Generation of Human Monocyte-derived Dendritic Cells from Whole Blood
Summary
Here, we demonstrate how monocytes are isolated by magnetic bead separation from peripheral blood mononuclear cells after density gradient centrifugation of human anti-coagulated blood. Following incubation for 5 days, human monocytes are differentiated into immature dendritic cells and are ready for experimental procedures in a non-clinical setting.
Abstract
Dendritic cells (DCs) recognize foreign structures of different pathogens, such as viruses, bacteria, and fungi, via a variety of pattern recognition receptors (PRRs) expressed on their cell surface and thereby activate and regulate immunity.
The major function of DCs is the induction of adaptive immunity in the lymph nodes by presenting antigens via MHC I and MHC II molecules to naïve T lymphocytes. Therefore, DCs have to migrate from the periphery to the lymph nodes after the recognition of pathogens at the sites of infection. For in vitro experiments or DC vaccination strategies, monocyte-derived DCs are routinely used. These cells show similarities in physiology, morphology, and function to conventional myeloid dendritic cells. They are generated by interleukin 4 (IL-4) and granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulation of monocytes isolated from healthy donors. Here, we demonstrate how monocytes are isolated and stimulated from anti-coagulated human blood after peripheral blood mononuclear cell (PBMC) enrichment by density gradient centrifugation. Human monocytes are differentiated into immature DCs and are ready for experimental procedures in a non-clinical setting after 5 days of incubation.
Introduction
Dendritic cells (DCs) are the most important specialized antigen-presenting cells of our immune system. Immature DCs (iDCs) reside in the skin or in mucosal tissues and are therefore among the first immune cells to interact with invading pathogens. DCs represent the bridge between the innate and the adaptive immune system1, since they can activate T- and B-cell responses following pathogen detection. Furthermore, they contribute to pro-inflammatory immune responses because of the secretion of high amounts of cytokines, such as IL-1β, IL-6, and IL-12. DCs also activate NK cells and attract other immune cells to the site of infection by chemotaxis.
DCs can be divided into immature dendritic cells (iDCs) and mature dendritic cells (mDCs)2 based on their morphology and function. After the recognition of foreign antigens by one of the many pattern recognition receptors (e.g., toll-like receptors, C-type lectins, or complement receptors) abundantly expressed on the cell surface, iDCs undergo major changes and start to mature. During this maturation process, receptors for antigen capture are down-regulated, whereas molecules essential for antigen presentation are up-regulated3. Mature DCs up-regulate the major histocompatibility complexes I and II (MHC I and II), co-stimulatory molecules like CD80 and CD86, which are essential for antigen presentation and activation of T-lymphocytes. Additionally, the expression of chemokine receptor CCR7 on the cell surface is induced, which enables the migration of DCs from peripheral tissues to the lymph nodes. The migration is facilitated by the "rolling" of DCs along a chemokine ligand 19 (CCL19/MIP-3b) and chemokine ligand 21 (CCL21/SLC) gradient to the lymph nodes4-6.
Following migration, mDCs present the processed antigen to naïve CD4+ and CD8+ T cells, thus initiating an adaptive immune response against the invading pathogen7. This interaction with T cells in the lymph nodes is also associated with the spread of the virus8. Other in vitro studies revealed that DCs efficiently capture and transfer HIV to T cells and that this transmission results in a vigorous infection9-12. These experiments highlight that in vivo HIV exploits DCs as shuttles from the periphery to the lymph nodes. During antigen presentation, DCs secrete key interleukins that shape the differentiation of effector T helper cells, and therefore, the outcome of the entire immune response against the microbe is determined at this very interaction. Apart from type 1 (Th1) and type 2 (Th2) effector T cells, other subsets of CD4+ T helper cells (e.g., type 17 (Th17) and type 22 (Th22) T cells) have been described, and their induction and function have been investigated thoroughly. DCs are furthermore involved in the generation of regulatory T cells (Tregs)13,14. These cells are immunosuppressive and can stop or down-regulate induction or proliferation of effector T cells and are thus crucial for developing immunity and tolerance.
