vaginal pH of 6.546, which may impact GBS gene expression and subsequent factors contributing to colonization. Lastly, native vaginal flora is distinct between humans and murine model counterparts, and future work should examine GBS colonization in gnotobiotic mice carrying human vaginal flora.
In summary, these studies have sought to examine host and bacterial factors that govern GBS vaginal colonization. Primarily, the robust, innovative animal model of GBS vaginal colonization developed in this work can be utilized to describe complex host-microbe interactions in an in vivo vaginal environment. The information obtained from this model has already greatly increased the knowledge of host immune components and specific GBS genes that control GBS vaginal persistence. Moreover, these results have raised additional questions and will be useful for further development of novel therapeutics to limit maternal GBS vaginal colonization and subsequent exposure of the newborn.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We would like to thank the vivarium manager and staff at San Diego State University for support with animal husbandry. During this work, K.A.P. was supported by an ARCS scholarship and a fellowship from the Inamori Foundation. K.S.D. is supported by an R01 grant, NS051247, from the National Institutes of Health.
Materials
| Sesame oil | Sigma Aldrich | S3547-250ML | |
| β-Estradiol | Sigma Aldrich | E8875-1G | CAUTION: Wear appropriate PPE. β-estradiol can be absorbed through the skin and mucosal surfaces. |
| 200 μL gel loading pipette tips | USA Scientific | 1252-0610 | |
| Urethro-genital, sterile, calcium alginate swabs | Puritan | 25-801 A 50 | |
| CHROMagar StrepB | DRG International | SB282 | |
| Todd Hewitt Broth | Hardy Diagnostics | 7161C | |
| 18 G, 1.5 inch needles | BD | 305199 | |
| 26 G, 0.5 inch needles | BD | 305111 | |
| 10 mL syringes | BD | 309604 | |
| 1 mL syringes | BD | 309659 | |
| 0.45 μm PVDF syringe filters | Whatman | 6900-2504 | |
| Dulbecco's Phosphate-Buffered Salt Solution 1X | Corning | 21-031-CV |
Referencias
- Verani, J. R., McGee, L., Schrag, S. J. Prevention of perinatal group B streptococcal disease–revised guidelines from CDC. MMWR. Recomm. Rep. 59 (RR-10), 1-36 (2010).
- Maisey, H. C., Doran, K. S., Nizet, V. Recent advances in understanding the molecular basis of group B Streptococcus virulence. Expert Rev. Mol. Med. 10, e27 (2008).
- Regan, J. A., Klebanoff, M. A., Nugent, R. P. The epidemiology of group B streptococcal colonization in pregnancy. Vaginal Infections and Prematurity Study Group. Obstet. Gynecol. 77 (4), 604-610 (1991).
- Stoll, B. J., Schuchat, A. Maternal carriage of group B streptococci in developing countries. Pediatr. Infect. Dis. J. 17 (6), 499-503 (1998).
- Brzychczy-Wloch, M., et al. Dynamics of colonization with group B streptococci in relation to normal flora in women during subsequent trimesters of pregnancy. New Microbiol. 37 (3), 307-319 (2014).
- Manning, S. D., Lewis, M. A., Springman, A. C., Lehotzky, E., Whittam, T. S., Davies, H. D. Genotypic diversity and serotype distribution of group B streptococcus isolated from women before and after delivery. Clin. Infect. Dis. 46 (12), 1829-1837 (2008).
- Le Doare, K., Heath, P. T. An overview of global GBS epidemiology. Vaccine. 31 (Suppl 4), D7-D12 (2013).
- Jensen, N. E., Andersen, B. L. The prevalence of group B streptococci in human urogenital secretions. Scand. J. Infect. Dis. 11 (3), 199-202 (1979).
- Honig, E., Mouton, J. W., van der Meijden, W. I. Can group B streptococci cause symptomatic vaginitis?. Infect. Dis. Obstet. Gynecol. 7 (4), 206-209 (1999).
- Muller, A. E., Oostvogel, P. M., Steegers, E. A., Dorr, P. J. Morbidity related to maternal group B streptococcal infections. Acta Obstet. Gynecol. Scand. 85 (9), 1027-1037 (2006).
