An Efficient Method for the Isolation of Highly Purified RNA from Seeds for Use in Quantitative Transcriptome Analysis
Summary
We have succeeded in establishing a method for RNA isolation from plant seeds containing large amounts of oils, proteins, and polyphenols, which have inhibitory effects on high-purity RNA isolation. Our method is suitable for monitoring the expression of genes with low level transcripts in seeds.
Abstract
Plant seeds accumulate large amounts of storage reserves comprising biodegradable organic matter. Humans rely on seed storage reserves for food and as industrial materials. Gene expression profiles are powerful tools for investigating metabolic regulation in plant cells. Therefore, detailed, accurate gene expression profiles during seed development are required for crop breeding. Acquiring highly purified RNA is essential for producing these profiles. Efficient methods are needed to isolate highly purified RNA from seeds. Here, we describe a method for isolating RNA from seeds containing large amounts of oils, proteins, and polyphenols, which have inhibitory effects on high-purity RNA isolation. Our method enables highly purified RNA to be obtained from seeds without the use of phenol, chloroform, or additional processes for RNA purification. This method is applicable to Arabidopsis, rapeseed, and soybean seeds. Our method will be useful for monitoring the expression patterns of low level transcripts in developing and mature seeds.
Introduction
Plants produce seeds, which give rise to the next generation. Seeds accumulate large amounts of storage reserves, such as oils, carbohydrates, and proteins, for post-germinative growth. Humans utilize seed storage reserves as sources of food and animal feed, and thus plant seeds are one of the major suppliers of edible organic matter worldwide. Increasing seed yields is an important challenge in plant science.
Since seed storage reserves are commercially valuable sources of food and industrial materials, the molecular mechanisms underlying the regulation of the metabolism of these reserves have been widely investigated1-6. Further elucidating these mechanisms will be useful for increasing seed yields in crops. Seeds develop in plant ovaries after fertilization, and they mature through a series of developmental stages1,6,7. Further understanding the molecular mechanism underlying seed development requires detailed, precise gene expression profiles from a series of developing seeds to be produced. However, the high amounts of oils, proteins, carbohydrates, and polyphenols in plant seeds make it difficult to isolate highly purified RNA, which precludes precise profiling of gene expression.
Here, we introduce an efficient method for RNA isolation from oilseeds containing large amounts of oils, proteins, and polyphenols. Using this method, researchers will be able to prepare highly purified RNA. Such RNA will be useful for monitoring transcriptional changes in key genes controlling the metabolic regulation of seed storage reserves in developing and mature oilseeds.
Protocol
1. Extraction of Total RNA from Plant Seeds
- Prepare buffer sets, spin columns, 1.5 and 2.0 mL polypropylene tubes, and nuclease-free 1.5 mL polypropylene tubes.
- Add 1% (w/v) molecular biology grade polyvinylpyrrolidone (hereafter referred to as PVP) to cell lysis buffer for RNA extraction and vortex vigorously. Incubate for 20 min at 25 °C to dissolve completely. After 20 min incubation, mix the buffer gently by turning the tube upside down to prevent the formation of bubbles. Store at room temperature (15-25 °C) before use.
- Harvest fruits from Arabidopsis thaliana plants and place in 1.5 mL polypropylene tubes on ice. Place fruits on aluminum plates kept at 4 °C and isolate seeds from the fruits under a stereo microscope.
- Place the isolated seeds (approximately 200 seeds) into 1.5 mL polypropylene tubes that have been stored in an aluminum rack on ice and immediately place the tubes in liquid nitrogen.
- Remove the tubes from the liquid nitrogen and return them to the aluminum rack on ice. Add 100 µL of the buffer containing 1% PVP and centrifuge for 1 min at 1,000 x g at 4 °C.
- Homogenize the sample with a stainless steel pestle using a motor-grinder for 60 s while keeping the tube in the aluminum rack on ice.
- Add 550 µL of the buffer containing 1% PVP, mix the buffer gently by turning the tube upside down, and incubate for 10 min at 25 °C.
- Centrifuge for 5 min at 8,000 x g at 25 °C and transfer 550 µL of the supernatant to a new 1.5 mL polypropylene tube.
- Centrifuge for 5 min at 10,000 x g at 25 °C and transfer 450 µL of the supernatant to a new 1.5 mL polypropylene tube.