Human conventional DCs (cDCs) comprise several subsets of cells with a myeloid origin (i.e., Langerhans Cells (LCs) and dermal and interstitial DCs) or a lymphoid origin (i.e., plasmacytoid DCs (pDCs)). For in vitro experiments or DC vaccination strategies, monocyte-derived DCs are routinely used as a model for dermal DCs. These cells show similarities in physiology, morphology, and function to conventional myeloid dendritic cells. They are generated by the addition of interleukin 4 (IL-4) and granulocyte-macrophage colony-stimulating factor (GM-CSF) to monocytes isolated from healthy donors12,15-18. Dendritic cells can also be directly isolated from dermal or mucosal biopsies, or can even be developed from CD34+ hematopoietic progenitor cells isolated from umbilical cord blood samples obtained ex utero. Here, we demonstrate how monocytes are isolated and stimulated from anti-coagulated human blood after peripheral blood mononuclear cell (PBMC) enrichment by density gradient centrifugation. After incubation for 5 days, human monocytes under specific conditions are differentiated into iDCs and are ready for experimental procedures in a non-clinical setting.
Protocol
Ethics statement: Written informed consent was obtained from all participating blood donors by the Central Institute for Blood Transfusion & Immunological Department, Innsbruck, Austria. The use of anonymized leftover specimens for scientific purposes was approved by the Ethics Committee of the Medical University of Innsbruck.
1. Enrichment of Peripheral Blood Mononuclear Cells (PBMCs)
- Concentration of PBMCs by centrifugation.
- Open the blood pack and split blood in sterile 50 ml centrifuge tubes according to the amount of blood received.
- If necessary, adjust the volume to 50 ml for each tube with sterile Dulbecco's Phosphate-Buffered Saline (D-PBS).
- Place the tubes into a centrifuge and spin them at 400 x g for 15 min at room temperature (RT) without brake.
- Draw off the upper layer containing plasma and platelets using a sterile 10 ml serological pipette, leaving the boundary layer undisturbed.
NOTE: If autologous plasma instead of FCS is used to supplement the culture medium, the plasma must be collected and heat-inactivated in this step. - Collect the mononuclear cells (boundary layers) from two 50 ml centrifuge tubes and transfer them into one sterile 50 ml tube using a sterile 10 ml serological pipette.
- Adjust the volume to 50 ml for each tube using sterile D-PBS.
- Place the tubes into a centrifuge and spin them at 400 x g for 15 min at RT without brake.
- Draw off the upper layer containing the plasma and platelets using a sterile 10 ml serological pipette, leaving the boundary layer undisturbed.
- Collect the mononuclear cells (boundary layers) from the 50 ml centrifuge tubes and transfer them into two sterile 50 ml collection tubes using a sterile 10 ml serological pipette.
- Adjust the volume to 50 ml for each tube with sterile D-PBS.
- Separation of PBMCs by density gradient centrifugation (Figure 1).
- Prepare four 50 ml tubes and pipette 15 ml of density gradient medium into each tube.
- Take 25 ml of pooled cells/blood from the collection tube (step 1.1.10) and carefully overlay the density gradient medium.
- Place the tubes into a centrifuge and spin them at 700 x g for 30 min at RT without brake.
- Collection and purification of PBMCs.
- Draw off the upper layer containing the plasma and platelets using a sterile 10 ml serological pipette, leaving the PBMC layer undisturbed.
- Collect the PBMC interphase from all tubes and pool the cells in two sterile 50 ml tubes using a sterile 25 ml serological pipette.
- Adjust the volume to 50 ml for each tube with sterile D-PBS. Place the tubes into a centrifuge and spin them at 400 x g for 15 min at 4 °C with the brake.
- Aspirate the supernatant and re-suspend the cells in sterile D-PBS. Pool the cells from both tubes and adjust the volume to 50 ml with sterile D-PBS.
- Place the tubes into the centrifuge and spin them at 400 x g for 10 min at 4 °C with brake.
- Aspirate the supernatant and re-suspend the cells in sterile D-PBS. Adjust the volume to 50 ml with sterile D-PBS and pipette 50 µl to a 1.5 ml tube containing 450 µl of D-PBS.