- Ulett, K. B., et al. Diversity of group B streptococcus serotypes causing urinary tract infection in adults. J. Clin. Microbiol. 47 (7), 2055-2060 (2009).
- Kessous, R., et al. Bacteruria with group-B streptococcus: is it a risk factor for adverse pregnancy outcomes?. J. Matern. Fetal. Neonatal. Med. 25 (10), 1983-1986 (2012).
- Jelìnková, J., Grabovskaya, K. B., Rýc, M., Bulgakova, T. N., Totolian, A. A. Adherence of vaginal and pharyngeal strains of group B streptococci to human vaginal and pharyngeal epithelial cells. Zentralbl. Bakteriol. Mikrobiol. Hyg. A. 262 (4), 492-499 (1986).
- Zarate, G., Nader-Macias, M. E. Influence of probiotic vaginal lactobacilli on in vitro adhesion of urogenital pathogens to vaginal epithelial cells. Lett. Appl. Microbiol. 43 (2), 174-180 (2006).
- Johri, A. K., et al. Transcriptional and proteomic profiles of group B Streptococcus type V reveal potential adherence proteins associated with high-level invasion. Infect. Immun. 75 (3), 1473-1483 (2007).
- Park, S. E., Jiang, S., Wessels, M. R. CsrRS and environmental pH regulate group B streptococcus adherence to human epithelial cells and extracellular matrix. Infect. Immun. 80 (11), 3975-3984 (2012).
- Sheen, T. R., Jimenez, A., Wang, N. Y., Banerjee, A., van Sorge, N. M., Doran, K. S. Serine-rich repeat proteins and pili promote Streptococcus agalactiae colonization of the vaginal tract. J. Bacteriol. 193 (24), 6834-6842 (2011).
- Wang, N. Y., et al. Group B streptococcal serine-rich repeat proteins promote interaction with fibrinogen and vaginal colonization. J. Infect. Dis. 210 (6), 982-991 (2014).
- Patras, K. A., et al. Group B Streptococcus CovR regulation modulates host immune signalling pathways to promote vaginal colonization. Cell. Microbiol. 15 (7), 1154-1167 (2013).
- Furtado, D. Experimental group B streptococcal infections in mice: hematogenous virulence and mucosal colonization. Infect. Immun. 13 (5), 1315-1320 (1976).
- Cox, F. Prevention of group B streptococcal colonization with topically applied lipoteichoic acid in a maternal-newborn mouse model. Pediatr. Res. 16 (10), 816-819 (1982).
- Ancona, R. J., Ferrieri, P. Experimental vaginal colonization and mother-infant transmission of group B streptococci in rats. Infect. Immun. 26 (2), 599-603 (1979).
- Cheng, Q., Nelson, D., Zhu, S., Fischetti, V. A. Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob. Agents Chemother. 49 (1), 111-117 (2005).
- Faralla, C., et al. Analysis of two-component systems in group B Streptococcus shows that RgfAC and the novel FspSR modulate virulence and bacterial fitness. mBio. 5 (3), e00870-e00814 (2014).
- Patras, K. A., Rösler, B., Thoman, M. L., Doran, K. S. Characterization of host immunity during persistent vaginal colonization by. Group B Streptococcus. Mucosal Immunol. 8 (6), 1339-1348 (2015).
- Cavaco, C. K., et al. A novel C5a-derived immunobiotic peptide reduces Streptococcus agalactiae colonization through targeted bacterial killing. Antimicrob. Agents Chemother. 57 (11), 5492-5499 (2013).
- Patras, K. A., Wescombe, P. A., Rösler, B., Hale, J. D., Tagg, J. R., Doran, K. S. Streptococcus salivarius K12 limits group B Streptococcus vaginal colonization. Infect. Immun. 83 (9), 3438-3444 (2015).
- Shimizu, S. Routes of administration. The Laboratory Mouse. Chapter. 32, 534-535 (2004).
- Caligioni, C. S. Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. 48, A.4I.1-A.4I.8 (2009).