- Use the supernatant as the cell lysate for RNA extraction. Hereafter, follow the procedure described in the manufacturer's instructions in the commercially available kit.
- Elute the RNA using a minimum volume of the elution buffer to ensure a sufficiently high concentration of RNA for monitoring the expression patterns of low level transcripts. Store the RNA in a deep freezer until used.
2. Verification of RNA Quality
- Thaw the total RNA, mix by gentle tapping, and place the tube in an aluminum rack on ice.
- Measure the RNA concentration, A260/A280 ratio, and A260/A230 ratio using a microvolume spectrophotometer.
- Dilute the total RNA in RNase-free water to a final concentration of 70 ng/µL.
3. Reverse Transcription of Total RNA from Seeds
- Thaw the total RNA and buffer sets from the commercial kit. Keep the enzymes from the kit on ice after gentle tapping. Prepare nuclease-free 0.2 mL polypropylene tubes.
- Add 5 µL of total RNA, 1 µL of Oligo dT primer (50 µM), and 1 µM of Random 6-mer (50 µM) to the 0.2 mL polypropylene tube on ice.
- Incubate for 15 min at 37 ºC and place on ice.
- Hereafter, follow the procedure described in the manufacturer's instructions in the reverse transcription-PCR kit.
- Place the reverse transcription products on ice before use.
4. Quantitative Real-time PCR Analysis
- Construct plasmids harboring the target gene sequences using the manufacturer's protocol.
- Adjust the concentrations to 100-500,000 copies/µL for DNA templates for the standard curves.
- Dilute the cDNA solutions (1:100) with distilled water.
- Add 2 µL of the diluted cDNA solutions and the plasmids for the standard curves to the master mix from the quantitative real-time PCR kit.
- Set up real-time PCR using the following cycling conditions: 95 °C for 30 s and then 40 cycles at 95 °C for 5 s and 60 °C for 35 s.
- Analyze the copy numbers according to the manufacturer's instructions for the real-time PCR system.
Representative Results
We first investigated the optimal concentration of PVP using Arabidopsis mature seeds. Total RNA was isolated from approximately 1,000 seeds according to the protocol described above using cell lysis buffer containing 0%, 0.25%, 0.5%, 1.0% or 2.0% PVP. After homogenization and centrifugation, the supernatant was collected while avoiding the oil layer and seed debris (Figure 1A).
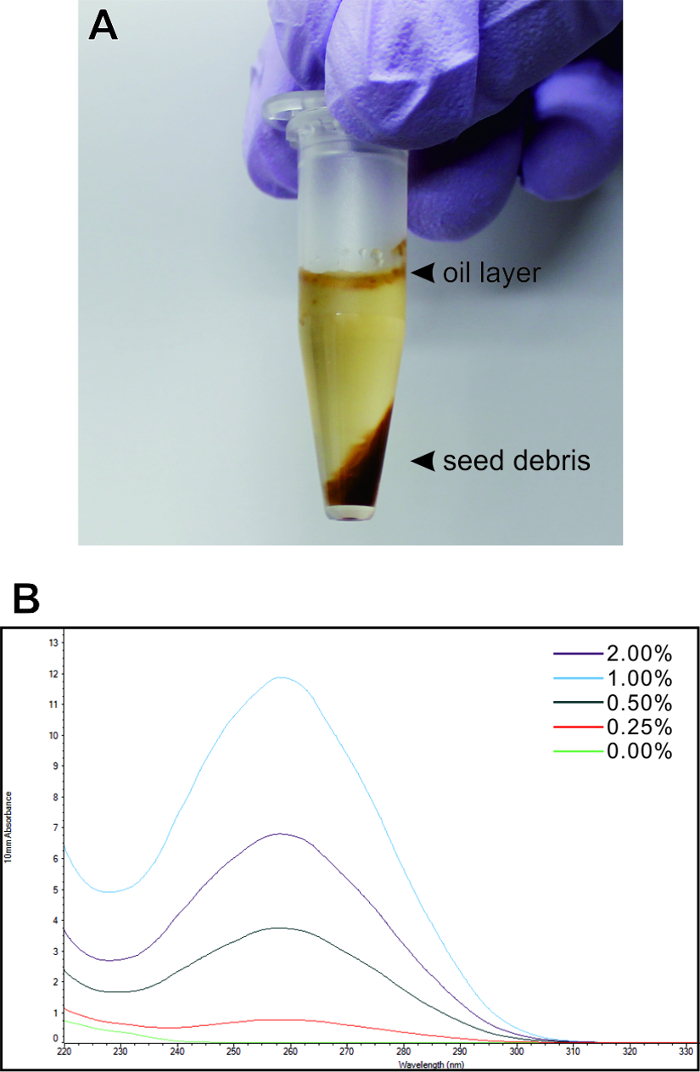
Figure 1: Estimation of the optimal polyvinylpyrrolidone concentration in the cell lysis buffer. Total RNA was extracted with the cell lysis buffer containing different concentrations of PVP. (A) A photograph of the seeds after homogenization and centrifugation. (B) The graphs of wavelength absorbance for each sample are indicated. Please click here to view a larger version of this figure.