- Vortex the 1.5 ml tube vigorously and count the cells in a Neubauer chamber or any other cell counting instrument.
- Place the 50 ml tubes into the centrifuge and spin them at 400 x g for 10 min at 4 °C with brake.
2. Isolation of Monocytes by Anti-human CD14 Magnetic Particles
- Labeling of PBMCs with magnetic particles.
- Adjust the PBMC concentration to 8 x 107 cells/ml using isolation buffer.
- Vortex the anti-human CD14 magnetic particles thoroughly and add 50 µl of particles for every 1 x 107 PBMCs.
- Mix the cell-particle suspension thoroughly and incubate it at RT for 30 min.
- Separation of the positive and negative fractions.
- Adjust the volume up to 12 ml using isolation buffer.
- Prepare six sterile round-bottom tubes and pipette 2 ml of the cell-particle suspension into each tube.
- Immediately place the tubes on the magnet. Incubate them for 10 min at RT.
- Keep the tubes on the magnet and carefully draw off the supernatant. The supernatant contains the negative fraction.
- Remove the tube from the magnet and add 2 ml of isolation buffer.
- Gently re-suspend the cells by pipetting up and down.
- Place the tubes back on the magnet. Incubate for 5 min at RT.
- Keep the tubes on the magnet and carefully draw off the supernatant. The supernatant contains the negative fraction.
- Remove the tubes from the magnet and add 2 ml of isolation buffer to each tube. Gently re-suspend the cells by pipetting up and down.
- Transfer the positive fraction to a sterile 50 ml tube and adjust the volume to 50 ml using sterile D-PBS.
3. Stimulation of Isolated Monocytes with IL-4 and GM-CSF
- Place the tube into the centrifuge and spin it at 400 x g for 10 min at 4 °C with brake.
- Aspirate the supernatant and re-suspend the cells in 50 ml of D-PBS.
- Pipette 50 µl into a 1.5 ml tube containing 450 µl of D-PBS. Vortex the 1.5 ml tube vigorously and count the cells in a Neubauer chamber or another cell counting instrument.
- Place the 50 ml tube into the centrifuge and spin it at 400 x g for 10 min at 4 °C with brake.
NOTE: If the negative fraction, containing peripheral blood lymphocytes (PBLs), is also required, perform washing and counting steps similar to those of the positive fraction (steps 3.1-3.5). - Adjust the cell concentration to 1 x 106/ml with pre-warmed culture medium (RPMI 1640 medium supplemented with 10% heat-inactivated FBS and 1% L-Glutamine solution) and pipette 3 ml/well into 6-well plates.
NOTE: If using heat-inactivated autologous plasma, replace the 10% FCS with 1-5% autologous plasma, according to the experimental set-up. - Stimulate the monocytes with recombinant human IL-4 (250 U/ml) and recombinant human GM-CSF (1,000 U/ml) by pipetting the cytokines directly into the culture medium. Incubate at 37 °C and 5% CO2.
- Re-stimulate the cells with IL-4 (250 U/ml) and GM-CSF (1000 U/ml) after 48 hr by pipetting the cytokines directly into the culture medium.
NOTE: If there are any signs of acidification shown by the pH indicator in the medium, replace half of the medium with pre-warmed culture medium. - Harvest immature dendritic cells on day 5 for down-stream experiments by pipetting the cultured cells out of the 6-well plate and into a 50 ml centrifuge tube after pipetting the culture medium up and down a few times.
- Count the cells and stain a sample of isolated monocytes with anti-human antibodies against CD11b, CD11c, and DC-SIGN/CD209, together with a cell viability dye. To avoid unspecific binding of the antibodies during surface staining, the use of Fc receptor blocking reagents or bovine serum albumin (BSA) buffers is highly recommended.
- Analyze the sample using the cell viability dye, according to the manufacturer's protocol, by performing flow cytometry to assess the purity and viability of the generated iDCs.