- Furr, P. M., Hetherington, C. M., Taylor-Robinson, D. The susceptibility of germ-free, oestradiol-treated, mice to Mycoplasma hominis. J. Med. Microbiol. 30 (3), 233-236 (1989).
- Mosci, P., et al. Mouse strain-dependent differences in estrogen sensitivity during vaginal candidiasis. Mycopathologia. 175 (1-2), 1-11 (2013).
- Poisson, D. M., Chandemerle, M., Guinard, J., Evrard, M. L., Naydenova, D., Mesnard, L. Evaluation of CHROMagar StrepB: a new chromogenic agar medium for aerobic detection of Group B Streptococci in perinatal samples. J. Microbiol. Methods. 82 (3), 238-242 (2010).
- Carey, A. J., et al. Infection and cellular defense dynamics in a novel 17beta-estradiol murine model of chronic human group B streptococcus genital tract colonization reveal a role for hemolysin in persistence and neutrophil accumulation. J. Immunol. 192 (4), 1718-1731 (2014).
- Randis, T. M., et al. Group B Streptococcus beta-hemolysin/cytolysin breaches maternal-fetal barriers to cause preterm birth and intrauterine fetal demise in vivo. J. Infect. Dis. 210 (2), 265-273 (2014).
- Gendrin, C., et al. Mast cell degranulation by a hemolytic lipid toxin decreases GBS colonization and infection. Sci Adv. 1 (6), e1400225 (2015).
- Santillan, D. A., Rai, K. K., Santillan, M. K., Krishnamachari, Y., Salem, A. K., Hunter, S. K. Efficacy of polymeric encapsulated C5a peptidase-based group B streptococcus vaccines in a murine model. Am. J. Obstet. Gynecol. 205 (3), e1-e8 (2011).
- De Gregorio, P. R., Juárez Tomás, M. S., Nader-Macìas, M. E. Immunomodulation of Lactobacillus reuteri CRL1324 on Group B Streptococcus Vaginal Colonization in a Murine Experimental Model. Am. J. Reprod. Immunol. 75 (1), 23-35 (2016).
- Whidbey, C., et al. A streptococcal lipid toxin induces membrane permeabilization and pyroptosis leading to fetal injury. EMBO Mol. Med. 7 (4), 488-505 (2015).
- Santillan, D. A., Andracki, M. E., Hunter, S. K. Protective immunization in mice against group B streptococci using encapsulated C5a peptidase. Am. J. Obstet. Gynecol. 198 (1), e1-e6 (2008).
- Cheng, Q., Fischetti, V. A. Mutagenesis of a bacteriophage lytic enzyme PlyGBS significantly increases its antibacterial activity against group B streptococci. Appl. Microbiol. Biotechnol. 74 (6), 1284-1291 (2007).
- De Gregorio, P. R., Juárez Tomás, M. S., Leccese Terraf, M. C., Nader-Macìas, M. E. In vitro and in vivo effects of beneficial vaginal lactobacilli on pathogens responsible for urogenital tract infections. J. Med. Microbiol. 63 (Pt 5), 685-696 (2014).
- De Gregorio, P. R., Juárez Tomás, M. S., Leccese Terraf, M. C., Nader-Macìas, M. E. Preventive effect of Lactobacillus reuteri CRL1324 on Group B Streptococcus vaginal colonization in an experimental mouse model. J. Appl. Microbiol. 118 (4), 1034-1047 (2015).
- Carey, A. J., et al. Interleukin-17A Contributes to the Control of Streptococcus pyogenes Colonization and Inflammation of the Female Genital Tract. Sci. Rep. 31 (6), 26836 (2016).
- Hickey, D. K., Patel, M. V., Fahey, J. V., Wira, C. R. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: stratification and integration of immune protection against the transmission of sexually transmitted infections. J. Reprod. Immunol. 88 (2), 185-194 (2011).
- Boskey, E. R., Telsch, K. M., Whaley, K. J., Moench, T. R., Cone, R. A. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect. Immun. 67 (10), 5170-5175 (1999).
- Meysick, K. C., Garber, G. E. Interactions between Trichomonas vaginalis and vaginal flora in a mouse model. J. Parasitol. 78 (1), 157-160 (1992).