The quantities and purity of the isolated RNAs were estimated from the graph of wavelength absorbance (Figure 1B), which showed that 1.0% PVP was the most effective concentration of isolating large amounts of purified RNA from seeds.
Developing seeds were isolated from Arabidopsis thaliana fruits on pre-chilled aluminum plates on ice under a stereomicroscope (Figures 2A, 2B). The seeds were homogenized with a stainless steel pestle and motor-grinder in a tube on an aluminum rack on ice (Figures 2C, 2D).
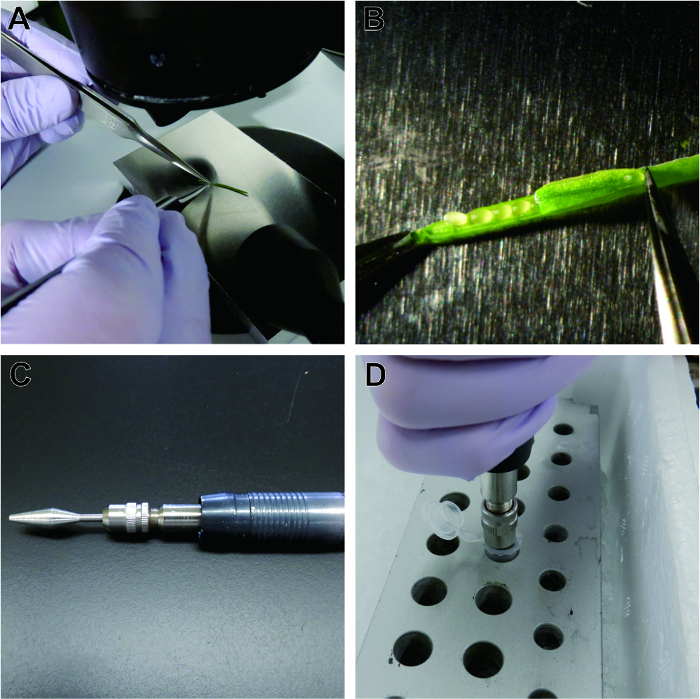
Figure 2: Isolation and homogenization of developing seeds of Arabidopsis thaliana. Developing seeds are isolated from fruits on a cold aluminum plate under a stereomicroscope (A). Carpels are peeled from the pedicle (B), and developing seeds are collected. The seeds are homogenized in lysis buffer containing 1% (w/v) polyvinylpyrrolidone using a stainless steel pestle (C) in tubes in an aluminum rack chilled on ice (D). Please click here to view a larger version of this figure.
Total RNA was isolated from the seeds at 4, 8, and 12 days after flowering (hereafter referred to as DAF) using our newly developed method or the conventional method. The concentrations, A260/A280 ratios, and A260/A230 ratios of RNA isolated from 200 seeds are shown in Table 1.