Representative Results
After centrifugation of anti-coagulated blood using a sucrose cushion, peripheral blood mononuclear cells (PBMCs) are enriched in an interphase on top of the density gradient medium (Figure 1). After the PBMCs are drawn off, FACS analysis is performed to characterize the different cell populations within the PBMCs using lineage markers (e.g., CD3 for T lymphocytes, CD14 for monocytes, and CD19 for B lymphocytes). Figure 2A shows the results of a representative FACS analysis of PBMCs collected and stained after density gradient centrifugation. Out of the gated population (Figure 2A, upper plot), we detected 4.03% B lymphocytes, 28.20% monocytes, and 67.62% other cells (Figure 2A, middle plot), of which 43.62% were T lymphocytes (Figure 2A, lower plot).
PBMCs are then incubated with CD14 magnetic particles and, following incubation on a magnet, the negative fraction containing the peripheral blood lymphocytes (PBLs) is analyzed by FACS using the selection of abovementioned lineage markers. Compared to PBMCs, we measured similar percentages of B lymphocytes (4.65%, Figure 2B, middle plot), higher percentages of other cells (88.61%, Figure 2B, middle plot), and strongly reduced percentages of monocytes (6.59%, Figure 2B, middle plot). Characterization of the positive fraction (Figure 3) demonstrated a high separation efficiency, since the purity of the isolated cells is over 97% (Figure 3, left plot), of which 99.56% (Figure 3, right plot) expressed high levels of CD14 on the cell surface.
IL4 and GM-CSF stimulation of monocytes for 5 days results in differentiation into monocyte-derived dendritic cells, which are comparable to dermal dendritic cells in morphology, behavior, and receptor expression19. FACS analysis of monocyte-derived dendritic cells on day 5 shows a homologous population (Figure 4, left plot) expressing high levels of CD11b, CD11c (not shown), and the C-type lectin DC-SIGN. No expression of the DC maturation marker CD83 is detected (Figure 4, right plot), and cells also lose the monocyte marker CD14 (not shown).
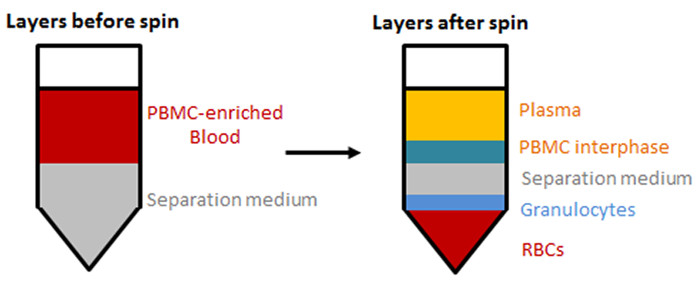
Figure 1: Separation of blood components by density gradient centrifugation. PBMCs are enriched in an interphase on top of the separation medium by density gradient centrifugation. Layers before (left) and after (right) centrifugation are shown. Please click here to view a larger version of this figure.
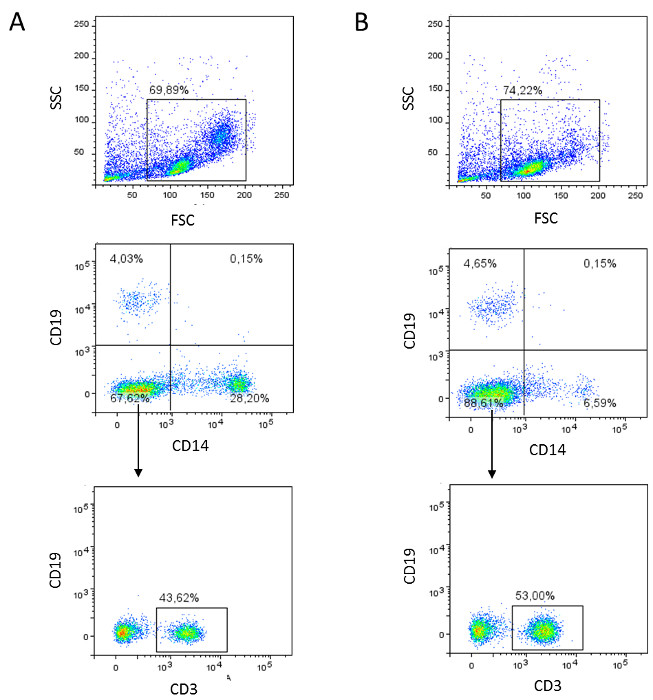
Figure 2: Flow cytometric analyses of PBMCs (A) and PBLs (B). (A) PBMCs are harvested, stained for lineage markers (mouse-anti-human CD3, CD14, and CD19 antibodies), and analyzed by flow cytometry. Dot plots of a representative result are shown. (B) The negative fraction containing PBLs is characterized by lineage markers (mouse-anti-human CD3, CD14, and CD19 antibodies) and analyzed by flow cytometry. Dot plots of a representative result are shown. Please click here to view a larger version of this figure.
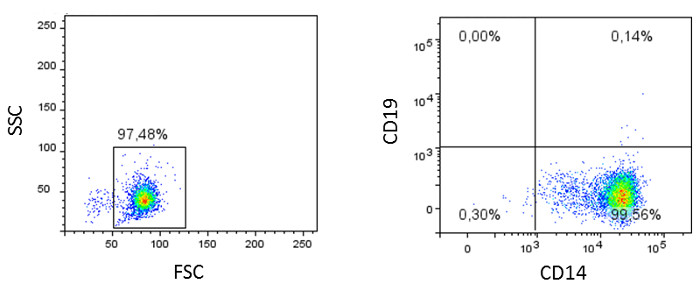
Figure 3: Flow cytometric analyses of monocytes. The isolated monocytes (positive fraction) are characterized using lineage markers (mouse-anti-human CD3, CD14, and CD19 antibodies) and analyzed by flow cytometry. Dot plots of a representative result are shown.
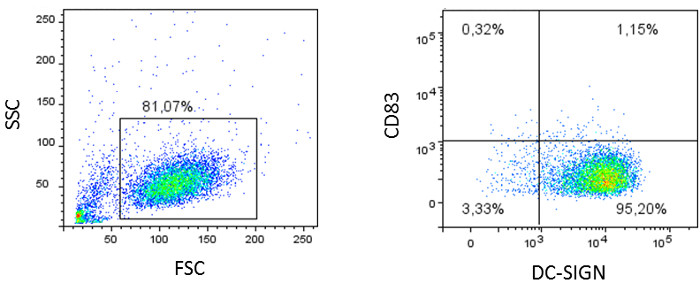
Figure 4: Flow cytometric analyses of monocyte-derived dendritic cells. Monocytes are stimulated for 5 days with IL4 and GM-CSF and stained for characteristic DC markers, like CD11b, CD11c (not shown), the C-type lectin DC-SIGN, and the maturation marker CD83. Dot plots of a representative result are shown.
Discussion
This protocol describes the generation of monocyte-derived dendritic cells (MDDCs) through the isolation of human monocytes from anti-coagulated blood using a magnetic nanoparticle-based assay. In this protocol, the centrifugation steps are performed upstream the cell isolation procedure, which leads to an enrichment of the PBMC fraction. Although cells are lost during centrifugation, to overlay the content of a whole blood pack on density gradient medium would require 200-300 ml of the density gradient medium and therefore cannot be considered cost efficient. Additionally, the enrichment of PBMCs by centrifugation results in a cleaner cell population, which positively influences the attachment of the anti-human CD14 magnetic particles during the isolation process. Magnetic-nanoparticle isolation of monocytes results in a viable monocyte population, which can be further used for in vitro differentiation to various DC subsets or macrophages.