| Method | DAF | RNA concentration | A260/A280 | A260/A230 |
| Conventional | 4 | 72.4 ± 7.4 | 1.90 ± 0.02 | 2.27 ± 0.06 |
| 8 | 10.8 ± 3.0 | 1.50 ± 0.08 | 1.40 ± 0.17 | |
| 12 | 4.9 ± 1.7 | 1.57 ± 0.10 | 0.76 ± 0.18 | |
| Nuevo | 4 | 63.7 ± 2.6 | 2.10 ± 0.08 | 2.29 ± 0.03 |
| 8 | 46.3 ± 4.9 | 2.15 ± 0.05 | 2.23 ± 0.09 | |
| 12 | 41.7 ± 3.1 | 2.09 ± 0.04 | 2.17 ± 0.07 |
Table 1: Concentrations, A260/A280 ratios, and A260/A230 ratios of RNA isolated from Arabidopsis seeds. Total RNA was isolated from approximately 200 seeds (from 190 to 206 seeds) at 4, 8, and 12 DAF using the conventional method or our newly developed method. The RNA concentrations, A260/A280 ratios, and A260/A230 ratios were measured. Values represent mean ± SD of three independent experiments.
The results show that our method enabled us to isolate highly purified RNA from 8 and 12 DAF seeds. The total RNA was reverse-transcribed to cDNA, and the cDNA solutions were diluted 100-fold. The copy numbers of WRI1 (WRINKLED 1: AT3G54320), SDP1 (SUGAR DEPENDENT1: AT5G04040)7, and EIF3K (eukaryotic translation initiation factor 3K: AT4G33250) as an internal control8 were measured, and the relative amounts of the transcripts were estimated by quantitative real-time PCR analyses (Figure 3).
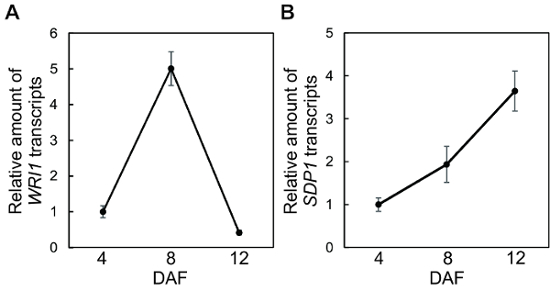
Figure 3: Quantification of low level transcripts in developing seeds. Total RNA was extracted from 4, 8, and 12 DAF seeds using our method, and cDNA solutions were prepared. The relative amounts of WRI1 (A) and SDP1 (B) transcripts were measured by quantitative real-time PCR. EIF3K was used as an internal control. Values represent mean SD of three independent experiments. DAF; days after flowering. Please click here to view a larger version of this figure.
Although the amounts of WRI1 and SDP1 transcripts are relatively low in developing seeds, it was possible to detect expression changes between seeds at different developmental stages. To verify that our method can be utilized in other oilseeds, total RNA was isolated from mature seeds of Brassica napus and Glycine max. The RNA concentrations, A260/A280 ratios, and A260/A230 ratios are shown in Table 2.
| Method | Plant | RNA concentration | A260/A280 | A260/A230 |
| Conventional | B. napus | 7.7 ± 0.8 | 1.5 ± 0.12 | 0.75 ± 0.11 |
| G. max | 9.7 ± 2.3 | 1.61 ± 0.06 | 0.69 ± 0.09 | |
| New | B. napus | 143.2 ± 19.0 | 2.17 ± 0.03 | 2.23 ± 0.13 |
| G. max | 152.3 ± 4.4 | 2.17 ± 0.02 | 2.30 ± 0.02 |
Table 2: Concentrations, A260/A280 ratios, and A260/A230 ratios of RNA isolated from mature seeds of Brassica napus and Glycine max. Total RNA was isolated from approximately 100 mg of Brassica napus and Glycine max seeds using the conventional method or our newly developed method. The seeds were previously crushed and roughly homogenized with a mortar and pestle chilled in liquid nitrogen. The RNA concentrations, A260/A280 ratios, and A260/A230 ratios were measured. Values represent mean ± SD of three independent experiments.
Discussion
Gene expression profiles contribute to our understanding of plant physiology; therefore, specific RNA isolation methods have been developed for each sample condition9-12. We investigated the processes that were inhibited during RNA isolation from seeds and found that RNA binding to silica membranes was severely inhibited. Large amounts of oil, proteins, and polyphenols inhibit RNA isolation. We modified the RNA extraction process to remove these compounds with a lysis solution before the process of RNA binding to silica membranes. The important modifications included the addition of 1% PVP (step 1.2) and the collection of the supernatant from centrifuged lysis solution (steps 1.7, 1.8). The aim of the addition of 1% PVP is to remove polyphenols. We examined the most effective concentration of PVP to use and confirmed that 1% PVP is the most effective concentration for this process. The aim of collecting the supernatant from centrifuged lysis solution is to remove oil and proteins. The supernatant should be collected carefully to avoid retaining an oil layer in the supernatant and the pellet in the tube. These two simple modifications drastically improved the recovery, A260/A280 ratios, and A260/A230 ratios of the isolated RNA from seeds at the middle and late phases of development (Table 1).