Advantages of this technique compared to other protocols used in the past, like adherence-mediated purification on tissue culture or gelatin-coated plastic dishes, are the high purity and homogeneity of the isolated cell population, as well as the convenience and speed. In general, it is important to work quickly but thoroughly when working with cells in order to avoid prolonged exposure to non-physiological conditions. The anti-human CD14 magnetic particles used in this protocol are optimized for the positive selection or depletion of CD14-bearing leukocytes; therefore, the magnetic nanoparticles bind directly to the cells of interest and are not removed after the procedure. This could be an issue that researchers must address before certain experiments or analyses are performed, since results could be influenced or altered by cell-bound magnetic nanoparticles. Alternatively, negative selection assays are available, which are designed to deplete unwanted cells and to keep the cells of interest untouched. For the generation of MDDCs, the anti-human CD14 magnetic nanoparticles used to isolate monocytes present no complication because, during the differentiation into MDDCs, cytokine-stimulated monocytes down-regulate the CD14 receptor. Thus, the magnetic nanoparticles are removed without further elution or detaching procedures, and the MDDCs are tested negative for CD14 receptor expression by flow cytometry. Since culture medium containing FCS is used during the differentiation in this protocol, MDDCs obtained here are for research use only. For MDDCs in clinical settings, special media without the need of serum have been developed. In addition, autologous plasma can be collected after the first centrifugation step (step 1.1.4), heat-inactivated, and used in the culture medium. Besides autologous plasma, commercially-available human plasma can also be added to the culture medium to avoid possible background reactivity in long-term assays due to xenogeneic proteins in FCS.
Alternatively, dendritic cells can be directly isolated from dermal or mucosal biopsies or can be developed from CD34+ hematopoietic progenitor cells isolated from umbilical cord blood samples obtained ex utero.
Nonetheless, MDDCs are the most widely-used model for dendritic cells, since the direct isolation of DCs from biopsies is more complex to perform. It can also lead to inefficient cell numbers that often vary due to differences in the donor quality. Similar problems can occur when DCs are generated from CD34+ stem cells isolated from cord blood.
For future applications, inducible pluripotent stem cells (iPSCs) could be used instead of CD14-magnetic bead isolation of monocytes from blood. The advantages of using iPSCs are that not only DC subsets, but all hematopoietic cells of interest, can be generated from one donor (T cells, B cells, and NK cells) and that patient-specific iPSCs can be used to characterize the interactions of autologous immune cells in a personalized manner.
The most critical step during the isolation of monocytes is the density gradient centrifugation step, since accurate separation of the red and white blood cells is only achieved when the brake of the centrifuge is turned off. This determines the outcome of the down-stream isolation efficiency.
In conclusion, this protocol is a very fast, efficient, and cost-effective way to generate a viable and homogenous monocyte-derived dendritic cell population through the isolation of human monocytes from anti-coagulated blood.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We would like to thank our technician Karolin Thurnes, Divison of Hygiene and Medical Microbiology, and Dr. Annelies Mühlbacher and Dr. Paul Hörtnagl, Central Institute for Blood Transfusion and Immunological Department, for their valuable help and support regarding this manuscript. We thank the Austrian Science Fund for supporting this work (P24598 to DW, P25389 to WP).
Materials
| APC Mouse Anti-Human CD19 Clone HIB19 | BD Biosciences | 555415 | |
| APC Mouse Anti-Human CD83 Clone HB15e | BD Biosciences | 551073 | |
| BD Imag Anti-Human CD14 Magnetic Particles | BD Biosciences | 557769 | |
| BD Imagnet | BD Biosciences | 552311 | |
| BSA (Albumin Fraction V) | Carl Roth | EG-Nr 2923225 | |
| Costar 6 Well Clear TC-Treated Multiple Well Plates | Costar | 3506 | |
| Density gradient media: Ficoll-Paque Premium | GE Healthcare Bio-Sciences | 17-5442-03 | |
| Dulbecco’s Phosphate Buffered Saline (D-PBS) | Sigma-Aldrich | D8537 | |
| Falcon 10mL Serological Pipet | Corning | 357551 | |
| Falcon 25mL Serological Pipet | Corning | 357525 | |
| Falcon 50mL High Clarity PP Centrifuge Tube | Corning | 352070 | |
| Falcon Round-Bottom Tubes | Corning | 352054 | |
| FITC Mouse Anti-Human CD3 Clone HIT3a | BD Biosciences | 555339 | |
| Ghost Dye Violet 510 (Cell Viability Reagent) | Tonbo biosciences | 13-0870 | |
| GM-CSF | MACS Miltenyi Biotec | 130-093-862 | |
| Heat Inactivated FBS (Fetal Bovine Serum), EU Approved Origin (South America) | Gibco | 10500-064 | |
| Hettich Rotanta 460R | Hettich | — | |
| IL-4 CC | PromoKine | C-61401 | |
| Isolation buffer: BD IMag Buffer (10X) | BD Biosciences | 552362 | |
| L-Glutamine solution | Sigma-Aldrich | G7513 | |
| Microcentrifuge tubes, 1,5 ml, SuperSpin | VWR | 211-0015 | |
| PE Mouse Anti-Human CD14 Clone M5E2 | BD Biosciences | 555398 | |
| PE Mouse Anti-Human CD209 Clone DCN46 | BD Biosciences | 551265 | |
| RPMI-1640 medium | Sigma-Aldrich | R0883 | |
| UltraPure 0.5M EDTA, pH 8.0 | Invitrogen | 15575020 |
Referencias
- Banchereau, J., Steinman, R. M. Dendritic cells and the control of immunity. Nature. 392, 245-252 (1998).
- Steinman, R. M., Hemmi, H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 311, 17-58 (2006).
- Steinman, R. M., Inaba, K., Turley, S., Pierre, P., Mellman, I. Antigen capture, processing, and presentation by dendritic cells: recent cell biological studies. Hum Immunol. 60, 562-567 (1999).
- Schwarz, J., Sixt, M. Quantitative Analysis of Dendritic Cell Haptotaxis. Methods Enzymol. 570, 567-581 (2016).
- Weber, M., Sixt, M. Live cell imaging of chemotactic dendritic cell migration in explanted mouse ear preparations. Methods Mol Biol. 1013, 215-226 (2013).
- Sixt, M., Lammermann, T. In vitro analysis of chemotactic leukocyte migration in 3D environments. Methods Mol Biol. 769, 149-165 (2011).
- Randolph, G. J., Angeli, V., Swartz, M. A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 5, 617-628 (2005).
- Wilflingseder, D., Banki, Z., Dierich, M. P., Stoiber, H. Mechanisms promoting dendritic cell-mediated transmission of HIV. Mol Immunol. 42, 229-237 (2005).
- Pope, M., Gezelter, S., Gallo, N., Hoffman, L., Steinman, R. M. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J Exp Med. 182, 2045-2056 (1995).
- McDonald, D., et al. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 300, 1295-1297 (2003).
- Pruenster, M., et al. C-type lectin-independent interaction of complement opsonized HIV with monocyte-derived dendritic cells. Eur J Immunol. 35, 2691-2698 (2005).
- Wilflingseder, D., et al. IgG opsonization of HIV impedes provirus formation in and infection of dendritic cells and subsequent long-term transfer to T cells. J Immunol. 178, 7840-7848 (2007).
- Kapsenberg, M. L. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 3, 984-993 (2003).
- Mills, K. H. TLR-dependent T cell activation in autoimmunity. Nat Rev Immunol. 11, 807-822 (2011).
- Banki, Z., et al. Complement as an endogenous adjuvant for dendritic cell-mediated induction of retrovirus-specific CTLs. PLoS Pathog. 6, e1000891 (2010).
- Posch, W., et al. Antibodies attenuate the capacity of dendritic cells to stimulate HIV-specific cytotoxic T lymphocytes. J Allergy Clin Immunol. 130, 1368-1374 (2012).
- Posch, W., et al. Complement-Opsonized HIV-1 Overcomes Restriction in Dendritic Cells. PLoS Pathog. 11, e1005005 (2015).
- Wilflingseder, D., et al. Immediate T-Helper 17 Polarization Upon Triggering CD11b/c on HIV-Exposed Dendritic Cells. J Infect Dis. 212, 44-56 (2015).
- Grassi, F., et al. Monocyte-derived dendritic cells have a phenotype comparable to that of dermal dendritic cells and display ultrastructural granules distinct from Birbeck granules. J Leukoc Biol. 64, 484-493 (1998).