Our method, which represents a simple modification of the standard protocol, uses commercially available kits for RNA isolation. Thus, our method is practical for isolating highly purified RNA from seeds without the need for cumbersome additional steps such as phenol-chloroform extraction and ethanol precipitation using lithium chloride. Our method is also effective for use with mature rapeseed and soybean seeds (Table 2), indicating that the modifications work well for oil- and protein-rich crop seeds. The RNA recovery rates from 4 DAF seeds (Table 1) and young roots, leaves and stems were lower using our method, indicating that our method is not suitable for RNA isolation from tissues that are poor in oil and proteins. Therefore, the method is advantageous only for the isolation of RNA from tissues containing oil, proteins, and polyphenols, such as seeds and fruits.
Overall, our method is suitable for acquiring highly purified RNA from plant seeds without the use of phenol, chloroform, or additional processes for RNA purification. This method will facilitate more precise profiling of gene expression in seeds.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We thank the staff of Functional Genomics Facility and Spectrography and Bioimaging Facility, NIBB Core Research Facilities, and Model Plant Research Facility, NIBB Bioresource Center.
Materials
| RNeasy Plant Mini Kit | QIAGEN | 74904 | |
| polyvinylpyrrolidone | Sigma-Aldrich | P5288-100G | |
| HOMOGENIZER S-303 | AS ONE | 1-1133-02 | |
| NanoDrop Lite | Thermo Scientific | ND-NDL-US-CAN | |
| PrimeScript RT reagent Kit (Perfect Real Time) | TAKARA | RR037A | |
| KAPA SYBR Fast qPCR kit | Kapa biosystems | KK4601 |
Referencias
- Hills, M. J. Control of storage-product synthesis in seeds. Curr Opin Plant Biol. 7 (3), 302-308 (2004).
- Li-Beisson, Y., et al. Acyl-lipid metabolism. Arabidopsis Book. 11, e0161 (2013).
- Bates, P. D., Stymne, S., Ohlrogge, J. Biochemical pathways in seed oil synthesis. Curr Opin Plant Biol. 16 (3), 358-364 (2013).
- Santos-Mendoza, M., et al. Deciphering gene regulatory networks that control seed development and maturation in Arabidopsis. Plant J. 54 (4), 608-620 (2008).
- Durrett, T. P., Benning, C., Ohlrogge, J. Plant triacylglycerols as feedstocks for the production of biofuels. Plant J. 54 (4), 593-607 (2008).
- Kanai, M., et al. The Plastidic DEAD-box RNA helicase 22, HS3, is essential for plastid functions both in seed development and in seedling growth. Plant Cell Physiol. 54 (9), 1431-1440 (2013).
- Kanai, M., et al. Extension of oil biosynthesis during the mid-phase of seed development enhances oil content in Arabidopsis seeds. Plant Biotechnol J. 14 (5), 1241-1250 (2016).
- Dekkers, B. J., et al. Identification of reference genes for RT-qPCR expression analysis in Arabidopsis and tomato seeds. Plant Cell Physiol. 53 (1), 28-37 (2012).
- Salzman, R. A., et al. An improved RNA isolation method for plant tissues containing high levels of phenolic compounds or carbohydrates. Plant Mol Biol Rep. 17 (1), 11-17 (1999).
- Vicient, C. M., Delseny, M. Isolation of total RNA from Arabidopsis thaliana seeds. Anal Biochem. 268 (2), 412-413 (1999).
- Wang, G. F., et al. Isolation of high quality RNA from cereal seeds containing high levels of starch. Phytochem Analysis. 23 (2), 159-163 (2012).
- Birtic, S., Kranner, I. Isolation of high-quality RNA from polyphenol-, polysaccharide- and lipid-rich seeds. Phytochem Analysis. 17 (3), 144-148 (2006).

